Abstract
Live prey used by the aquaculture industry are usually poor in some essential nutrients including long chain polyunsaturated fatty acids (LC-PUFA) and must be enriched to improve their nutritional value prior to larval feeding. Standard enrichment protocols are commonly based on lipid emulsions, being associated to a high oxidative stress condition. The combination of microalgae and lipid emulsion can palliate this situation, where the oxidative stress can be partially compensated by the antioxidant compounds present in microalgae. The maintenance of living microalgae in culture facilities is laborious, and the produced biomass may present fluctuating properties, leading to a serious bottleneck in the cultivation of live prey. Hence, substitutes for live microalgae including pastes or dried formats are receiving increasing research attention due to its nutritional stability, longer shelf-life and easy handling. In this study four different microalgae formats combined with a lipid emulsion are tested as enrichment products for Brachionus plicatilis and Artemia. Thus, fresh, frozen and spray-dried Navicula salinicola (NFRE, NFRO and NSD, respectively), and spray-dried Isochrysis galbana (ISD) were mixed with a commercial oil concentrate (IncromegaTM) or a marine lecithin (LC 60®), and added for 5 h to the rotifer or Artemia culture media. The antioxidant capacity of the microalgae extracts and the live prey activity of antioxidant enzymes, peroxides index (PxI) and thiobarbituric acid reactive substances (TBARS) were evaluated. The lipid profile of microalgae formats and enriched live preys was also determined. Ethyl acetate extract was the most antioxidant active extract of all microalgae formats. In addition, overall, I. galbana seems to be better than any N. salinicola format for a more effective protection against oxidative stress and for live prey lipid enrichment. Both rotifer and Artemia cultured with the mixture of I. galbana and the lipid emulsion generally showed higher DHA/EPA and EPA/ARA ratios. Moreover, the combination of the microalgae with LC 60® lipid emulsion highly favored Artemia´s polar lipid and DHA incorporation. Among microalgae products, both spray-dried formats better enhanced live prey n-3 LC-PUFA content. Our results highlight the great potential of new microalgae-derived products to improve effectiveness of current live prey lipid enrichment protocols used in aquaculture.
Similar content being viewed by others
Introduction
After consuming the yolk sac the larvae of most marine species are in an undeveloped state with a still rudimentary digestive system unable to effectively process formulated diets (Gisbert et al. 2004; Pérez et al. 2020). Consequently, live feed, namely zooplankton such as the rotifer Brachionus plicatilis and Artemia, are commonly provided sequentially to avoid compromising larval growth and survival (Bengston 2003; Oliver et al. 2017; Eryalçın, 2019; Samat et al. 2020).
However, these organisms are not the natural prey of marine larvae and their nutritional profiles do not match the nutritional requirements of larvae during their first life stages (Oliver et al. 2017). In particular, rotifers and Artemia are deficient in some essential nutrients including long chain polyunsaturated fatty acids (LC-PUFA), compared with natural prey such as copepods (McEvoy et al. 1995; Viciano et al. 2015). LC-PUFA, especially eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and arachidonic acid (ARA), play significant roles in marine larval development (Salhi et al. 1997; Tocher 2010) and their deficiency promotes metabolic disorders, negatively affects growth and causs skeletal abnormalities in fish larvae (Radhakrishnan et al. 2020). The requirements for these essential fatty acids (FA) depend upon species, their genetic repertoire, and dietary and environmental factors, and must be incorporated through the diet as they cannot be endogenously synthesized at an adequate rate to meet larval needs (Samat et al. 2020). In this context, the improvement of the nutritional value of live feed with essential nutrients to better suit predator’s nutritional needs has been widely addressed (Rodríguez et al. 1997; Viciano et al. 2015; Eryalçın, 2018; Choi et al. 2022).
The type of lipid in which fatty acids are esterified seems to also be particularly relevant in marine larvae nutrition (Lund et al. 2018; Reis et al. 2021). Marine phyto- and zooplankton contain mostly of n-3 LC-PUFA-rich phospholipids, while most enrichment commercial products are rich in triacylglycerols (TAG), which usually have lower n-3 LC-PUFA proportions than polar lipids (Morais et al. 2007). Hence, live prey are frequently enriched with excess lipid, especially in its neutral lipid fraction. An overabundance of neutral lipids has been suggested to negatively influence absorption and digestion processes, compromising larval performance (Morais et al. 2007; Reis et al. 2017, 2019). On the other hand, phospholipids (mainly phosphatidylcholine; PC, commonly named as lecithin) are directly involved in lipid transportation through the circulatory system (Morais et al. 2007) aiming towards proper larval growth and survival.
The high metabolic activity during the first stages of larval development and the aeration systems used in live prey enrichment practices, generate a pro-oxidant environment that compromises the appropriate supply of LC-PUFA to the larvae (Viciano et al. 2017). Moreover, the most common and effective enrichment protocols for live prey are based in lipid emulsions rich in PUFA (Sorgeloos et al. 2001), highly susceptible to oxidation, promoting the bioaccumulation of lipid peroxides which are potentially toxic for the larvae (Viciano et al. 2015).
Microalgal biomass can be a rich source of protein and in some cases, can also provide certain quantities of EPA or DHA, and also containing compounds with antioxidant capacity such as pigments, being commonly used alone or in combination with lipid emulsions to feed live prey, helping to reduce lipid peroxidation in the enrichment protocols (Siddik et al. 2023). In fact, the aquaculture practice known as “green water” contributes to maintaining microbial load and water quality, reduces water exchange, and improves growth and immunity (Neori 2011). The ability to produce antibacterial substances by algae is also found to be effective in controlling diseases (Goud et al. 2023). Nowadays, microalgae are increasingly exploited as animal feeds for their nutritional value, as well as in several other sectors for the production of food, nutraceutical substances, cosmetics, biostimulants and fertilizers for agriculture crops, and as bioremediation vectors and biofuels, among others (Raja et al. 2018; Braun and Colla 2023). Several species of microalgae are currently used in the aquaculture industry to feed invertebrates and fish larvae (Eryalçın, 2019; Sibonga et al. 2021; Siddik et al. 2023). However, microalgae require a lipid supplementation prior to be added to the culture media in order to successfully increase the n-3 LC-PUFA levels of live prey (Eryalçın, 2019) that, in addition to their high production costs (up to 30-40% of total cost), entail a serious bottleneck in hatcheries (Eryalçın, 2019; Siddik et al. 2023). This, together with the difficulty in producing, concentrating and storing live microalgal biomass, has encouraged the search for alternatives to live microalgae in recent years (Raja et al. 2018; Samat et al. 2020; de la Cruz-Huervana et al. 2022). The most interesting substitutes are microalgal products such as pastes, dried and spray-dried microalgae, microencapsulates, and cryopreserved or flocculated microalgae, among other formats (Raja et al. 2018).
