Abstract
Purpose
This study aimed to analyze the impact of different biopsy protocols on the rate of mosaic blastocysts.
Methods
This is a retrospective cohort study which included 115 cycles with pre-implantation genetic testing for aneuploidy (PGT-A). Two groups were allocated based on the biopsy protocols: method 1 group, the zona pellucida (ZP) was drilled on day 3 embryos followed by trophectoderm (TE) biopsy; and method 2 group, the ZP was opened on day 5 or 6 blastocysts followed by TE biopsy. All biopsy samples were assessed using next-generation sequencing (NGS) at a single reference laboratory. The euploid, aneuploid, and mosaic blastocyst rates and clinical outcomes were compared.
Results
The mosaicism rate in the method 1 group was 19.58%, significantly higher than the method 2 group (8.12%; P < 0.05). No statistically significant difference was observed in euploid, aneuploid blastocyst rates, and clinical pregnancy rates between the two groups. Logistic regression analysis indicated that the biopsy protocols were independently associated with the mosaicism rates among all the variables.
Conclusions
The present study showed that different biopsy protocols may have an impact on the mosaic blastocyst rate. ZP opening on day 3 combined with TE biopsy might increase the incidence of mosaic blastocysts.
Similar content being viewed by others
Introduction
Although assisted reproductive technology (ART) has been established for > 40 years, the clinical pregnancy rate per cycle in in vitro fertilization (IVF) remains low. It is widely accepted that aneuploidy is one of the main reasons for implantation failure of embryos. Pre-implantation genetic testing for aneuploidies (PGT-A) strives to improve the clinical outcomes by identification of aneuploid and transfer of euploid embryos. However, the utility of this technology is currently debated [1,2,3,4,5].
With the introduction of next-generation sequencing (NGS), it is now possible to detect pre-implantation embryos with chromosomal mosaicism [6,7,8,9,10]. Chromosomal mosaicism is defined as two or more chromosomally different cell lines within an embryo. Studies showed that the transfer of mosaic embryos gives rise to healthy pregnancies, but may be associated with reduced implantation, higher miscarriage rates, and an increased risk of fetal abnormalities compared to euploid embryo transfer [6, 8, 11,12,13,14,15]. The incidence of mosaicism in blastocysts ranged from 1 to 40% [11, 16,17,18]. However, less than 50% of clinics transfer mosaic embryos [19]. This results in a large number of discarded embryos. The above factors lead to the application of PGT-A more controversial. Thus, a means by which to reduce the mosaicism rate is urgently needed.
The factors leading to the different mosaicism rate remain to be identified. Embryo culture conditions (e.g., oxygen concentration, temperature fluctuations, and culture medium composition) may affect chromosome segregation. Specifically, higher oxygen concentrations have been shown to increase non-disjunction events in an embryo mouse model [20]. Insemination methods have been shown to be associated with mosaicism rates; IVF had a trend toward higher rates of mosaicism compared to intracytoplasmic sperm injection (ICSI) [21]. Mosaicism is mainly derived from the first few mitotic errors after fertilization. Components of the fertilizing sperm, such as the centrosome and associated proteins, play an important role in chromosome segregation mechanisms during the post-fertilization stages [22]. A recent report showed that compromised sperm quality is associated with increased rates of mosaic blastocysts, verifying the important role of sperm in the formation of mosaic embryos [23]. The influence of ovarian stimulation on mosaicism rate remains debated. The research by Munne et al. [24] showed that stimulation might impact mosaicism rate. However, a recent study by Alba Cascales et al. [25] did not find an association between embryonic mosaicism and any of the stimulation protocol variables. Algorithms used for normalizing the chromosome mapping bins can also potentially alter the profiles [26]. In addition, biopsy technique was also suggested to result in various technical effects on mosaicism rate [26].
