Abstract
Objective
Assisted oocyte activation (AOA) can restore fertilization rates after IVF/ICSI cycles with fertilization failure. AOA is an experimental technique, and its downstream effects remain poorly characterized. Clarifying the relationship between AOA and embryo, morphokinetics could offer complementary insights into the quality and viability of the embryos obtained with this technique. The aim of this study is to compare the preimplantation morphokinetic development of embryos derived from ICSI-AOA (experimental group) vs. ICSI cycles (control group).
Methods
A retrospective cohort study was carried out with 141 embryos from fresh oocyte donation cycles performed between 2013 and 2017; 41 embryos were derived from 7 ICSI-AOA cycles and 100 embryos from 18 ICSI cycles. Morphokinetic development of all embryos was followed using a time-lapse system.
Results
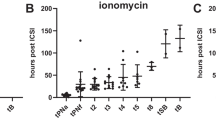
We show that embryos from both groups develop similarly for most milestones, with the exception of the time of second polar body extrusion (tPB2) and the time to second cell division (t3).
Conclusions
We conclude that ionomycin mediated AOA does not seem to affect the morphokinetic pattern of preimplantation embryo development, despite the alterations found in tPB2 and t3, which could directly reflect the use of a Ca2+ ionophore as a transient and quick non-physiologic increase of free intracytoplasmic Ca2+.

Similar content being viewed by others
References
Plachot M, Mandelbaum J. Oocyte maturation, fertilization and embryonic growth in vitro. Br Med Bull. 1990;46(3):675–94. https://doi.org/10.1093/oxfordjournals.bmb.a072424.
Neri QV, Lee B, Rosenwaks Z, Machaca K, Palermo GD. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell Calcium. 2014;55(1):24–37. https://doi.org/10.1016/j.ceca.2013.10.006.
Putney JW Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7(1):1–12.
Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, et al. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129(15):3533–44.
Malcuit C, Kurokawa M, Fissore RA. Calcium oscillations and mammalian egg activation. J Cell Physiol. 2006;206(3):565–73. https://doi.org/10.1002/jcp.20471.
Esfandiari N, Javed MH, Gotlieb L, Casper RF. Complete failed fertilization after intracytoplasmic sperm injection--analysis of 10 years' data. Int J Fertil Women's Med. 2005;50(4):187–92.
Combelles CM, Morozumi K, Yanagimachi R, Zhu L, Fox JH, Racowsky C. Diagnosing cellular defects in an unexplained case of total fertilization failure. Hum Reprod. 2010;25(7):1666–71. https://doi.org/10.1093/humrep/deq064.
Heindryckx B, Van der Elst J, De Sutter P, Dhont M. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod. 2005;20(8):2237–41. https://doi.org/10.1093/humrep/dei029.
Borges E Jr, de Almeida Ferreira Braga DP, de Sousa Bonetti TC, Iaconelli A Jr, Franco JG Jr. Artificial oocyte activation using calcium ionophore in ICSI cycles with spermatozoa from different sources. Reprod BioMed Online. 2009;18(1):45–52. https://doi.org/10.1016/s1472-6483(10)60423-3.
Bonte D, Ferrer-Buitrago M, Dhaenens L, Popovic M, Thys V, De Croo I, et al. Assisted oocyte activation significantly increases fertilization and pregnancy outcome in patients with low and total failed fertilization after intracytoplasmic sperm injection: a 17-year retrospective study. Fertil Steril. 2019;112(2):266–74. https://doi.org/10.1016/j.fertnstert.2019.04.006.
Torra-Massana M, Cornet-Bartolome D, Barragan M, Durban M, Ferrer-Vaquer A, Zambelli F, et al. Novel phospholipase C zeta 1 mutations associated with fertilization failures after ICSI. Hum Reprod. 2019;34(8):1494–504. https://doi.org/10.1093/humrep/dez094.
Tejera A, Molla M, Muriel L, Remohi J, Pellicer A, De Pablo JL. Successful pregnancy and childbirth after intracytoplasmic sperm injection with calcium ionophore oocyte activation in a globozoospermic patient. Fertil Steril. 2008;90(4):1202 e1–5. https://doi.org/10.1016/j.fertnstert.2007.11.056.
Murugesu S, Saso S, Jones BP, Bracewell-Milnes T, Athanasiou T, Mania A, et al. Does the use of calcium ionophore during artificial oocyte activation demonstrate an effect on pregnancy rate? A meta-analysis. Fertil Steril. 2017;108(3):468–82 e3. https://doi.org/10.1016/j.fertnstert.2017.06.029.
D'Haeseleer E, Vanden Meerschaut F, Bettens K, Luyten A, Gysels H, Thienpont Y, et al. Language development of children born following intracytoplasmic sperm injection (ICSI) combined with assisted oocyte activation (AOA). Int J Language Commun Disord. 2014;49(6):702–9. https://doi.org/10.1111/1460-6984.12100.
Vanden Meerschaut F, Nikiforaki D, Heindryckx B, De Sutter P. Assisted oocyte activation following ICSI fertilization failure. Reprod BioMed Online. 2014;28(5):560–71. https://doi.org/10.1016/j.rbmo.2014.01.008.
Deemeh MR, Tavalaee M, Nasr-Esfahani MH. Health of children born through artificial oocyte activation: a pilot study. Reprod Sci. 2015;22(3):322–8. https://doi.org/10.1177/1933719114542017.
Vanden Meerschaut F, D'Haeseleer E, Gysels H, Thienpont Y, Dewitte G, Heindryckx B, et al. Neonatal and neurodevelopmental outcome of children aged 3-10 years born following assisted oocyte activation. Reprod BioMed Online. 2014;28(1):54–63. https://doi.org/10.1016/j.rbmo.2013.07.013.