This research aims to compare fresh, frozen and spray-dried formats of the marine diatom Navicula salinicola Hustedt (Bacillariophyta, Bacillariophyceae) and a spray-dried format of Isochrysis galbana Parke (Haptophyta, Coccolithophyceae), combined with two different lipid emulsions, for their potential application in rotifer and Artemia enrichment protocols. N. salinicola has elevated biomass productivity (Rachmayanti et al. 2020), and tolerates strong salinity variations and high irradiation (Sylvestre et al. 2004). Its resistance to biological contamination (M. Venuleo, personal communication) and benthic nature (Rachmayanti et al. 2020) facilitate its culture and harvest. It also improves water quality in cultivation systems (Khatoon et al. 2007), being used as food for cultured organisms such as shrimps and sea urchins (Xing et al. 2007; Zupo and Messina 2007). I. galbana, is the microalga most commonly used as live feed in aquaculture (El-Tohamy et al. 2021).
Materials and methods
Microalgal cultivation and processing
Fresh paste, frozen paste and spray-dried formats of N. salinicola, and spray-dried I. galbana were produced at the facilities of Instituto Tecnológico de Canarias (ITC), located in Pozo Izquierdo (27°48’52’’ N, 15°25’25’’ W; Gran Canaria, Spain).
N. salinicola strain BEA 2004B (GenBank access ID: MT012298.1; https://www.ncbi.nlm.nih.gov/nuccore/MT012298; accessed on: 24 April, 2023), was isolated by the ITC from samples taken of a desalination plant (“Desaladora del Sureste”, Gran Canaria). I. galbana strain BEA 1751B (GenBank access ID: MN867789.1, https://www.ncbi.nlm.nih.gov/nuccore/MN867789.1, accessed on: 24 April, 2023), was isolated by the Spanish Bank of Algae (BEA, Telde, Gran Canaria) from natural water samples collected at the “Bocacangrejo” saltpans (Agüimes, Gran Canaria).
Briefly, the microalgae were aseptically grown at ITC’s facilities in 200 mL Erlenmeyer flasks and transferred progressively into increasingly larger flasks and to 8 L Nalgene polycarbonate carboys until a minimum biomass concentration of 0.5 g L-1. Cultures were then used to inoculate 250 L raceway-type cultivation systems placed under a 1,500 m2 greenhouse and transferred to increasingly larger fiberglass reinforced polyester (250 L and 1,600 L) or concrete 10,000 L raceways covered by PVC. Culture depth was maintained ≤ 12 cm and outdoor cultures were supplied with CO2 to maintain the pH between 7.0 and 8.0. Both algae were harvested during the exponential growth phase, when they reached a biomass concentration ≥ 0.7 g L-1.
Microalgae pastes obtained by centrifugation had a water content between 75 and 85% (w/w). In the case of N. salinicola, this paste was used to obtain the fresh format or frozen at -20°C to obtain the frozen format. Fractions of both N. salinicola and I. galbana pastes were further dehydrated by spray drying (L-12, Ohkawara Kakohki Co, Ltd, Japan) to obtain a dried biomass with a water content between 5 and 7% (w/w). Frozen format was maintained for approximately 20 days after collection until the beginning of the experiment.
Extract preparation
Independently of the format, 25 g of microalgae were extracted by maceration with 96% ethanol (3 × 250 mL × 24 h) and continuous stirring at room temperature (20 ± 4 ºC). The extract was filtered and the solvent was removed under reduced pressure at 40 ºC on a rotary evaporator, yielding ethanolic extracts (Figure 1, Supplementary Material). Extraction yields are shown in Table S1, Supplementary Material.
Liquid-liquid partition of EtOH extract
The ethanol extract was further fractionated by liquid-liquid partition (Figure 1, Supplementary Material) by sequentiall fractionation using a series of solvents of increasing polarity. Briefly, the ethanol extract (8-12 g) was suspended in 300 mL distilled water and successively extracted with n-hexane (3 × 300 mL) and ethyl acetate (3 × 300 mL). The organic phases were concentrated under reduced pressure at 40 ºC on a rotary evaporator to give the n-hexane and ethyl acetate fractions, whereas the aqueous residue was lyophilized providing the water fraction. Partition yields are shown in Table S1, Supplementary Material.
Total antioxidant capacity of microalgae formats
Ethanol extracts, n-hexane, ethyl acetate and water fractions, and a standard solution of Trolox were dissolved in sterile dimethyl sulfoxide (DMSO) using a sonication bath for 3-4 min at a final concentration of 50 mg mL-1 (Zárate et al. 2020).
Total antioxidant capacity was evaluated through the study of both 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging assays.
The ABTS radical scavenging assay was conducted on samples dissolved at different concentrations (0.244 to 250 µg mL-1 in methanol), using 96-well microplates and adding a control in each plate (Re et al. 1999; Zárate et al. 2020). Equal volumes of 7 mM ABTS and 2.4 mM potassium persulfate were mixed for 12-16 h at room temperature in the dark and dissolved in methanol until an absorbance of 0.7 at 734 nm was obtained. ABTS solution was added to the microwells and after 8 min of incubation the absorbance was recorded at 750 nm with a BioRad Microplate Reader Model 680 (Bio-Rad Laboratories, USA).
Similarly, DPPH radical scavenging assay was assessed using DPPH dissolved in methanol (45 µg mL-1). The absorbance was measured at 515 nm after 30 min in darkness (Blois 1958).
For both analyses the percentage of antioxidant activity was calculated following the equation:
where: Abscontrol is the absorbance of ABTS or DPPH radical + methanol; Abssample is the absorbance of ABTS or DPPH radical + sample/standard.
Concentration scavenging 50% of radicals (IC50) of each sample was calculated by the interpolation of the antioxidant activity percentage vs. concentration curve.
Experimental conditions
The enrichment protocols were performed in triplicate in 10 L cylinder-conical fiberglass tanks at the rearing facilities of Centro Oceanográfico de Canarias from Instituto Español de Oceanografía (IEO-CSIC, Santa Cruz de Tenerife, Tenerife), for 5 h. Water temperature was 22-23°C and salinity 28-30 ppt for rotifer, and 36 ppt for Artemia. Rotifers were cultivated at a density of 180 ± 20 individuals mL-1 whereas 12-24 h-old Artemia nauplii obtained from decapsulated BF Artemia cysts (INVE Aquaculture, Belgium) were reared at 10 ± 2 individuals mL-1. Rotifers were enriched with 0.06 g L-1 of a commercial lipid based on DHA, Incromega™ DHA500 (Croda, Inc., UK), while 0.6 g L-1 of Marine Lecithin LC 60® (PhosphoTech Laboratoires, France) was used for Artemia as recommended by the manufacturer. Regardless of the species, the lipid emulsions without microalgae supplementation constituted the control group. In addition, the same lipid emulsion supplemented with one of the formats of N. salinicola or I. galbana was used for the experimental treatments. Thus, experimental rotifers were supplemented with either 0.04 g L-1 of spray-dried I. galbana (ISD) or N. salinicola (NSD), 0.07 g L-1 of fresh (NFRE) or frozen (NFRO) N. salinicola paste, while 0.10 g L-1 of ISD and NSD, and 0.20 g L-1 of NFRE or NFRO were used for Artemia. Microalgae doses were based upon survival and oxidative stress response of live prey recorded in preliminary assays.