Currently, blastocyst trophectoderm (TE) biopsy is the most widely used technique to obtain genetic material for PGT-A. There are two blastocyst biopsy protocols that differ by the time of zona pellucida (ZP) opening before biopsy. In the first method, the ZP is drilled by a laser pulse on day 3 cleavage embryos, followed by extended culture to the blastocyst stage [23, 27]. On day 5 or 6, the blastocysts with herniating TE cells from the artificial hole are biopsied [28]. In the second method, the embryo is left in culture medium up to the blastocyst stage. The ZP is opened on day 5 or 6 blastocysts, immediately followed by TE biopsy [18, 29,30,31].
Recently, some researchers have demonstrated that the second method improved the frozen blastocyst survival rate and clinical outcomes [31]. Only a few studies, however, have demonstrated the possible influence of different biopsy protocols on the mosaicism rate, and the results are controversial [18, 32].
Mosaicism is not a negligible phenomenon in human embryos. The study of mosaicism has a profound significance not only to understand human embryonic development but also for the practice of ART. The true incidence of mosaicism remains controversial and an active area of study. The represent study aims to investigate whether biopsy protocols could have an influence on the mosaic blastocyst rate by comparing the mosaic blastocyst rate of PGT-A between two biopsy protocols.
Materials and methods
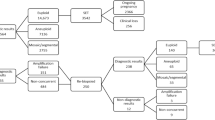
All procedures in this study were performed in accordance with the relevant guidelines and regulation [33]. This retrospective cohort study was approved by the Institutional Review Board on 24 June 2020 at our clinic. This was a retrospective single-center study including patients who underwent PGT-A at our clinic from January 2018 to May 2020. There were 206 patients who underwent PGT-A during the study period. Based on a previous publication showing that compromised sperm quality is associated with an increased rate of mosaic blastocysts [23]; cycles with abnormal sperm parameters were excluded (n = 28). To avoid the possible influence of embryo vitrification on the rate of mosaicism, cycles with thawed embryos were also excluded (n = 14). The biopsy samples were sent to two different sequencing companies for testing in our clinic. To exclude the influence of different sequencing platforms, only samples sequenced by one company were selected for this study (n = 115). The subject selection process is detailed in Fig. 1. All of the patients signed a consent form for all of the procedures performed during PGT-A treatment.
There were two different biopsy protocols during the study period in our clinic. Method 1 was used from January to October 2018, in which artificial ZP opening was performed on day 3 embryos. According to the literature, this protocol may disturb embryo development and result in premature blastocyst hatching. Therefore, we switched the biopsy protocol from method 1 to 2 since November 2018, in which artificial ZP opening was performed immediately before biopsy on day 5 or 6 blastocysts.
A total of 115 PGT-A cycles recruited in this study, and 474 blastocysts were biopsied: 240 from the method 1 group and 234 from the method 2 group. The primary endpoints were euploidy, aneuploidy, and mosaicism rates. The secondary endpoints were clinical pregnancy and miscarriage rates within each group.
There were two experienced embryologists who specialized in blastocyst biopsies using the same micromanipulation equipment during the study period. There were no statistical differences with respect to the no-results and clinical pregnancy rates between the two embryologists. We checked the quality control records at our clinic during the study period. There were no notable variations regarding the culture condition (PH, temperature, and gas composition of the incubators), media, and disposables. The protocols for handling the specimens, kit for whole-genome amplification (WGA), internal algorithms, and software for sequencing analysis remained the same during the study period.
However, due to the influence of the COVID-19 epidemic, the ovarian stimulation protocols changed; more cycles used antagonist protocols from February 2020 on.
Controlled ovarian stimulation and oocyte retrieval
Ovarian stimulation and oocyte retrieval were carried out as previously described [34]. In brief, ovarian stimulation was performed by recombinant FSH (Gonal F; Merck Serono, Switzerland) and monitored by plasma estradiol and transvaginal ultrasonography. Human chorionic gonadotropin (hCG, Ovidrel; Merck Serono, Switzerland) was administered when at least 3 follicles measuring > 18 mm in diameter. Transvaginal oocyte retrievals were performed 36 h after hCG injection. The retrieved oocytes were placed in 2.5 ml of G-IVF medium (Vitrolife Sweden AB, Sweden) and cultured at 37 °C in a 5% O2/6% CO2 incubator until insemination.
