Abstract
Objective
Our objective was to evaluate the time course and the predictive value of the extrusion of the second polar body after intracytoplasmic injection (ICSI) related to the fertilization rate, embryo cleavage and quality.
Setting
The setting was the in vitro fertilization program of a university hospital.
Patients
Twenty-one patients were treated with intracytoplasmic single sperm injection either for fertilization failure in IVF, low fertilization in IVF (<5%), or severe male factors.
Design
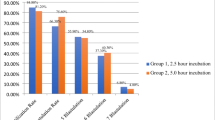
One hundred thirty-five of 205 metaphase 2 oocytes treated with intracytoplasmic single sperm injection were observed 1, 2, and 3 hr after the assisted fertilization procedure. Extrusion of the second polar body was recorded. For each of these oocytes, fertilization was noted 18 hr after ICSI and cleavage and embryo quality were assessed 24 hr later. The 70 remaining oocytes were used to assess a possible negative effect of repeated exposure to light microscopy.
Results
The extrusion of the second polar body 3 hr after injection was an observation with a sensitivity of 0.87, a specificity of 0.58, and a high positive predictive value (0.90) toward oocyte fertilization. Twenty-nine and four-tenths percent of the oocytes extruded a second polar body within the first hour, 56.6% within the first 2 hr, and 78.3% had a second polar body 3 hr after injection. This time course was related neither to the speed of embryo cleavage nor to the embryo quality. Fertilization, cleavage, and embryo quality were not affected by repeated observation as deduced from comparison with the control group and confirmed by a high pregnancy (62% per oocyte retrieval) and implantation rate (22% per replaced embryo).
Conclusion
Oocytes can be checked, in all safety, 3 hr after a single sperm injection for the presence of a second polar to predict oocyte fertilization with a high certainty.
Similar content being viewed by others
References
Van Steirteghem A, Nagy Z, Joris H, Liu J, Staessens C, Stnitz J, Wisanto A, Devroey P: High fertilization and implantation rates after intracytoplasmic sperm injection. Hum Reprod 1993;8:1061–1066
Van den Bergh M, Bertrand E, Englert Y: Intracytoplasmic single sperm injection preclinical training and first clinical results. J Assist Reprod Genet 1994;11(6):1–5
Legendre L, Stewart J: Effect of cumulus maturity on sperm penetration in the golden hamster. Biol Reprod 1993;49:82–88
Bavister B: Fertilization of hamster eggs in vitro at sperm: egg ratios close to unity. J Exp Zool 1979;210:259–264
Philips D, Shalgi R: Sperm penetration into rat ova fertilized in vivo. J Exp Zool 1982;221:373–378
Hoppe P, Pitt S: Fertilization in vitro and development of mouse ova. Biol Rep 1973;420–426
Hanada A, Chamg M: In vitro fertilization of hamster eggs in different media and the stimulating effect of heterologous and homologous spermatozoa. J Reprod Fert 1976;XX:104–114
Plachot M, Junca A, Mandelbaum J, Cohen J, Salat-Baroux J, Da Lage C: Timing of in vitro fertilization of cumulus-free and cumulus enclosed human oocytes. Hum Reprod 1986;1:237–242
Lavy G, Bayers S, Decherney A: Hyaluronidase removal of the cumulus oophorus increases in vitro fertilization. J Vitro Fert Embryo Trans 1988;5:257–260
Mahadevan M, Trounson A: Removal of the cumulus oophorus of the human oocyte for in vitro fertilization. Fertil Steril 1985;43:263–267
Tesarik J, Pilka L, Drahoràd J, Cechovà D, Veselsky I: The role of cumulus cell-secreted proteins in the development of human sperm fertilizing ability: Implication in IVF. Hum Reprod 1988;3:129–132
Tesarik J, Kopecny V: Developmental control of the human male pronucleus by ooplasmic factors. Hum Reprod 1989;4:962–968
Marston J, Kelly W: Time relationship of spermatozoon penetration into the egg of the rhesus monkey. Nature 1968;217:1073–1074
Uehara T, Yanagimachi R: Microsurgical injection of spermatozoa into hamster eggs with subsequent transformation of sperm nuclei into male pronuclei. Biol Reprod 1976;15:467–470
Tesarik J, Kopecny V: Development of human male pronucleus: Ultrastructure and timing. Gamete Res 1989;24:135–149
Edwards R, Bavister D, Steptoe P: Early stages of fertilization of human oocytes matured in vitro. Nature 1969;221:632–635
McMaster R, Yanagimachi R, Lopata A: Penetration of human eggs by human spermatozoa in vitro. Biol Reprod 1978;19:212–216
Lopata A, McMaster R, Mcbain J, Johnston W: In vitro fertilization of preovulatory human eggs. J Reprod Fertil 1978;52:339–342
Lopata A, Sathananthan A, McBain J, Johnston W, Speirs A: The ultrastructure of the human egg fertilized in vitro. Fertil Steril 1980;33:12–20
Nagy ZP, Liu J, Joris H, Devroey P, Van Steirteghem A: Time-course of oocyte activation, pronucleus formation and cleavage in human oocytes fertilized by intracytoplasmic sperm injection. Hum Reprod 1994;9:1743–1748
Englert Y, Van den Bergh M, Rodesch C, Vandervorst P, Berberoglugil P, Laruelle C, Biramane J, Gervy C, Schwers J: Nouveau programme de fécondation in vitro à l'hôpital Erasme: premiers résultats et aspects originaux. Rev Med Brux 1991;12:305–314
Englert Y, Van den Bergh M, Rodesch C, Bertrand E, Biramane J, Legreve A: Comparative auto-controlled study between swim-up and Percoll preparation of fresh semen samples for in-vitro-fertilization. Hum Reprod 1992;7:399–402
Puissant F, Van Rysselberge M, Barlow P, Deweze J, Leroy F: Embryo scoring as a prognostic tool in IVF treatment. Hum Reprod 1987;2:705–708
Ohsumi K, Katagiri C, Yanagimachi R: Human sperm nuclei can transform into condensed chromosomes in Xenopus egg extracts. Gamete Res 1988;20:1–9
Perrault S, Naish S, Zirkin B: The timing of hamster nuclear decondensation and male pronucleus formation is related to sperm nuclear disulfide bond content. Biol Reprod 1987;36:239–244
Balakier H, MacLusky N, Casper R: Characterization of the first cell cycle in human zygotes: Implications for cryopreservation. Fertil Steril 1993;59:359–365
Tesarik J, Kopecny V: Nucleic acid synthesis and development of human male pronucleus. J Reprod Fertil 1989;86:549–558
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van Den Bergh, M., Bertrand, E. & Englert, Y. Second polar body extrusion is highly predictive for oocyte fertilization as soon as 3 hr after intracytoplasmic sperm injection (ICSI). J Assist Reprod Genet 12, 258–262 (1995). https://doi.org/10.1007/BF02212928
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02212928