Survival and sample preparation
At the beginning and at the end of the experimental period, rotifers and Artemia nauplii population parameters were volumetrically determined from the culture density (individuals mL-1) and final survival calculated. At the end of the experiments, culture media containing rotifers and Artemia were respectively filtered through a 60 or 100 µm mesh, washed with clean seawater in order to eliminate remaining microalgae and lipid emulsion, and immediately stored at -80°C until further analysis.
Lipid composition
The total lipid (TL) of samples was extracted in chloroform/methanol (2:1, v/v) by the Folch method (Folch et al. 1957) with small modifications as described by Christie and Han (2010), and Reis et al. (2019).
Lipid classes were analysed by high-performance thin-layer chromatography (HPTLC) in a one dimensional double-development to separate polar and neutral lipids (Olsen and Henderson 1989). Lipid classes identification was performed by comparison to external lipid standards (cod roe lipid extract; digalactosyl-diacylglycerol, monogalactosyl-diacylglycerol, and sulfoquinovosyl-diacylglycerol (Avanti Polar Lipids, Inc., USA)) placed on the same HPTLC plate. Lipid classes were quantified by calibrated densitometry as described by Reis et al. (2019).
Fatty acid methyl esters (FAME) were obtained from 1 mg TL by acid-catalyzed transmethylation using toluene and 1% sulphuric acid in methanol (v/v) for 16 h at 50°C (Christie and Han 2010). FAME were purified by thin layer chromatography using 20 × 20 cm plates coated with silicagel (Macherey-Nagel, Germany) and subsequently separated and quantified using a TRACE-GC Ultra Gas Chromatograph (Thermo Scientific, Italy) as detailed by Galindo et al. (2022a). Individual FAME were determined by comparison to a mixture of commercial standards (Mix C4-C24 and PUFA No. 3 from menhaden oil (Supelco Inc.)), and the identity confirmed by GC-MS (DSQ II, Thermo Scientific) when necessary.
Antioxidant response
Peroxide index (PxI, meqO2 kg-1) was determined following Shantha and Decker (1994) with small modifications as described previously (Galindo et al. 2022b). The concentration of lipid peroxides was calculated by monitoring ferric chloride (FeCl3) synthesis at 500 nm, using a standard curve.
For the analysis of thiobarbituric acid reactive substances (TBARS) and antioxidant enzymes, rotifer and Artemia samples were homogenized in an ice-cold 20 mM Tris-Cl (w/v) buffer (pH 7.4) with protease inhibitors (Complete®, Sigma, Spain), and supernatants aliquoted and stored at -80°C until analysis (Galindo et al. 2022b).
Malondialdehyde (MDA) content of samples was obtained by TBARS assay (Ohkawa et al. 1979). Homogenates were incubated in a solution containing thiobarbituric acid (Galindo et al. 2022b) and after reaction, they were fluorimetrically determined (excitation 530 nm/emission 550 nm) (Thermo Scientific Appliskan, Thermo Fisher Scientific, Finland). MDA content was calculated using a standard curve of 1,1,3,3-tetramethoxipropane and expressed as nmol MDA mg-1 protein.
Regarding antioxidant enzymes, superoxide dismutase (SOD, EC 1.15.1.1) activity was calculated using 30 mM pyrogallol as substrate. The auto-oxidation of pyrogallol and the inhibition of this reaction were monitored at 420 nm for 10 min. One unit of SOD activity is equivalent to the amount of enzyme that produces a 50% inhibition of the auto-oxidation of pyrogallol (Mesa-Herrera et al. 2019).
Catalase (CAT, EC 1.11.1.6) activity was established using the molar extinction coefficient of H2O2 (Ɛ = 42.6 M-1 cm-1), with 485 mM H2O2 as substrate. Degradation of H2O2 was read at 240 nm during 15 min (Clairborne 1985).
Glutathione reductase (GR, EC 1.6.4.2) activity was measured following Chung et al. (1991) with 1 mM GSSG and 60 μM NADPH as substrates. Oxidation of NADPH was determined at 340 nm for 15 min, using a molar extinction coefficient of -6.22 mM-1 cm-1.
The activity of glutathione-S-transferase (GST, EC 2.5.1.18) was determined by the conjugation of 5 mM GSH with 1 mM 1-chloro-2,4-dinitrobenzene (CDNB), and the absorbance read at 340 nm for 15 min (Habdous et al. 2002). GST activity was quantified using the molar extinction coefficient of Mesenheimer complex (Ɛ = 9.6 mM-1 cm-1).
Absorbances were measured in a spectrophotometer and one unit of activity (U) defined as µmol min-1 unless otherwise stated. Soluble protein of homogenized samples was quantified following Bradford (1976) using bovine serum albumin as standard.
Statistical analysis
Prior to analysis, normality and homoscedasticity of data were confirmed and variance stabilizing transformations (arcsine and logarithm) were used when needed. If both assumptions were satisfied, one-way ANOVA followed by a Tukey HSD post-hoc test were performed to establish significant differences between treatments. When variance stabilizing transformations did not succeed, Welch test followed by the Dunnett T3 test, or Kruskall-Wallis nonparametric test followed by pair-wise comparison Mann–Whitney test with Bonferroni correction were developed for no homoscedastic data, or non-normal distribution, respectively.
Results are presented as means ± standard deviation (SD). The statistical significance was set at p<0.05. Statistical analysis was carried out with IBM SPSS Statistics 25.0 software package (IBM Corp., USA) for Windows.
Results
Total antioxidant activity of microalgae formats
DPPH antioxidant activity was generally low in all microalgae formats, with neither of the extracts showing more than 50% of activity at the maximum concentration tested (250 µg mL-1). The highest values were mostly recorded in ethyl acetate extracts from both N. salinicola (42-44%) and I. galbana (~39%) (p<0.05; Table 1).