Insemination, embryo culture, and grading
To minimize the risk of maternal contamination from residual cumulus cells and paternal contamination from surplus sperm attached to the ZP, all the mature oocytes were inseminated by ICSI under an inverted microscope by three experienced embryologists. To limit the exposure of the embryos to sub-optimal conditions, a time-lapse monitoring system (EmbryoScope Plus; Vitrolife, Sweden) was used to culture embryos after insemination. From days 1 to 3, embryos were cultured in G1 medium (Vitrolife Sweden AB). From days 3 to 5 or day 6, embryos were cultured in G2 medium (Vitrolife Sweden AB). Blastocyst quality was evaluated on days 5 and 6 based on the criteria by Gardner and Lane [35].
Blastocyst biopsy procedures
For method 1, a ~ 10-μm hole on the ZP was created on day 3 embryos using a 180-μs laser pulse (ZILOS-tk Laser, Hamilton Thorne Biosciences, MA, USA). After ZP opening, embryos were rinsed in G2 medium several times, transferred to fresh G2 medium, and cultured in a time-lapse monitoring incubator until the blastocyst stage. Blastocysts with the TE cells herniated from the artificial opening on day 5 or 6 were transferred to a culture dish containing 4 microdroplets of G-mops medium (Vitrolife Sweden AB) and overlaid with 5 ml of mineral oil (Vitrolife Sweden AB) for further biopsy. The biopsies were performed as follows. The holding pipette aspirated the blastocysts with herniated TE cells at the 3 o’clock position. The herniated TE cells were then aspirated with the biopsy pipette, while laser pulses were applied to cut the TE cells. After 3–5 laser pulses, TE cells were removed by a quick flicking movement of the biopsy pipette against the holding pipette. Usually, no more than 5 laser pulses were used for each blastocyst in method 1.
For method 2, day 3 embryos were rinsed in G2 medium several times, transferred to fresh G2 medium, and cultured in a time-lapse monitoring incubator until the blastocyst stage. On day 5 or 6, a ~ 10-μm hole on the ZP was drilled immediately before biopsy with the use of the same laser. The biopsies were performed as follows. To avoid injuring the inner cells (ICM), the holding pipette aspirated the blastocyst with the ICM at the 7 o’clock position (i.e., away from the biopsy pipette), as described by Capalbo [31]. An opening on the ZP was made with one or two laser pulses, working inwards from the outer surface of the zona taking care to avoid damaging the embryo, creating a small hole (~ 10 μm) on the ZP at the 3 o’clock position. After the blastocyst collapsed and the TE cells crumbled, the TE cells were aspirated into the biopsy pipette and withdrawn from the ZP. Finally, the TE cells were removed by a quick flicking movement of the biopsy pipette against the holding pipette. We usually do not use the laser to cut the TE cells in method 2.
The high-quality blastocysts were defined as blastocysts with at least grade 4BB according to the criteria established by Gardner and Lane [35]. A mean of 4–8 TE cells were removed from blastocysts. After biopsy, TE cells were thrice-rinsed in G-mops medium, then transferred to the PCR tubes. WGA of the TE cells was carried out within 1 week after biopsy; then, the samples were sent to two different sequencing company for NGS (only cycles sequenced in one company was included in this study).
Next-generation sequencing pre-implantation genetic testing procedures
All biopsy samples were assessed using NGS at a single sequencing company (Yikon Genomics). After WGA with the WGA kit (genome sequencing universal sample processing kit, Yikon Genomics, Suzhou, China), DNA samples were subjected to NGS on the Illumina Hiseq 2500 platform (Illumina, San Diego, CA, USA) with 2.0-Mb raw reads generated for each sample. ChromGo (Yikon Genomics) software was used to analyze sequencing data and report chromosomal abnormalities. Biopsy diagnostic decisions were based on copy number variations: a chromosome with two copies was assigned a value of 2 and determined to be euploid, a chromosome with one copy was assigned a value of 1 and determined to be a monosomy, and a chromosome with three copies was assigned a value of 3 and determined to be a trisomy. Values detected between 1 and 2 or 2 and 3 were considered to be mosaic.
