Abstract
Purpose
To investigate if needle-immersed vitrification or slow-freezing yields better implantation results for human ovarian tissue and which method benefits more when combined with the “improvement protocol” of host melatonin treatment and graft incubation with biological glue + vitamin E + vascular endothelial growth factor-A.
Methods
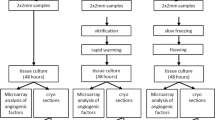
Human ovarian tissue was preserved by needle-immersed vitrification or slow-freezing and transplanted into immunodeficient mice, either untreated (groups A and C, respectively) or treated with the improvement protocol (groups B and D, respectively). Grafted and ungrafted slices were evaluated by follicle counts, apoptosis assay and immunohistochemistry for Ki67 and platelet endothelial cell adhesion molecule (PECAM).
Results
Follicle number in the recovered grafts was limited. The number of atretic follicles was significantly higher after vitrification with/without the improvement protocol and slow-freezing than that after slow-freezing + the improvement protocol. Stroma cell apoptosis was the lowest in the group D. PECAM staining showed a peripheral and diffuse pattern in the group D (mostly normal follicular morphology) and a diffuse pattern in all other groups (few follicles, mostly atretic), with significantly higher diffuse levels in the vitrification groups. Ki67 staining was identified in all normal follicles. Follicles did not survive transplantation in the vitrification groups.
Conclusions
Ovarian sample preparation with slow-freezing + the improvement protocol appears to yield better implantation outcomes than needle-immersed vitrification with/without the improvement protocol. The real quality of frozen tissue can be assessed only after grafting and not after thawing/warming.





Similar content being viewed by others
References
Feigin E, Freud E, Fisch B, Orvieto R, Kravarusic D, Avrahami G, et al. Fertility preservation in female adolescents with malignancies. In: Moorland MT, editor. Cancer in female adolescents. Hauppauge: Nova; 2008. p. 103–38.
Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2016. doi:10.1007/s10815-016-0843-9.
Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474–4749.
Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto M, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–15.
Amorim CA, Curaba M, Van Langendonckt A, Dolmans MM, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod Biomed Online. 2011;23:160–86.
Wang Y, Xiao Z, Li L, Fan W, Li SW. Novel needle immersed vitrification: a practical and convenient method with potential advantages in mouse and human ovarian tissue cryopreservation. Hum Reprod. 2008;23:2256–65.
Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online. 2009;18:568–77.
Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24:1670–83.
Xiao Z, Wang Y, Li L, Luo S, Li SW. Needle immersed vitrification can lower the concentration of cryoprotectant in human ovarian tissue cryopreservation. Fertil Steril. 2010;94:2323–8.
Amorim CA, Jacobs S, Devireddy RV, Van Langendonckt A, Vanacker J, Jaeger J, et al. Successful vitrification and autografting of baboon (Papio anubis) ovarian tissue. Hum Reprod. 2013;28:2146–56.
Dolmans MM, Binda JF, Jacobs S, Dehoux JP, Squifflet JL, Ambroise J, et al. Impact of cryopreservation technique and vascular bed on ovarian tissue transplantation in cynomolgus monkeys. J Assist Reprod Genet. 2015;32:1251–62.
Abir R, Fisch B, Jessel S, Felz C, Ben-Haroush A, Orvieto R. Improving posttransplantation survival of human ovarian tissue by treating the host and graft. Fertil Steril. 2011;95:1205–10.
Friedman O, Orvieto R, Fisch B, Felz C, Freud E, Ben-Haroush A, et al. Possible improvements in human ovarian grafting by various host and graft treatments. Hum Reprod. 2012;27:474–82.
Gavish Z, Peer G, Roness H, Cohen Y, Meriow D. Follicle activation and ‘burn-out’ contribute to post-transplantation follicle loss in ovarian tissue grafts: the effect of graft thickness. Hum Reprod. 2016;29:989–96.
Lerer-Serfaty G, Samara N, Fisch B, Shachar M, Kossover O, Seliktar D, et al. Attempted application of bioengineered/biosynthetic supporting matrices with phosphatidylinositol-trisphosphate-enhancing substances to organ culture of human primordial follicles. J Assist Reprod Genet. 2013;30:1279–88.
Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–8.
Abir R, Biron-Shental T, Orvieto R, Garor R, Krissi H, Fisch B. Transplantation of frozen-thawed late-gestational-age human fetal ovaries into immunodeficient mice. Fertil Steril. 2009;92:770–7.
Gougeon A. Regulations of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–54.
Gumina RJ, Kirschbaum NE, Rao PN, Kenny D, Warltier DC, Newman PJ, et al. The human PECAM1 gene maps to 17q23. Genomics. 1996;34:229–32.
Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006;12:4–18.
Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27:2514–23.
Privratsky JR, Newman DK, Newman PJ. PECAM-1: conflicts of interest in inflammation. Life Sci. 2010;87:69–82.
Rahimi G, Isachenko V, Kreienberg R, Sauer H, Todorov P, Tawadros S, et al. Re-vascularisation in human ovarian tissue after conventional freezing or vitrification and xenotransplantion. Eur J Obstet Gynecol Reprod Biol. 2010;149:63–7.
Isachenko V, Lapidus I, Isachenko E, Krivokharchenko A, Kreienberg R, Woriedh M, et al. Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological and molecular biological evaluation. Reproduction. 2009;138:319–27.
Hewitt SM, Baskin DG, Frevert CW, Stahl WL, Rosa-Molinar E. Controls for immunohistochemistry: the Histochemical Society’s standards of practice for validation of immunohistochemical assays. J Histochem Cytochem. 2014;62:693–7.
Ehrbar M, Djonov VG, Schnell C, Tschanz SA, Martiny-Baron G, Schenk U, et al. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ Res. 2016;94:1124–32.
Tang J, Hua Y, Su J, Zhang P, Zhu X, Wu L, et al. Expression of VEGF and neural repair after alprostadil treatment in a rat model of schiatic nerve crush injury. Neurol India (Serial: Online). 2009;57:387–94.
Schmidt KL, Byskov AG, Nyboe-Andersen A, Müller J, Andersen Yding C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18:1158–64.
Van Eyck A-S, Bouzin C, Feron O, Romeu L, Van Langendonckt A, Donnez J, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93:1676–85.
Demeestere I, Simon P, Dedeken L, Moffa F, Tsepelidis S, Branchet C, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod. 2015;30:2107–9.
Anderson RA, McLaughlin M, Wallace WH, Albertini DF, Telfer EE. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod. 2014;29:97–106.
Lande Y, Fisch B, Tsur A, Farhi J, Prag-Rosenberg R, Ben-Haroush A, et al. Short term exposure of human ovarian follicles to cyclophosphamide metabolites seems to promote follicular activation in vitro. RBM Online. 2016;34:104–14.
Li YB, Zhou CQ, Yang GF, Wang Q, Dong Y. Modified vitrification method for cryopreservation of human ovarian tissues. Chin Med J. 2007;120:110–4.
Huang L, Mo Y, Wang W, Li Y, Zhang Q, Yang D. Cryopreservation of human ovarian tissue by solid-surface vitrification. Eur J Obstet Gynecol Reprod Biol. 2008;139:193–8.
Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E. Micro-organ ovarian transplantation enables pregnancy: a case report. Hum Reprod. 2011;26:1097–103.
Acknowledgments
The authors are indebted to Ms. G. Ganzach from the Editorial Board of Rabin Medical Center for the English editing. The authors are also grateful to our laboratory technician Carmela Felz for the histological sections.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the local institutional ethics committee. Informed consent to donate tissue for the present study was obtained from the patients or the parents of minors.
Additional information
Dr. Noa Fisher D.M.D. performed most of the experiments as a partial requirement for her M.Sc. degree at Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
An erratum to this article is available at http://dx.doi.org/10.1007/s10815-017-0918-2.
Rights and permissions
About this article
Cite this article
Abir, R., Fisch, B., Fisher, N. et al. Attempts to improve human ovarian transplantation outcomes of needle-immersed vitrification and slow-freezing by host and graft treatments. J Assist Reprod Genet 34, 633–644 (2017). https://doi.org/10.1007/s10815-017-0884-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-0884-8