Similarly, ABTS scavenging assay showed that all ethyl acetate extracts and also the fresh/frozen N. salinicola ethanol extract were capable of inhibiting more than 50% of the radicals at 250 µg mL-1. The lowest IC50 values were obtained in spray-dried and fresh/frozen N. salinicola ethyl acetate extracts (p<0.05), despite which they were near 100-fold less active than Trolox (87-89 µg mL-1 vs. 0.87 ± 0.18 µg mL-1) (Table 1).
Survival of live prey
Neither rotifer (69-93%) nor Artemia survival (over 92% in all cases) was significantly affected by the enrichment with any blend of microalgae and lipid emulsions assayed.
Lipid profile of microalgae formats and lipid emulsions
Microalgal total lipid content varied between 15 and 21% dry weight (DW), of which 61-75% were total neutral lipids (TNL). Free fatty acids + phytosterols (FFA+PTS) were the main neutral lipid fraction (31-45%), although TAG was especially relevant in NFRO (~30%). Pigments (P) were remarkably abundant in ISD (~25%) (Table 2).
As shown in Table 3, total PUFA constituted the main FA group of ISD, while total monounsaturated FA was in N. salinicola. ISD contained high n-3 PUFA (~37% of total FA) and n-3 LC-PUFA (~16%) levels, mainly due to DHA (~15%). On the other hand, EPA (~10%) and EPA/ARA ratios were remarkably high in all N. salinicola formats.
Finally, both LC 60® and Incromega™ emulsions were mainly formed by PUFA (~53 and ~77% of total FA, respectively) as a result of high EPA (~13 and ~9%) and DHA (~36 and ~55%) contents (Table 3).
Total lipid and lipid class composition of live prey
Regardless of the microalgae format, the TL content of rotifers remained unchanged (23-27% DW) (Table 4). TNL encompassed 82-88% of TL, mainly formed by TAG (33-37% of TL). Cholesterol (CHO) was higher in NFRE-rotifers (~15%) than in the control treatment (~12%; p<0.05), while FFA was highest in NSD-rotifers (~27%) and lowest in NFRO-rotifers (~18%; p<0.05).
Both PC and phosphatidylethanolamine (PE) were the dominant polar lipids in all rotifer groups (4-5%). NFRO-rotifers had the highest proportions of phosphatidylglycerol (PG), phosphatidylserine (PS) and lysophosphatidylcholine + sphingomyelin (LPC+SM), while NSD- and NFRE-rotifers showed the lowest values (p<0.05; Table 4).
The TL content of Artemia nauplii (18-19% DW) was not affected by the microalgae feeding regime (Table 5). TNL ranged from ~57% in the control treatment to ~63% in ISD-Artemia. TAG was higher in both groups fed the spray-dried microalgae (29-30%) than in the control-Artemia (~25%; p<0.05), while FFA was highest in NFRE and ISD-groups (~7%), and lowest in NFRO (~4%; p<0.05). Finally, sterol esters (SE) reached its greatest proportion in ISD-Artemia (4.06 ± 0.41%; p<0.05).
PC (10-15% of TL) and PE (10-11%) were the most abundant lipid fractions within polar lipids. PG was highest in NFRO and NSD-Artemia (3.56 ± 0.14 and 3.37 ± 0.03%, respectively), and lowest in ISD (2.89 ± 0.16%; p<0.05) (Table 5).
FA composition of live prey
Rotifer FA profile was greatly affected by the enrichment protocol (Table 6). Thus, PUFA was the most relevant group of FA (56-64% of total), being higher in ISD and control groups, and lower in NFRE-rotifers (p<0.05). A similar pattern was onserved for total n-6 PUFA (8-9%), total n-3 PUFA (50-58%) and total n-3 LC-PUFA (47-53%) (p<0.05; Table 6).
ARA and DHA were remarkably high in control and ISD-rotifers (2.22 ± 0.03% and 2.15 ± 0.03%; 42.36 ± 0.58% and 42.39 ± 0.69%, respectively). EPA was also high in all treatments, mainly in NSD-rotifer (8.48 ± 0.04%; p<0.05). Consequently, all rotifers had high DHA/EPA ratios (5.0-5.6), mainly control and ISD-rotifers, while EPA/ARA (3.5-4.1) ratio reached its peak in the groups enriched with NSD and NFRO (p<0.05; Table 6).
Monounsaturated was the most abundant FA family in Artemia nauplii, ranging from ~38% in ISD-Artemia to ~44% in NFRO-Artemia, followed by PUFA (from 29.31 ± 0.06% in the control group to 36.17 ± 0.56% in ISD-Artemia), of which total n-3 was 3-4 fold higher than total n-6 (22-28% vs. 6-7%). Total saturated was higher in the control group (~29%) than in the experimental ones (22-25%; p<0.05).
Total n-6 PUFA (7.11 ± 0.15%), 18:2n-6 (3.92 ± 0.09%), 18:3n-3 (2.73 ± 0.07%), ARA (2.88 ± 0.06%), and DHA (10.80 ± 0.34%) were greatest in ISD-Artemia (p<0.05). In addition, both groups fed spray-dried microalgae contained the most n-3 LC-PUFA (23-24%) and EPA (~13%; p<0.05).
ISD-Artemia showed the highest DHA/EPA ratio (0.85) while NSD- and NFRO-Artemia had the highest EPA/ARA ratio (4.4-4.8; p<0.05).
Live prey antioxidant activities and lipid peroxidation
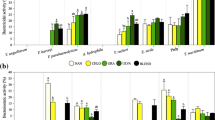
The activity of rotifer’s antioxidant enzymes CAT (4.14-7.67 U mg-1 protein), GR (6.60-9.24 mU mg-1 protein), GST (471.96-991.95 mU mg-1 protein) and SOD (714.15-4135.77 U mg-1 protein) was not affected by the dietary treatment (Fig. 1A-D). By contrast, PxI was lowest in the control group (15.24 ± 2.00 meqO2 kg-1), ranging between 57.88 and 91.28 meqO2 kg-1 in the experimental groups (p<0.05; Fig. 1E). TBARS were reduced in both control and ISD-rotifers (2.02 ± 0.89 and 1.14 ± 0.90 nmol MDA mg-1 protein, respectively) compared to rotifers fed N. salinicola formats (4.88-6.05 mg-1 protein; p<0.05; Fig. 1F).