The reference laboratory states that mosaicism less than 20% or greater than 80% cannot be differentiated from technical noise. Thus, samples with less than 20% mosaicism are classified as euploid, samples with greater than 80% mosaicism are classified as aneuploid, and samples between 20 and 80% mosaicism are classified as mosaic.
Blastocyst vitrification, warming, and embryo transfer
The Cryotop and Kitazato Vitrification Kit (Kitazato BioPharma Co., Shizuoka, Japan) were used in the process of blastocyst vitrification and warming. Vitrification and warming was carried out as described previously [36]. Blastocysts were warmed 2 h before transfer. Embryo transfer was performed under transabdominal ultrasound guidance. The women were advised to rest for 30 min after transfer. The luteal phase was supported by a combination of estrogen and progesterone.
Diagnosis of pregnancy
The serum β-hCG was measured 14 d after embryo transfer. A clinical pregnancy was established when at least one fetal heartbeat was detected by transvaginal ultrasound after embryo transfer. Miscarriage was defined as a pregnancy that did not progress after a fetal heartbeat was visualized by ultrasonography.
Statistical analysis
The software used for statistical analysis was Stata (version 12.0). Quantitative data were expressed as the mean ± SD and compared using ANOVA. Differences among groups were compared using the least square method. The clinical pregnancy rates were presented as a percentage and compared using the trend chi-square test. A p value < 0.05 was considered statistically significant.
Results
Baseline characteristics
The baseline characteristics of the cycles are shown in Table 1. As Table 1 indicates, there were no differences between the two method groups with respect to female and male ages, years of infertility, BMI, basal serum FSH level, AMH level, and indications for PGT. However, the ovarian stimulation protocols, E2 level on the hCG trigger day, and duration of gonadotropin treatment were significantly different between the two method groups (P < 0.05).
Embryology laboratory data
The embryology laboratory data were compared between the two method groups (Table 2). The mean number of oocytes retrieved and blastocysts biopsied were significantly different, and the cycles in the method 1 group presented with a greater number of oocytes retrieved and blastocysts biopsied (P < 0.05); however, the 2PN and good-quality embryo rates on day 3 were similar between the two groups. Furthermore, the blastocyst biopsy rate was 64.11% in the method 2 group, which was slightly higher than the method 1 group (59.41%; P > 0.05), although the difference did not reach statistical significance.
NGS result analysis
There were 240 blastocysts biopsied in the method 1 group and 234 in the method 2 group. There were no significant differences in the cycle cancellation and no-results blastocyst rates between the two methods (Table 3). All blastocysts without results after NGS were re-biopsied at our clinic. The NGS results showed that the euploid and aneuploid blastocyst rates were similar between the two method groups (47.50% vs. 55.13%; 32.92% vs. 36.75%; P > 0.05). The overall mosaic blastocyst rate was 13.92% (66/474) in the two method groups. However, the mosaicism rate in the method 2 group was 8.12%, significantly lower than the method 2 group (19.58%; P<0.05). There were no statistical differences regarding the simple and complex mosaicism rates between the two method groups (P > 0.05).
Comparison of long stimulation protocols between the two methods
The long protocol was main ovarian stimulation protocols, which were further compared between the two methods. The results showed that there were no statistical differences in terms of E2 on hCG trigger day, total dosage of gonadotropin, and duration of gonadotropin administration between the two methods. However, the euploid blastocyst rate in method 1 was significantly lower than that in method 2 (48.07% vs. 59.26%, P = 0.038, Table 4). The mosaicism rate in method 1 was significantly higher than that in method 2 (18.88% vs. 7.41%, P = 0.003).
