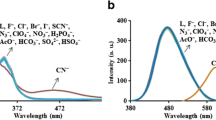
The synthesis and spectral characteristics of a new fluorescent N-allyl-4-iminodi(N-benzylacetamide)-1,8-naphthalimide (Zabe) sensor were reported. The ability of this new compound to detect anions was evaluated by spectrophotometrically monitoring the changes in the fluoresence intensity performed on its N,N-dimethylformamide (DMF) solution. Compared to other anions examined, only picrate (Pic–) generated a prominent fluorescence quenching at 516 nm. No significant fluorescence change was observed in the presence of other anions. The emission quenching was due to the enhanced photoinduced electron transfer (PET) from the receptor to the excited state of the fluorophore upon the recognition of picrate. The chemosensor can be applied to the quantification of picrate with a linear range from 4.97 × 10−6 to 6.82 × 10−5 M and a detection limit of 6.6 × 10−7 M. Most importantly, this sensor can be utilized for the spectroscopic detection of picrate in the presence of other competing anions. Moreover, the response time of the chemo sensor is less than 1 min.
Similar content being viewed by others
References
P. D. Beer and P. A. Gale, Angew. Chem. Int. Ed., 40, 486–516 (2001).
L. Fabbrizzi, M. Licchelli, and G. Rabaioli, A. Taglietti, Coordin. Chem. Rev., 205, 85–120 (2000).
J. Zhao, T. M. Fyles, and T. D. James, Angew. Chem. Int. Ed., 43, 3461–3464 (2004).
P. A. Gale, Accoun. Chem. Res., 39, 465–475 (2006).
V. Pimienta, R. Etchenique, and T. Buhse, J. Phys. Chem. A, 105, 10037–10044 (2001).
J. Y. Shen, J. F. Zhang, Y. Zuo, L. J. Wang, X. Y. Sun, J. S. Li, W. Q. Han, and R. He, J. Hazard. Mater., 163, 1199–1206 (2009).
R. Mantha, K. E. Taylor, N. Biswas, and J. K. Bewtra, Environ. Sci. Technol., 35, 3231–3236 (2001).
F. D. Marvin-Sikkema and J. A. M. de Bont, Appl. Microbiol. Biotechnol., 42, 499–507 (1994).
C. Beyer, U. Böhme, C. Pietzsch, and G. Roewer, J. Organomet. Chem., 654, 187–201 (2002).
H. Muthurajan, R. Sivabalan, M. B. Talawar, and S. N. Asthana, J. Hazard. Mater., 112, 17–33 (2004).
T. H. Ma, M. Dong, Y. M. Dong, Y. W. Wang, and Y. Peng, Chem. Eur. J., 16, 10313–10318 (2010).
M. Dong, Y. W. Wang, and Y. Peng, Org. Lett., 12, 5310–5313 (2010).
Y. Salinas, R. Martínez-Máñez, M. D. Marcos, F. Sancenón, A. M. Costero, M. Parra, and S. Gil, Chem. Soc. Rev., 41, 1261–1296 (2012).
Y. Q. Xu, B. H. Li, W. W. Li, J. Zhao, S. G. Sun, and Y. Pang, Chem. Commun., 49, 4764–4766 (2013).
S. Singh, J. Hazard. Mater., 144, 15–28 (2007).
M. E. Germain and M. J. Knapp, Chem. Soc. Rev., 38, 2543–2555 (2009).
J. F. Callan, A. P. de Silva, and D. C Magri, Tetrahedron, 6 1, 8551–8588 (2005).
W. Zhu, N. Minami, S. Kazaoui, and Y. Kim, J. Mater. Chem., 13, 2196–2201 (2003).
Z. F. Tao and X. Qian, Dyes and Pigments, 43, 139–145 (1999).
V. B. Bojinov, N. I. Georgiev, and N. V. Marinova, Sens. Actuators, B, Chem., 148, 6–16 (2010).
L. Patrick and A. Whiting, Dyes and Pigments, 55, 123–132 (2002).
V. B. Bojinov and I. P. Panova, Dyes and Pigments, 74, 551–560 (2007).
J. Kollár, P. Hrdlovič, and Š. Chmela, J. Photochem. Photobiol. A: Chem., 195, 64–71 (2008).
W. H. Zhu, C. Hu, K. C. Chen, and H. Tian, Synth. Met., 96, 151–154 (1998).
V. Gruzinskii, A. Kukhto, and G. Shakkah, J. Appl. Spectrosc., 65, 463–465 (1998).
W. H. Zhu, M. Hu, R. Yao, and H. Tian, J. Photochem. Photobiol., A, Chem., 154, 169–177 (2003).
W. W. Stewart, J. Am. Chem. Soc., 103, 7615–7620 (1981).
H. Tian, J. Gan, K. C. Chen, J. He, Q. L. Song, and X. Y. Hou, J. Mater. Chem., 12, 1262–1267 (2002).
C. Y. Li, F. Xua, Y. F. Li, K. Zhou, and Y. Zhou, Anal. Chim. Acta, 717, 122–126 (2012).
I. Grabchev, X. H. Qian, V. Bojinov, and Y. Xiao, W. Zhang, Polymer, 43, 5731–5736 (2002).
V. B. Bojinov and T. N. Konstantinova, Sens. Actuators, B, Chem., 123, 869–876 (2007).
N. I. Georgiev and V. B. Bojinov, J. Lumin., 132, 2235–2241 (2012).
H. Yang, H. S. Song, Y. C. Zhu, and S. P. Yang, Tetrahedron Lett., 53, 2026–2029 (2012).
P. G. Jones, J. Ossowski, and P. Kus, Z. Naturforsch. B, 57, 914–921 (2002).
T. N. Konstantinova, P. Meallier, and I. Grabchev, Dyes and Pigments, 22, 191–198 (1993).
C. G. Niu, X. Yang, W. Q. Lin, G. L. Shen, and R. Q. Yu, Analyst, 127, 512–517 (2002).
R. Parkesh, T. C. Lee, and T. Gunnlaugsson, Org. Biomol. Chem., 5, 310–317 (2007).
C. C. Wang, S. Feng, L. Y. Wu, S. Y. Yan, C. Zhong, P. Guo, R. Huang, X. C. Weng, and X. Zhou, Sens. Actuators, B, Chem., 190, 792–799 (2014).
E. H. Lu, X. J. Peng, F. L. Song, and J. L. Fan, Bioorg. Med. Chem. Lett., 15, 255–257 (2005).
C. G. Niu, G. M. Zeng, L. X. Chen, G. L. Shen, and R. Q. Yu, Analyst, 129, 20–24 (2004).
V. B. Bojinov, I. P. Panova, and J. M. Chovelon, Sens. Actuators, B, Chem., 135, 172–180 (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Zhurnal Prikladnoi Spektroskopii, Vol. 84, No. 1, pp. 33–38, January–February, 2017.
Rights and permissions
About this article
Cite this article
Wu, HL., Aderinto, S.O., Xu, YL. et al. A Highly Selective Fluorescent Chemosensor for the Detection of Picrate Anion Based on 1,8-Naphthalimide Derivatives. J Appl Spectrosc 84, 25–30 (2017). https://doi.org/10.1007/s10812-017-0421-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-017-0421-7