Antioxidant activities: (A) catalase (CAT); (B) glutathione reductase (GR); (C) glutathione-S-transferase (GST); (D) superoxide dismutase (SOD); (E) peroxides index (PxI) and (F) TBARS of rotifers enriched with the different dietary treatments. Results are presented as mean ± SD (n = 3). NSD, spray-dried N. salinicola; NFRE, fresh N. salinicola; NFRO, frozen N. salinicola; ISD, spray-dried I. galbana. Different letters denote significant differences (p<0.05)
Artemia nauplii presented lower CAT activity in both ISD- and NFRO-groups (3.22 ± 0.49 and 3.49 ± 0.47 U mg-1 protein, respectively) than in the control treatment (6.76 ± 1.82 U mg-1 protein; p<0.05; Fig. 2A). By contrast, enzymatic activities of GR, GST, and SOD, the lipid peroxidation indexes and TBARS were not affected by the enrichment protocol (Fig. 2B-F).
Antioxidant activities: (A) catalase (CAT); (B) glutathione reductase (GR); (C) glutathione-S-transferase (GST); (D) superoxide dismutase (SOD); (E) peroxides index (PxI) and (F) TBARS of Artemia enriched with the different dietary treatments. Results are presented as mean ± SD (n = 3). NSD, spray-dried N. salinicola; NFRE, fresh N. salinicola; NFRO, frozen N. salinicola; ISD, spray-dried I. galbana. Different letters denote significant differences (p<0.05)
Discussion
Rotifers and Artemia must be enriched with LC-PUFA in order to boost their nutritional value and successfully contribute to proper larval development (Seychelles et al. 2009; Viciano et al. 2015, 2017). However, LC-PUFA-rich lipid emulsions are highly susceptible to oxidation, requiring the addition of antioxidant compounds (Viciano et al. 2017). In this context, microalgae may exert several beneficial effects when used to feed zooplankton including the improvement of live prey protein content and FA profile and/or antioxidant protection (Matsui et al 2020; Turcihan et al. 2021).
N. salinicola extracts showed lower DPPH scavenging activity (22-44%; Table 1) than the values (~62.8%) reported by Affan et al. (2007) for this species (previously known as Navicula incerta; Hong et al. (2019)) although this might be due to the different solvent used in their study (methanol). Nevertheless, the ABTS test yielded more interesting results, with all ethyl acetate extracts, and the ethanol extract from fresh/frozen N. salinicola capable of inhibiting more than 50% of the radicals at 250 µg mL-1. Antioxidant capacity of I. galbana has been previously attributed to its carotenoid, polysaccharides and phenolic compounds content (Saranya et al. 2014; Sun et al. 2014), and ethyl acetate has been recommended as the best solvent to obtain most of the valuable FA and carotenoids in this species (Bustamam et al. 2021).
Under our experimental conditions, the mixture of different N. salinicola formats or the spray-dried I. galbana with a lipid emulsion did not affect live prey survival or TL content, as reported previously in works testing mixed microalgal diets and formats (Palmtag et al. 2006; Thépot et al. 2016). However, dietary treatments directly impacted the FA profiles of rotifers and Artemia. Thus, spray-dried formats generally improved PUFA retention in both species (Tables 6 and 7) supporting the higher availability of nutrients in spray-dried formats pointed out in a previous study with Nannochloropsis oculata (Eryalçın, 2019). In particular, Artemia fed ISD showed the highest DHA content suggesting it is the best treatment to preserve DHA incorporation, protecting against the preferential oxidation of DHA described in Artemia (Reis et al. 2019). In addition, the reduced DHA incorporation in all rotifers fed N. salinicola formats might be related to a poorer ability to retain C22 PUFA into tissue lipids (Pérez et al. 2022) due to the lower availability of DHA in their final enrichment products compared to those of the control and I. galbana (Table 3).
As expected, the mixed enrichment protocol was more effective in terms of LC-PUFA incorporation than other feeding practices using commercial products or just microalgae. For instance, ARA, EPA and DHA proportions in rotifer and Artemia nauplii raised compared to values reported when fed the commercial Ori-Green™ (algae + lipid emulsion-based product) (Rocha et al. 2017). Our experimental treatments also improved the rates of LC-PUFA incorporation by live prey when fed with I. galbana for 24 h in a previous study performed by our group (data not shown). The present results indicate that the combination of microalgae and a lipid emulsion with a short-term enrichment period (5 h) may be a valuable enrichment protocol, even better than some commercial products or microalgae alone, as previously suggested by Eryalçın (2019). Furthermore, diatoms are not commonly used to feed live prey. The effectiveness of using N. salinicola with a lipid emulsion as an enrichment protocol for rotifers and Artemia, is reported for the first time in this study.
The DHA/EPA ratio is a common indicator to evaluate the nutritional suitability of a diet for marine larvae nutrition, as inadequate ratios of these two FA may impair membrane function and larval growth (Rodríguez et al. 1997; Viciano et al. 2017). Although DHA/EPA requirement is species-specific (Rodríguez et al. 1997), the recommended ratio for marine fish larval normal development is similar or even higher than that present in natural preys such as copepods (~2) (Sargent et al. 1997; Viciano et al. 2017). In our study, rotifers fed the mixture of microalgae and lipid emulsion had higher DHA/EPA ratios (4.64-5.69) than rotifers fed fresh or frozen T-ISO (Seychelles et al. 2009), N. oculata, or a mixture of N. oculata and Chlorella vulgaris (Thépot et al. 2016). However, DHA/EPA ratios in Artemia were generally low (0.7-0.8), in accordance to other assays with either lipid emulsion or microalgae enrichment (Viciano et al. 2015, 2017). The enrichment of Artemia with DHA is actually a great research challenge in the aquaculture sector, as it is well established that they retro-convert DHA to EPA (Navarro et al. 1999).
ARA is also a relevant LC-PUFA in fish nutrition, being important for growth, survival, pigmentation, stress tolerance, egg/larval quality and improved feed utilization efficiency (Bell and Sargent 2003). Moreover, ARA and EPA are involved in eicosanoids production, both competing for the same enzymes, and consequently, their ratio must be properly balanced (Magalhães et al. 2020). In our experiments, rotifers and Artemia presented EPA/ARA ratios of around 4 (Tables 6 and 7), which is in line to values recommended for European seabass (Dicentrarchus labrax) and gilthead seabream (Sparus aurata) larvae (Hamre et al. 2013).
Several studies suggest that phospholipids are preferred over TAG as the main lipid source by most marine fish larvae species (Reis et al. 2019; Lund et al. 2021; Turcihan et al. 2021). Diets formulated with TAG may reduce fish growth and survival, and promote lipid accumulation in the liver and intestine. By contrast, phospholipids are highly abundant in marine fish eggs, being essential structural components of membranes, also involved in digestion, absorption and transport of lipids (Sargent et al. 1999), that are required for the correct growth and survival of a wide range of aquatic species (Hamre et al. 2013; Lund et al. 2018). An adequate balance between energy and essentiality for live prey can only be achieved by a suitable equilibrium between phospholipids and TAG (Sargent et al. 1999). While rotifers and Artemia have a natural polar/neutral lipid ratio of 30:70, copepods have a more favorable proportion of approximately 50:50 (Hamre et al. 2013). Under our experimental conditions, Artemia showed average polar/neutral lipid ratios of 40:60, more similar to copepods than that of rotifers (12-18/88-82). Although recent studies demonstrate that Artemia tends to metabolize dietary phospholipids into TAG (Reis et al. 2017, 2019), the short enrichment period used in our present work (5 h), together with the lipid emulsion based on phospholipids (LC-60) probably favored Artemia´s polar lipid and DHA incorporation.