Factors related to mosaicism by logistic regression analysis
Because the ovarian stimulation protocols, E2 level on the hCG trigger day, duration of gonadotropin treatment, and oocytes retrieved were statistically different between the two groups, logistic regression analysis was carried out to identify the factors related to mosaicism. The results indicated that the biopsy protocol is the only factor associated with the mosaic blastocyst rate (OR, 0.237; 95% CI = 0.084–0.668; P < 0.05). However, other factors, including female age, male age, AMH level, BMI, PGT indication, stimulation protocols, E2 level on the hCG trigger day, total dosage of gonadotropin, duration of gonadotropin, and mean number of oocytes were not associated with mosaicism rates (P > 0.05) (Table 5).
Clinical outcomes after FET
To analyze the influence of different biopsy protocols on the clinical outcomes, cycles with single day 5 euploid blastocysts were compared. There were 34 cycles in which frozen embryos were transferred in the method 1 group and 35 in the method 2 group (Table 6). The survival rates after vitrification-warming were 100% in both groups. There were no statistically significant differences in female age or endometrial thickness. The clinical pregnancy rate in the method 2 group was 65.71%, which was similar to the method 1 group (64.71%; P > 0.05). There was 1 miscarriage in the method 1 group and 3 miscarriages in the method 2 group. The results did not demonstrate an association between the biopsy protocols and clinical pregnancy rate.
Discussion
The results presented that different biopsy protocols might have an influence on mosaic blastocyst rates. Biopsy with method 1 resulted in significantly higher mosaic blastocyst rates compared to method 2 (19.58% vs. 8.12%; P < 0.05). There were no statistical differences in terms of the rates of blastocysts biopsied, aneuploid, euploid, and clinical pregnancy between the two methods.
In our unreported data, the mosaicism rate was 13.92% in one sequencing company and 24% in another company, indicating the influence of different sequencing platforms on mosaicism rates. Thus, only cycle in one sequencing company was included in this study.
At present, two protocols are usually utilized for blastocyst biopsies. Antonio Capalbo et al. firstly published the direct biopsy method without ZP opening on day 3 in 2014 [31]; Yang et al. [29] provided a step-by-step demonstration for the new biopsy method (mechanical blunt dissection). The influence of different biopsy protocols on the clinical pregnancy rate was reported [30, 37]; however, neither study mentioned the mosaicism rate.
Recently, several studies have noted the possible effects of different biopsy protocols on mosaic blastocyst rates, the results of which are controversial [18, 32]. Zhao et al. [18] compared the two TE biopsy protocols in a randomized controlled trial. However, they did not find an association between biopsy protocols and mosaicism rates. In contrast, Grassa et al. [32] indicated the possible relationship between biopsy methods and mosaic blastocyst rates. They suggested that > 4 laser shots during biopsy could modify the genetic constitution, thus increasing the mosaicism rate.
Our results showed that the mosaic blastocyst rates were related to the biopsy protocols. Biopsy with method 1 produced a significantly higher mosaic blastocyst rate compared to method 2. The potential reasons are unclear.
One possible explanation might be that ZP manipulation on day 3 might affect, directly (by physical action) or indirectly (by affecting culture conditions, although transiently), the fidelity of chromosome segregation during the day 3–day 5 intervals. In addition, the ZP opening on day 3 might result in the prematurely hatching of blastocysts on day 5 and trigger biological events that could result in higher rates of true mosaicism in embryos. However, our data showed that there were no statistical differences regarding the stage and quality of blastocysts biopsied between the two method groups, which did not support the above hypothesis.
