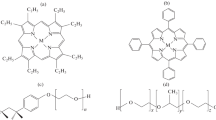
A study has been carried out on the spectral and photophysical parameters of a series of hydrophobic metallocomplexes of phthalocyanine and porphyrins encapsulated in polymeric micelles in aqueous solution at 293 K. The fluorescence characteristics of the free bases and metallocomplexes with light ions [Mg(II) and Zn(II)] are only slightly altered upon going from solution in organic solutions to aqueous micellar media. In contradistinction, encapsulation of porphyrin compounds with heavy ions [Pd(II) and Pt(II)] in polymeric micelles leads to much greater quantum yields and phosphorescence lifetimes in aqueous micellar media in comparison with these parameters in organic solvents. This behavior was attributed to enhancement of the spin-orbital interaction for the compounds with Pd(II) and Pt(II) compounds as well as to a significant reduction of quenching in the polymeric micelles. The luminescence parameters of the compounds studied depend on their structure and the nature of the polymeric micelles.
Similar content being viewed by others
References
J. Park, K. Hong, H. Lee, and W. Jang, Acc. Chem. Res., 54, No. 9, 2249–2260 (2021).
Y. Shi, F. Zhang, and R. Linhardt, Dyes Pigments, 188, No. 4, Article ID 109136 (2021).
P. Gujarathi, Pharm. Innovat., 9, No. 4, 80–86 (2020).
J. Park, J. Lee, and W. Jang, Coord. Chem. Rev., 407, 213157 (2020).
D. Monti, S. Nordis, M. Stefanelli, R. Paolesse, C. Natale, and A. D’Amico, J. Sensors, Article ID 856053 (2009).
Y. F. Huan, Q. Fei, H. V. Shan, B. J. Wang, H. Xua, and G. D. Feng, Analyst, 140, 1655–1661 (2015).
D. Wöhrle, G. Schnurpfeil, S. Makarov, A. Kazarin, and O. Suvorova, Macroheterocycles, 5, 191–202 (2012).
V. Lioret, S. Saon, A. Berrou, L. Lernerman, C. Arnould, and R. Decréau, Photochem. Photobiol. Sci. (2022); doi: https://doi.org/10.1007/s43630022003130.
X. Li, X. Peng, B. Zheng, J. Tang, Y. Zhao, B. Zheng, M. Ke, and J. Huang, Chem. Sci., 9, Article ID 20982104 (2018).
N. Sekkat, H. van den Bergh, T. Nyokong, and N. Lange, Molecules, 17, No.1, Article ID 98144 (2012).
M. Whalley, J. Chem. Soc., 866–869 (1961).
A. T. Gradyushko and M. P. Tsvirko, Opt. Spektrosk., 31, No. 4, 213–218 (1971).
A. S. Starukhin, A. V. Gorskii, and M. Z. Kiyak, Izv. Rossiisk. Akad. Nauk, Ser. Fiz., 82, No. 12, 1722–1727 (2018).
V. Verdree, S. Pakhomov, G. Su, M. Allen, A. Countryman, R. Hammer, and S. Soper, J. Fluoresc., 17 547–563 (2007).
E. Güzel, A. Kocac, and M. Koçak, Supramol. Chem., 29, 536–546 (2017).
S. Makhseed, M. Machacek, W. Alfadly, A. Tuhl, M. Vinodh, T. Simunek, V. Novakova, P. Kubat, E. Rudolf, and P. Zimcik, Chem. Commun., 49, 11149–11151 (2013).
J. Matsumoto, T. Shiragami, K. Hirakawa, and M. Yasuda, Int. J. Photoenergy, Article ID 148964 (2015).
S. Gungor, M. Tumer, M. Kose, O. Gungor, and S. Purtas, Appl. Organometal. Chem., 36, No. 3 (2022); doi: https://doi.org/10.1002/aoc.6534.
E. Yabas, S. SahinBolukhasi, and Z. D. SahinInan, J. Porphyrin. Phthaloc., 26, No. 1, 65–77 (2022).
H. P. Karaoğlu, Ӧ. Sağlam, S. Ӧzdemir, S. Gonca, and M. Koçak, Dalton Trans., 50, No. 28, 9700–9708 (2021).
M. L’her, Ӧ. Göktuğ, M. Durmuş, and V. Ahsen, Electrochim. Acta, 213, 655–552 (2016).
Y. Li, Y. Liu, H. Wang, Z. Li, and D. Zhang, ACS Appl. Bio. Mater., 5, No. 2, 881–888 (2022).
A. Starukhin, V. Apyari, A. Gorski, A. Ramanenka, and A. Furtletov, EPJ Web Conf., 220, Article ID 03003 (2019).
R. P. Linsstead and M. Whalley, J. Chem. Soc., 944, 4839–4846 (1952).
R. Sakamoto and E. OhnoOkumura, Materials, 2, No. 3, 1127–1135 (2009).
J. Sessler, A. Mozaffari, and A. Johnson, Org. Syntheses, 70, 68–73 (2003).
A. Adler, F. Longo, J. Finarelli, Alan D. Adler, J. Goldmacher, and L. Korsakoff, J. Org. Chem., 32, No. 2, 476 (1967).
A. S. Starukhin, Yu. D. Korol’, T. A. Pavich, A. A. Romanenko, and L. I. Gaina, Izv. Ross. Akad. Nauk, Ser. Fiz., 86, No. 6, 775–780 (2022).
A. S. Starukhin, A. A. Romanenko, and V. Yu. Plavskii, Opt. Spektrosk., 130, No. 5, 709–716 (2022)
M. Tanigucha, J. Lindsey, D. Bocian, and D. Holten, J. Photochem. Photobiol. C, 46, No. 3, Article ID 100401 (2021).
R. Basak and R. Bandyopadnyay, Langmuir, 6, No. 29, 4350–4356 (2013).
M. Managa and T. Nyokang, Macroheterocycles, 10, Nos. 4–5, 467–473 (2017).
M. Managa, J. Britton, E. Prinsloo, and T. Nyokonget, Polyhedron, 152, No. 15, 102–107 (2018).
C. Grewer and H. Brauer, J. Phys. Chem., 98, No. 16, 4250–4256 (1994).
M. Montalti, A. Credi, L. Prodi, and M. Gandolfi, Handbook of Photochemistry. Materials Science, 3rd edition, SBC, Taylor & Francis Group (2006), pp. 542–548.
A. Gorski, V. Knyuktshto, E. Zenkevich, A. Starukhin, M. Kijak, J. Solarski, A. Semelkin, and T. Lyubimova, J. Photochem. Photobiol., 354, 101–111 (2018).
R. Redmond and J. Gamlin, Photochem. Photobiol., 70, No. 4, 391–475 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 90, No. 2, pp. 211–219, March–April, 2023. https://doi.org/10.47612/051475062023902211219.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Starukhin, A.S., Korol’, Y.D., Pavich, T.A. et al. Spectral and Photophysical Parameters of a Series of Hydrophobic Phthalocyanines and Porphyrins in Aqueous Micellar Solutions. J Appl Spectrosc 90, 308–315 (2023). https://doi.org/10.1007/s10812-023-01537-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-023-01537-3