Live prey lipid-enrichment protocols promote auto-oxidation of PUFA and the formation of potentially toxic oxidative products, compromising health and survival of predators. Rotifers and Artemia naturally possess certain levels of antioxidant enzymes required to metabolize reactive oxygen species (ROS) and to repair damage caused by free radicals, including lipid oxidation. In this sense, SOD and CAT enzymes are considered the first line of defense in the detoxification process against ROS (Barata et al. 2005b). CAT activity was higher in Artemia fed the control treatment than in NFRO and ISD-Artemia (Fig. 2), suggesting a protective effect of these two last formats compared to the control, as organisms can up-regulate antioxidant defenses in response to increasing ROS production (Barata et al. 2005a, 2005b). In spite of these differences in CAT activity between Artemia groups, PxI was not affected by dietary treatments, indicating that the Artemia antioxidant capacity was able to efficiently fight against excessive ROS formation and oxidative stress. The presence in Artemia of carotenoids with recognized antioxidant capacity such as cantaxanthin (Rønnestad et al. 1998), can be attenuating the damaging effects of ROS.
On the other hand, and even though antioxidant enzyme activity was not increased in rotifers, all experimental treatments showed higher PxI and TBARS indexes than the control group, except for the TBARS values in the ISD-rotifers (Fig. 1). This suggests that lipid peroxidation was partially compensated by rotifers fed ISD or that the time of enrichment was enough to generate oxidative stress but not to maintain it. Thus, reducing the time of enrichment could potentially have decreased lipid peroxidation. In fact, short enrichment periods have been recommended in order to avoid lipid oxidation and to improve enrichment efficiency, especially in DHA-rich emulsions (McEvoy et al. 1995) for both Artemia and rotifers (Reis et al. 2019; Pérez et al. 2022).
In conclusion, both N. salinicola and I. galbana might be considered valuable microalgae to enrich rotifers and Artemia when combined with a suitable lipid emulsion. However, considering its n-3 LC-PUFA content and DHA/EPA ratio, I. galbana seems to be more adequate than N. salinicola to feed rotifers and Artemia for fish larvae. In addition, the extra risk of oxidative stress caused by the addition of a lipid emulsion seems to be partially compensated by the ISD format and the short period of enrichment. Finally, this study demonstrated that N. salinicola can be used as feed for rotifers and Artemia, even improving their lipid composition in combination with a lipid emulsion. These results emphasize the potential of microalgae biomass in combination with other agents for live prey enrichment protocols in aquaculture.
Availability of data and material
Data can be obtained from the corresponding author upon reasonable request
References
Affan A, Karawita R, Jeon YJ, Lee JB (2007) Growth characteristics and antioxidant properties of the benthic diatom Navicula incerta (Bacillariophyceae) from Jeju Island, Korea. J Phycol 43:823–832
Barata C, Lekumberri I, Vila-Escal M, Prat N, Porte C (2005a) Trace metal concentration, antioxidant enzyme activities and susceptibility to oxidative stress in the tricoptera larvae Hydropsyche exocellata from the Llobregat river basin (NE Spain). Aquat Toxicol 74:3–19
Barata C, Varo I, Navarro JC, Arun S, Porte C (2005) Antioxidant enzyme activities and lipid peroxidation in the freshwater cladoceran Daphnia magna exposed to redox cycling compounds. Comp Biochem Physiol C 140:175–186
Bell JG, Sargent J (2003) Arachidonic acid in aquaculture feeds: current status and future opportunities. Aquaculture 218:491–499
Bengston DA (2003) Status of marine aquaculture in relation to live prey: Past, present and future. In: Støttrup JG, McEvoy LA (eds) Live Feeds in Marine Aquaculture. Blackwell Science, Oxford, pp 1–13
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizaing the principle of protein-dye binding. Anal Biochem 72:248–254
Braun JCA, Colla LM (2023) Use of microalgae for the development of biofertilizers and biostimulants. Bioenergy Res 16:289–310
Bustamam MSA, Pantami HA, Azizan A, Shaari K, Min CC, Abas F, Nagao N, Maulidiani M, Banerjee S, Sulaiman F, Ismail IS (2021) Complementary analytical platforms of NMR spectroscopy and LCMS analysis in the metabolite profiling of Isochrysis galbana. Mar Drugs 19:139
Choi J, Han GS, Byun SG, Oh HY, Lee TH, Lee DY, Lee CH, Kim HS (2022) Effects of dietary docosahexaenoic acid enrichment in Artemia feed on the growth, survival, and fatty acid composition of Pacific cod (Gadus macrocephalus) larvae. Aquac Res 53:4353–4362
Christie WW, Han X (2010) Lipid analysis: Isolation, separation, identification and lipidomic analysis. Oily Press, Bridgwater
Chung PM, Cappel RE, Gilbert HF (1991) Inhibition of glutathione disulfide reductase by glutathione. Arch Biochem Biophys 288:48–53
Clairborne A (1985) Catalase activity. In: Greenwald RA (ed) Handbook of Methods for Oxygen Radical Research. CRC Press, Boca Ratón, pp 283–284
de la Cruz-Huervana JJ, Dionela C, Franco A (2022) Use of rotifers-fed microalgal paste in the seed production of Mangrove crab Scylla serrata in the Philippines. J Appl Phycol 34:3047–3057
El-Tohamy W, Qin J, Abdel-Aziz N, El-Ghobashy A, Dorgham M (2021) Suitable algal species and density for the culture of copepod Gladioferens imparipes as a potential live food for fish larvae. Aquac Int 29:105–125
Eryalçın KM (2018) Effects of different commercial feeds and enrichments on biochemical composition and fatty acid profile of rotifer (Brachionus plicatilis, Müller 1786) and Artemia franciscana. Turk J Fish Aquat Sci 18:81–90
Eryalçın KM (2019) Nutritional value and production performance of the rotifer Brachionus plicatilis Müller, 1786 cultured with different feeds at commercial scale. Aquac Int 27:875–890