Another possible explanation might be that the mosaic blastocyst rate is related to the application of the laser pulse. It is inevitable that cell damage from the thermal effect will be increased when using laser pulse. The genetic materials from these damaged cells might influence the sequencing results, leading to an overdiagnosis of normal embryos as mosaics. In method 1, TE cells were separated based on the combination of laser pulse and flicking in our study. In method 2, however, TE cells were most often biopsied by flicking. Laser pulse was usually not used. Grassa et al. [32] suggested that > 4 laser shots during biopsy could increase the mosaicism rate. The use of laser pulse might be one of the reasons for the higher mosaicism rate in the method 1 group. However, it should be noted that the power of laser was only 180-μs, no more than 5 laser pulses were used, which was also less than that in Grassa’s report (4–9 laser pulses) [32]. The lower laser power might result in less damage on TE cells. So the use of laser pulse might not be the main reason for the high mosaic blastocyst rate in this group. Further studies are needed to confirm the effects of the laser pulse on the mosaicism rates.
With respect to the euploid and aneuploid rates, our results showed that there was no statistically significant difference between the two method groups. This result is comparable to the study by Zhao et al. [18].
Studies have analyzed the relationship between biopsy protocols and clinical outcomes. Zhao et al. [18] showed that the clinical pregnancy rate using method 2 was slightly higher than method 1 (64.20% vs. 57.32%); however, the differences did not reach statistical significance. A recent report by Rubino [30] showed that biopsies with method 2 produced significantly higher clinical pregnancy, ongoing implantation, and live birth rates compared with method 1. However, the new biopsy protocol (method 2) was combined with removal of one-fourth of the ZP in their study [30], which might be related to the improved clinical outcomes. We did not find a correlation between the biopsy protocol and the clinical pregnancy rate, which might be related to the small sample size in our study. Further studies are warranted to confirm the effects of the different biopsy protocols on clinical outcomes.
It should be noted that the ovarian stimulation protocols, gonadotropin time, the E2 level on the hCG administration day, and the number of oocytes retrieved were statistically different between the method 1 and 2 groups in our study. Further investigation indicated that the method 1 group consisted of 94.23% of long stimulation protocol cycles, which was only 57.14% in the method 2 group (P = 0.000). That might account for the above differences between the two groups. The comparison of sequencing data in the long stimulation protocols showed that the mosaicism rate in method 1 was significantly higher than that in method 2, and the euploid rate was significantly lower than that in method 2, indicating the influence of different biopsies on sequencing outcomes. Logistic regression analysis was performed to eliminate the possible effects of confounders, and the results revealed that the biopsy protocols were the only factor associated with the mosaicism rate among all the variables. Cascales et al. [25] also reported similar results, and showed that ovarian stimulation protocols were not associated with the mosaic blastocyst rate.
Indeed, the mosaic blastocyst rates are influenced by a number of factors, such as the culture environment [20], sperm quality [23], and insemination method [21]. It has been shown that small alterations in the profiles generated by NGS, currently noted with the form “consistent with mosaicism” or “putative mosaicism",” could be artifacts of the PGT analysis or biopsy technique, and do not reflect an altered embryonic chromosomal status. Theoretically, in order to determine presence of a true chromosomal mosaicism, a second TE biopsy in the first specimen must be performed to confirm the specific variation in chromosomal copy number. Therefore, the presence of technical noise should be kept in mind when we interpret the above results.
Our study is certainly limited by the small sample size and retrospective nature. The influence of culture conditions and stimulation protocols on the mosaicism rate cannot be ruled out since this study used a historical control in different years. In addition, ll embryos from the PGT cycles are cultured in the time-lapse monitoring system (EmbryoScope) at our clinic, which might be related to the outcomes inconsistent with others. A more comprehensive study with a large sample size and a prospective randomized controlled trial will be required to confirm our results in the future.
Conclusion
This study showed the possible effects of different biopsy protocols on mosaicism rate; ZP opening on day 3 embryos followed by TE biopsy on day 5 or day 6 might increase the incidence of mosaic blastocysts.
References
Gleicher N, Orvieto R. Is the hypothesis of preimplantation genetic screening (PGS) still supportable? A review. J Ovarian Res. 2017;10:21.
Morin SJ, Kaser DJ, Franasiak JM. The dilemma of aneuploidy screening on low responders. Curr Opin Obstet Gynecol. 2018;30:179–84.