Meseguer M, Herrero J, Tejera A, Hilligsoe KM, Ramsing NB, Remohi J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26(10):2658–71. https://doi.org/10.1093/humrep/der256.
Herrero J, Tejera A, Albert C, Vidal C, de los Santos MJ, Meseguer M. A time to look back: analysis of morphokinetic characteristics of human embryo development. Fertil Steril. 2013;100(6):1602–9 e1–4. https://doi.org/10.1016/j.fertnstert.2013.08.033.
Chen AA, Tan L, Suraj V, Reijo Pera R, Shen S. Biomarkers identified with time-lapse imaging: discovery, validation, and practical application. Fertil Steril. 2013;99(4):1035–43. https://doi.org/10.1016/j.fertnstert.2013.01.143.
Desai N, Goldberg JM, Austin C, Falcone T. Are cleavage anomalies, multinucleation, or specific cell cycle kinetics observed with time-lapse imaging predictive of embryo developmental capacity or ploidy? Fertil Steril. 2018;109(4):665–74. https://doi.org/10.1016/j.fertnstert.2017.12.025.
Azzarello A, Hoest T, Mikkelsen AL. The impact of pronuclei morphology and dynamicity on live birth outcome after time-lapse culture. Hum Reprod. 2012;27(9):2649–57. https://doi.org/10.1093/humrep/des210.
Pujol A, Garcia D, Obradors A, Rodriguez A, Vassena R. Is there a relation between the time to ICSI and the reproductive outcomes? Hum Reprod. 2018;33(5):797–806. https://doi.org/10.1093/humrep/dey067.
Heindryckx B, De Gheselle S, Gerris J, Dhont M, De Sutter P. Efficiency of assisted oocyte activation as a solution for failed intracytoplasmic sperm injection. Reprod BioMed Online. 2008;17(5):662–8. https://doi.org/10.1016/s1472-6483(10)60313-6.
Ciray HN, Campbell A, Agerholm IE, Aguilar J, Chamayou S, Esbert M, et al. Proposed guidelines on the nomenclature and annotation of dynamic human embryo monitoring by a time-lapse user group. Hum Reprod. 2014;29(12):2650–60. https://doi.org/10.1093/humrep/deu278.
Coroleu B, Barri PN, Carreras O, Belil I, Buxaderas R, Veiga A, et al. Effect of using an echogenic catheter for ultrasound-guided embryo transfer in an IVF programme: a prospective, randomized, controlled study. Hum Reprod. 2006;21(7):1809–15. https://doi.org/10.1093/humrep/del045.
Nikiforaki D, Vanden Meerschaut F, de Roo C, Lu Y, Ferrer-Buitrago M, de Sutter P, et al. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril. 2016;105(3):798–806 e2. https://doi.org/10.1016/j.fertnstert.2015.11.007.
Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci U S A. 2012;109(11):4169–74. https://doi.org/10.1073/pnas.1112333109.
Van den Bergh M, Bertrand E, Englert Y. Second polar body extrusion is highly predictive for oocyte fertilization as soon as 3 hr after intracytoplasmic sperm injection (ICSI). J Assist Reprod Genet. 1995;12(4):258–62. https://doi.org/10.1007/BF02212928.
Martinez M, Obradors A, Vernaeve V, Santalo J, Vassena R. Oocyte vitrification does not affect early developmental timings after intracytoplasmic sperm injection for women younger than 30 years old. Mol Reprod Dev. 2016;83(7):624–9. https://doi.org/10.1002/mrd.22667.
Ferrer-Buitrago M, Bonte D, De Sutter P, Leybaert L, Heindryckx B. Single Ca(2+) transients vs oscillatory Ca(2+) signaling for assisted oocyte activation: limitations and benefits. Reproduction. 2018;155(2):R105–R19. https://doi.org/10.1530/REP-17-0098.
Nagy ZP, Liu J, Joris H, Devroey P, Van Steirteghem A. Time-course of oocyte activation, pronucleus formation and cleavage in human oocytes fertilized by intracytoplasmic sperm injection. Hum Reprod. 1994;9(9):1743–8. https://doi.org/10.1093/oxfordjournals.humrep.a138786.
Vassena R, Boue S, Gonzalez-Roca E, Aran B, Auer H, Veiga A, et al. Waves of early transcriptional activation and pluripotency program initiation during human preimplantation development. Development. 2011;138(17):3699–709. https://doi.org/10.1242/dev.064741.
Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332(6163):459–61. https://doi.org/10.1038/332459a0.
Conaghan J, Chen AA, Willman SP, Ivani K, Chenette PE, Boostanfar R, et al. Improving embryo selection using a computer-automated time-lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril. 2013;100(2):412–9 e5. https://doi.org/10.1016/j.fertnstert.2013.04.021.
Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Hickman CF. Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod BioMed Online. 2013;26(5):477–85. https://doi.org/10.1016/j.rbmo.2013.02.006.
Acknowledgments
The authors wish to thank Désirée García and Francesc Figueras for the statistical support and to Montserrat Barragan for the helpful discussion and comments.
Funding
This study was carried out with intramural fundings from Clinica Eugin.
Author information
Authors and Affiliations
Contributions
M. Martínez involved in study design, video analysis, data compilation and analysis, and manuscript preparation. M. Durban involved in study design. A. Rodriguez involved in manuscript supervision and expert knowledge. J. Santaló and R.Vassena involved in the study design, implementation and supervision, expert knowledge, and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Martínez, M., Durban, M., Santaló, J. et al. Assisted oocyte activation effects on the morphokinetic pattern of derived embryos. J Assist Reprod Genet 38, 531–537 (2021). https://doi.org/10.1007/s10815-020-02025-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-02025-9