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Galindo A, Reis DB, Rodríguez I, Pérez JA, Abdul-Jalbar B, Zárate R, Nunes N, Pinheiro De Carvalho MAA, Acosta NG, Rodríguez C (2022a) Lipid characterization of 14 macroalgal species from Madeira Archipelago: Implications for animal and human nutrition. Bot Mar 65:51–67
Galindo A, Rodríguez C, Reis DB, Marrero M, Acosta NG, Barreto MC, Jiménez IA, de Urioste J, Venuleo M, Pérez JA (2022b) Valorization of seaweed wracks: Inclusion as additive in diets for grass carp (Ctenopharyngodon idella). Aquac Nutr 2022:6992682
Gisbert E, Piedrahita RH, Conklin DE (2004) Ontogenetic development of the digestive system in California halibut (Paralichthys californicus) with notes on feeding practices. Aquaculture 232:455–470
Goud EA, Vaijnath A, Yadava KK (2023) Green water technology. Vigyan Varta 4:80–82
Habdous M, Vincent-Viry M, Visvikis S, Siest G (2002) Rapid spectrophotometric method for serum glutathione S-transferases activity. Clin Chim Acta 326:131–142
Hamre K, Yúfera M, Rønnestad I, Boglione C, Conceição LEC, Izquierdo M (2013) Fish larval nutrition and feed formulation: Knowledge gaps and bottlenecks for advances in larval rearing. Rev Aquac 5:S26–S58
Hong JW, Kang NS, Jang HS, Kim HJ, An YR, Yoon M, Kim HS (2019) Biotechnological potential of Korean marine microalgal strains and its future prospectives. Ocean Polar Res 41:289–309
Khatoon H, Yusoff FM, Banerjee S, Shariff M, Mohamed S (2007) Use of periphytic cyanobacterium and mixed diatoms coated substrate for improving water quality, survival and growth of Penaeus monodon Fabricius postlarvae. Aquaculture 271:196–205
Lund I, Kertaoui N. El, Izquierdo MS, Dominguez D, Hansen BW, Kestemont P (2018) The importance of phospholipids combined with long-chain PUFA in formulated diets for pikeperch (Sander lucioperca) larvae. Br J Nutr 120:628–644
Lund I, Reis DB, Tomkiewicz J, Benini E, Pérez JA, Kottmann JS, Politis SN, Rodríguez C (2021) Assessment of lipid uptake and fatty acid metabolism of European eel larvae (Anguilla anguilla) determined by 14C in vivo incubation. Aquaculture 531:735858
Magalhães R, Guerreiro I, Coutinho F, Moutinho S, Sousa S, Delerue-Matos C, Domingues VF, Olsen RE, Peres H, Oliva-Teles A (2020) Effect of dietary ARA/EPA/DHA ratios on growth performance and intermediary metabolism of gilthead sea bream (Sparus aurata) juveniles. Aquaculture 516:734644
Matsui H, Intoy MMB, Waqalevu V, Ishikawa M, Kotani T (2020) Suitability of Tisochrysis lutea at different growth phases as an enrichment diet for Brachionus plicatilis sp. complex rotifers. J Appl Phycol 32:3933–3947
McEvoy LA, Navarro JC, Bell JG, Sargent J (1995) Autoxidation of oil emulsions during the Artemia enrichment process. Aquaculture 134:101–112
Mesa-Herrera F, Quinto-Alemany D, Díaz M (2019) A sensitive, accurate, and versatile method for the quantification of superoxide dismutase activities in biological preparations. React Oxyg Sp 7:10–20
Morais S, Conceição LEC, Rønnestad I, Koven W, Cahu C (2007) Dietary neutral lipid level and source in marine fish larvae: Effects on digestive physiology and food intake. Aquaculture 268:106–122
Navarro JC, Henderson RJ, McEvoy LA, Bell MV, Amat F (1999) Lipid conversions during enrichment of Artemia. Aquaculture 174:155–166
Neori A (2011) “Green water” microalgae: the leading sector in world aquaculture. J Appl Phycol 23:143–149
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Oliver MP, Olivotto I, Turchi C (2017) Live prey production systems. In: Calado R, Olivotto I, Oliver MP, Holt GJ (eds) Marine Ornamental Species Aquaculture. John Wiley & Sons, New Jersey, pp 53–60
Olsen RE, Henderson RJ (1989) The rapid analysis of neutral and polar marine lipids using double-development HPTLC and scanning densitometry. J Exp Mar Biol Ecol 129:189–197
Palmtag MR, Faulk CK, Holt GJ (2006) Highly unsaturated fatty acid composition of rotifers (Brachionus plicatilis) and Artemia fed various enrichments. J World Aquac Soc 37:126–131
Pérez JA, Papadakis IE, Papandroulakis N, Cruces L, Cotou E, Gisbert E, Lorenzo A, Mylonas CC, Rodríguez C (2020) The ontogeny of greater amberjack digestive and antioxidant defence systems under different rearing conditions: A histological and enzymatic approach. Aquac Nutr 26:1908–1925
Pérez JA, Reis DB, Ramírez D, Acosta NG, Dorta-Guerra R, Jerez S, Rodríguez C (2022) In vivo biosynthesis of long-chain polyunsaturated fatty acids by the euryhaline rotifer (Brachionus plicatilis). Aquaculture 560:738415
Rachmayanti Y, Mwebaza H, Radjawane IM, Nurachman Z (2020) Tropical marine Navicula salinicola NBO: Morphology, genetic identification, and biochemical properties. IOP Conf Ser Earth Environ Sci 618:012033
Radhakrishnan DK, AkbarAli I, Schmidt BV, John EM, Sivanpillai S, Thazhakot Vasunambesan S (2020) Improvement of nutritional quality of live feed for aquaculture: An overview. Aquac Res 51:1–17
Raja R, Coelho A, Hemaiswarya S, Kumar P, Carvalho IS, Alagarsamy A (2018) Applications of microalgal paste and powder as food and feed: An update using text mining tool. Beni-Suef Univ J Basic Appl Sci 7:740–747
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Reis DB, Acosta NG, Almansa E, Navarro JC, Tocher DR, Andrade JP, Sykes AV, Rodríguez C (2017) Comparative study on fatty acid metabolism of early stages of two crustacean species: Artemia sp. metanauplii and Grapsus adscensionis zoeae, as live prey for marine animals. Comp Biochem Physiol B 204:53–60
Reis DB, Acosta NG, Almansa E, Garrido D, Andrade JP, Sykes AV, Rodríguez C (2019) Effect of Artemia inherent fatty acid metabolism on the bioavailability of essential fatty acids for Octopus vulgaris paralarvae development. Aquaculture 500:264–271
Reis DB, Pérez JA, Hamre K, Acosta NG, Norberg B, Harboe T, Rodríguez C (2021) The lipid metabolism of Atlantic halibut (Hippoglossus hippoglossus, L.) larvae determined by 14C in vivo incubations. Aquaculture 540:736733
Rocha GS, Katan T, Parrish CC, Gamperl AK (2017) Effects of wild zooplankton versus enriched rotifers and Artemia on the biochemical composition of Atlantic cod (Gadus morhua) larvae. Aquaculture 479:100–113