Sullivan-Pyke C, Dokras A. Preimplantation genetic screening and preimplantation genetic diagnosis. Obstet Gynecol Clin N Am. 2018;45:113–25.
Murphy LA, Seidler EA, Vaughan DA, Resetkova N, Penzias AS, Toth TL, et al. To test or not to test? A framework for counselling patients on preimplantation genetic testing for aneuploidy (PGT-A). Hum Reprod. 2019;34:268–75.
Somigliana E, Busnelli A, Paffoni A, Capalbo A, Vigano P, Riccaboni A, et al. Cost-effectiveness of preimplantation genetic testing for aneuploidies. Fertil Steril. 2019;111:1169–76.
Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373:2089–90.
Popovic M, Dheedene A, Christodoulou C, Taelman J, Dhaenens L, Van Nieuwerburgh F, et al. Chromosomal mosaicism in human blastocysts: the ultimate challenge of preimplantation genetic testing? Hum Reprod. 2018;33:1342–54.
Munne S, Blazek J, Large M, Martinez-Ortiz PA, Nisson H, Liu E, et al. Detailed investigation into the cytogenetic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil Steril. 2017;108:62–71 e68.
Sachdev NM, Maxwell SM, Besser AG, Grifo JA. Diagnosis and clinical management of embryonic mosaicism. Fertil Steril. 2017;107:6–11.
Capalbo A, Ubaldi FM, Rienzi L, Scott R, Treff N. Detecting mosaicism in trophectoderm biopsies: current challenges and future possibilities. Hum Reprod. 2017;32:492–8.
Spinella F, Fiorentino F, Biricik A, Bono S, Ruberti A, Cotroneo E, et al. Extent of chromosomal mosaicism influences the clinical outcome of in vitro fertilization treatments. Fertil Steril. 2018;109:77–83.
Liu YL, Yu TN, Chen CH, Wang PH, Chen CH, Tzeng CR. Healthy live births after mosaic blastocyst transfers with the use of next-generation sequencing. Taiwan J Obstet Gynecol. 2019;58:872–6.
Homer HA. Preimplantation genetic testing for aneuploidy (PGT-A): the biology, the technology and the clinical outcomes. Aust N Z J Obstet Gynaecol. 2019;59:317–24.
Lin PY, Lee CI, Cheng EH, Huang CC, Lee TH, Shih HH, et al. Clinical outcomes of single mosaic embryo transfer: high-level or low-level mosaic embryo, does it matter? J Clin Med. 2020;9:1695.
Kahraman S, Cetinkaya M, Yuksel B, Yesil M, Pirkevi CC. The birth of a baby with mosaicism resulting from a known mosaic embryo transfer: a case report. Hum Reprod. 2020;35:727–33.
Munne S, Weier HU, Grifo J, Cohen J. Chromosome mosaicism in human embryos. Biol Reprod. 1994;51:373–9.
Taylor TH, Gitlin SA, Patrick JL, Crain JL, Wilson JM, Griffin DK. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update. 2014;20:571–81.
Zhao H, Tao W, Li M, Liu H, Wu K, Ma S. Comparison of two protocols of blastocyst biopsy submitted to preimplantation genetic testing for aneuploidies: a randomized controlled trial. Arch Gynecol Obstet. 2019;299:1487–93.
Kim TG, Neblett MF, Shandley LM, Omurtag K, Hipp HS, Kawwass JF. National mosaic embryo transfer practices: a survey. Am J Obstet Gynecol. 2018;219:602.e1–7.
Bean CJ, Hassold TJ, Judis L, Hunt PA. Fertilization in vitro increases non-disjunction during early cleavage divisions in a mouse model system. Hum Reprod. 2002;17:2362–7.
Palmerola KL, Vitez SF, Amrane S, Fischer CP, Forman EJ. Minimizing mosaicism: assessing the impact of fertilization method on rate of mosaicism after next-generation sequencing (NGS) preimplantation genetic testing for aneuploidy (PGT-A). J Assist Reprod Genet. 2019;36:153–7.
Palermo G, Munne S, Cohen J. The human zygote inherits its mitotic potential from the male gamete. Hum Reprod. 1994;9:1220–5.
Tarozzi N, Nadalini M, Lagalla C, Coticchio G, Zaca C, Borini A. Male factor infertility impacts the rate of mosaic blastocysts in cycles of preimplantation genetic testing for aneuploidy. J Assist Reprod Genet. 2019;36:2047–55.
Munne S, Magli C, Adler A, Wright G, de Boer K, Mortimer D, et al. Treatment-related chromosome abnormalities in human embryos. Hum Reprod. 1997;12:780–4.
Cascales A, Lledó B, Ortiz JA, Morales R, Ten J, Llácer J, et al. Effect of ovarian stimulation on embryo aneuploidy and mosaicism rate. Syst Biol Reprod Med. 2021;07:1–8.
Fragouli E, Munné WD. The cytogenetic constitution of human blastocysts: insights from comprehensive chromosome screening strategies. Hum Reprod Update. 2019;25:15–33.
Hernandez-Nieto C, Lee JA, Slifkin R, Sandler B, Copperman AB, Flisser E. What is the reproductive potential of day 7 euploid embryos? Hum Reprod. 2019;34:1697–706.
McArthur SJ, Leigh D, Marshall JT, de Boer KA, Jansen RP. Pregnancies and live births after trophectoderm biopsy and preimplantation genetic testing of human blastocysts. Fertil Steril. 2005;84:1628–36.
Yang D, Feng D, Gao Y, Sagnelli M, Wang X, Li D. An effective method for trophectoderm biopsy using mechanical blunt dissection: a step-by-step demonstration. Fertil Steril. 2020;114:438–9.
Rubino P, Tapia L. Ruiz de Assin Alonso R, Mazmanian K, Guan L, Dearden L, et al: Trophectoderm biopsy protocols can affect clinical outcomes: time to focus on the blastocyst biopsy technique. Fertil Steril. 2020;113:981–9.
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott TA, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29:1173–81.
Herrero Grassa L, Aparicio Gonzalez M, Cascales Romero L, Ortiz JA, Castillo JC, Garcia-Ajofrin C, et al. Is mosaicism and its characteristics influenced by the method of trophectoderm-biopsy technique employed in PGT-A cycles? Genetic and reproductive outcomes after pulling versus flicking. Abstracts 36th virtual annual meeting of European Society of Human Reproduction and Embryology (ESHRE). 2020; P-246.
Preimplantation Genetic Diagnosis International Society (PGDIS). Guidelines for good practice in PGD: programme requirements and laboratory quality assurance. Reprod Biomed Online.2008;16:134-47.
Xiong S, Han W, Liu JX, Zhang XD, Liu WW, Liu H, et al. Effects of cumulus cells removal after 6 h co-incubation of gametes on the outcomes of human IVF. J Assist Reprod Genet. 2011;28:1205–11.
Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3:367–82.
Xiong S, Liu JX, Gao Y, Liu WW, Wu LH, Han W, et al. Shortened equilibration time can compromise clinical outcomes in human embryo vitrification. Hum Fertil (Camb). 2016;19:114–9.
Capalbo A, Poli M, Cimadomo D, Benini F, Patassini C, Rubio C, et al. Low-degree mosaicism profiles do not provide clinically useful predictive values: interim results from the first multicenter prospective non-selection study on the transfer of mosaic embryos. Eshre Poster Award. 2020;P-502.
Acknowledgement
I would like to express my gratitude to all those who helped me with the writing and amendment of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Disclosures
This work was supported by the Chongqing Health Committee (grant number: 2019MSXM067 and 2020MSXM086).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiong, S., Liu, W., Wang, J. et al. Trophectoderm biopsy protocols may impact the rate of mosaic blastocysts in cycles with pre-implantation genetic testing for aneuploidy. J Assist Reprod Genet 38, 1153–1162 (2021). https://doi.org/10.1007/s10815-021-02137-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02137-w