Rodríguez C, Pérez JA, Díaz M, Izquierdo MS, Fernández-Palacios H, Lorenzo A (1997) Influence of the EPA/DHA ratio in rotifers on gilthead seabream (Sparus aurata) larval development. Aquaculture 150:77–89
Rønnestad I, Helland S, Lie Ø (1998) Feeding Artemia to larvae of Atlantic halibut (Hippoglossus hippoglossus L.) results in lower larval vitamin A content compared with feeding copepods. Aquaculture 165:159–164
Salhi M, Izquierdo MS, Hernandez-Cruz CM, Socorro J, Fernandez-Palacios H (1997) The improved incorporation of polyunsaturated fatty acids and changes in liver structure in larval gilthead seabream fed on microdiets. J Fish Biol 51:869–879
Samat NA, Yusoff FM, Rasdi NW, Karim M (2020) Enhancement of live food nutritional status with essential nutrients for improving aquatic animal health: a review. Animals 10:2457
Saranya C, Hemalatha A, Parthiban C, Anantharaman P (2014) Evaluation of antioxidant properties, total phenolic and carotenoid content of Chaetoceros calcitrans Chlorella salina and Isochrysis galbana. Int J Curr Microbiol Appl Sci 3:365–377
Sargent J, McEvoy LA, Bell JG (1997) Requirements, presentation and sources of polyunsaturated fatty acids in marine fish larval feeds. Aquaculture 155:117–127
Sargent J, McEvoy L, Estevez A, Bell G, Bell M, Henderson J, Tocher DR (1999) Lipid nutrition of marine fish during early development: current status and future directions. Aquaculture 179:217–229
Seychelles LH, Audet C, Tremblay R, Fournier R, Pernet F (2009) Essential fatty acid enrichment of cultured rotifers (Brachionus plicatilis, Müller) using frozen-concentrated microalgae. Aquac Nutr 15:431–439
Shantha NC, Decker EA (1994) Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J AOAC Int 77:421–424
Sibonga RC, Laureta LV, Lebata-Ramos MJH, Nievales MFJ, Pedroso FL (2021) Single and mixed species of microalgae as larval food for the tropical sea cucumber sibonga scabra. J Appl Phycol 33:3103–3112
Siddik MAB, Sørensen M, Islam SMM, Saha N, Rahman MA, Francis DS (2023) Expanded utilisation of microalgae in global aquafeeds. Rev Aquac 16:6–33
Sorgeloos P, Dhert P, Candreva P (2001) Use of the brine shrimp, Artemia spp., in marine fish larviculture. Aquaculture 200:147–159
Sun Y, Wang H, Guo G, Pu Y, Yan B (2014) The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohydr Polym 113:22–31
Sylvestre F, Guiral D, Debenay JP (2004) Modern diatom distribution in mangrove swamps from the Kaw Estuary (French Guiana). Mar Geol 208:281–293
Thépot V, Mangott A, Pirozzi I (2016) Rotifers enriched with a mixed algal diet promote survival, growth and development of barramundi larvae, Lates calcarifer (Bloch). Aquac Rep 3:147–158
Tocher DR (2010) Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac Res 41:717–732
Turcihan G, Turgay E, Yardımcı RE, Eryalçın KM (2021) The effect of feeding with different microalgae on survival, growth, and fatty acid composition of Artemia franciscana metanauplii and on predominant bacterial species of the rearing water. Aquac Int 29:2223–2241
Viciano E, Monroig Ó, Salvador A, Amat J, Fiszman S, Navarro JC (2015) Enriching Artemia nauplii with a high DHA-containing lipid emulsion: Search for an optimal protocol. Aquac Res 46:1066–1077
Viciano E, Monroig Ó, Barata C, Peña C, Navarro JC (2017) Antioxidant activity and lipid peroxidation in Artemia nauplii enriched with DHA-rich oil emulsion and the effect of adding an external antioxidant based on hydroxytyrosol. Aquac Res 48:1006–1019
Xing R, Wang C, Cao X, Chang Y (2007) The potential value of different species of benthic diatoms as food for newly metamorphosed sea urchin Strongylocentrotus intermedius. Aquaculture 263:142–149
Zárate R, Portillo E, Teixidó S, Pinheiro De Carvalho MAA, Nunes N, Ferraz S, Seca AML, Rosa GP, Barreto MC (2020) Pharmacological and cosmeceutical potential of seaweed beach-casts of Macaronesia. Appl Sci 10:5831
Zupo V, Messina P (2007) How do dietary diatoms cause the sex reversal of the shrimp Hippolyte inermis Leach (Crustacea, Decapoda). Mar Biol 151:907–917
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was funded by the European Interreg Programme 2014-2020 (MACBIOBLUE; MAC/1.1b/086). A. Galindo was supported by Cajasiete, Ministerio de Ciencia, Innovación y Universidades, and Universidad de La Laguna (PhD contract). Thanks are due to FCT - Fundação para a Ciência e a Tecnologia for supporting G.P. Rosa’s grant (SFRH/BD/144446/2019), through national and European funds and co-financed by the European Social Fund through the Regional Operational Programme Centro 2020, as well as to FCT, the European Union, QREN, FEDER, and COMPETE, through funding the cE3c center (UIDB/00329/2020). This work received financial support from PT national funds (FCT/MCTES, Fundação para a Ciência e a Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through projects UIDB/50006/2020 and UIDP/50006/2020. Dr. Covadonga Rodríguez is member of the Instituto de Tecnologías Biomédicas de Canarias (ITB).
Author information
Authors and Affiliations
Contributions
A. Galindo: Writing-original draft preparation, Investigation, Formal analysis; J.A. Pérez: Supervision, Writing-Reviewing and Editing; E. Almansa: Methodology, Resources, Writing-Reviewing and Editing; G.P. Rosa: Formal analysis, Methodology, Supervision; I.A. Jiménez: Formal analysis, Methodology, Supervision; M. Venuleo: Resources, Funding acquisition, Reviewing and Editing; N.G. Acosta: Formal analysis, Methodology, Supervision; C. Rodríguez: Conceptualization, Project administration, Funding acquisition, Reviewing and Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galindo, A., Pérez, J.A., Almansa, E. et al. Antioxidant capacity and lipid composition of Brachionus plicatilis and Artemia enriched with a mixture of different post-processing formats of Navicula salinicola and Isochrysis galbana and lipid emulsions. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03223-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03223-z