Abstract
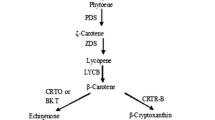
The unicellular green alga Haematococcus pluvialis accumulates large quantities of astaxanthin, a highly valuable carotenoid pigment in the aquaculture, food, and pharmaceutical industries. Biological diversity within a species with a biotechnological interest allows applying strain selection procedures avoiding genetic manipulation. The aim of this study was to determine growth and carotenogenic capacity among 13 strains isolated from a latitudinal range throughout south-central and southern Chile, in order to know their biological diversity and biotechnological potential. Strains were isolated from rain pools located in public squares and cemeteries of the cities: Concepción, Valdivia, Osorno, Puerto Varas, and Castro. All the Chilean strains were more carotenogenic than the reference strain Steptoe. Physiological attributes differed greatly among the strains. Principal component analysis (PCA) of growth and carotenogenesis parameters grouped the strains into four groups, two of them composed by only one strain (CCM-UdeC-038 and CCM-UdeC-039). These latter strains were also the most carotenogenic ones, being strain CCM-UdeC-039 the one that accumulated the highest amount of astaxanthin (7 mg astaxanthin L−1 culture) associated to the highest total carotenoid content (1.1 % by dry biomass) and carotenoids/chlorophyll ratio (2.6). These results demonstrate that a great physiological diversity exists at intraspecific level in H. pluvialis (even among strains coming from the same geographical origin), which allows performing genetic improvement by means of simple selection of new strains from nature. Further research will be focused on finding out the culture conditions that optimize the astaxanthin production of the most promising strains.


Similar content being viewed by others
References
Behra R, Genoni GP, Joseph AL (1999) Effect of atrazine on growth, photosynthesis, and between-strain variability in Scenedesmus subspicatus (Chlorophyceae). Arch Environ Contam Toxicol 37:36–41
Borowitzka MA (1992) Comparing carotenogenesis in Dunaliella and Haematococcus: implications for commercial production strategies. In: Villa TG, Abalde J (eds) Profiles on biotechnology. Universidad de Santiago de Compostela, Santiago de Compostela, pp 301–310
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Borowitzka MA, Huisman JM, Osborn A (1991) Culture of the astaxanthin-producing green alga Haematococcus pluvialis 1. Effects of nutrients on growth and cell type. J Appl Phycol 3:295–304
Boussiba S (2000) Carotenogenesis in the green alga Haematococcus pluvialis: cellular physiology and stress response. Physiol Plantarum 108:111–117
Bubrick P (1991) Production of astaxanthin from Haematococcus. Bioresource Technol 38:237–239
Carvalho AP, Monteiro CM, Malcata FX (2009) Simultaneous effect of irradiance and temperature on biochemical composition of the microalga Pavovla lutheri. J Appl Phycol 21:543–552
Chen Y, Li D, Lu W, Xing J, Hui B, Han Y (2003) Screening and characterization of astaxanthin-hyperproducing mutants of Haematococcus pluvialis. Biotechnol Lett 25:527–529
Christiansen R, Lie O, Torrisen OJ (1995) Growth and survival of Atlantic salmon, Salmo salar L., fed different dietary levels of astaxanthin. First-feeding fry. Aquacult Nutr 1:189–198
Christiansen R, Torrissen OJ (1997) Effects of dietary astaxanthin supplementation on fertilization and egg survival in Atlantic salmon (Salmo salar L.). Aquaculture 153:51–62
Cifuentes AS, González M, Conejeros M, Dellarossa V, Parra O (1992) Growth and carotenogenesis in eight strains of Dunaliella salina Teodoresco from Chile. J Appl Phycol 4:111–118
Del Campo JA, Rodríguez H, Moreno J, Vargas MA, Rivas J, Guerrero MG (2004) Accumulation of astaxanthin and lutein in Chlorella zofingensis (Chlorophyta). Appl Microbiol Biot 64:848–854
Del Río E, Acién FG, García-Malea MC, Rivas J, Molina-Grima E, Guerrero MG (2005) Efficient one-step production of astaxanthin by the microalga Haematococcus pluvialis in continuous culture. Biotechnol Bioeng 91:808–15
Di Rienzo JA, Casanoves F, Balzarini MG, González L, Tablada M, Robledo CW (2011) InfoStat versión. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina, http://www.infostat.com.ar
Fujii K, Imazato E, Nakashima H, Ooi O, Saeki A (2006) Isolation of the non- fastidious microalga with astaxanthin-accumulating property and its potential for application to aquaculture. Aquaculture 262:285–293
Gallagher JC (1980) Population genetics of Skeletonema costatum (Bacillariophyceae) in Narragansett Bay. J Phycol 16:464–474
Gallagher JC (1982) Physiological variation and electrophoretic banding patterns of genetically different seasonal populations of Skeletonema costatum (Bacillariophyceae). J Phycol 18:148–162
Giannelli L, Yamada H, Katsuda T, Yamaji H (2015) Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. J Biosci Bioeng 119(3):345–350
Gómez P, González M (2001) Genetic polymorphism in eight Chilean strains of the carotenogenic microalga Dunaliella salina Teodoresco (Chlorophyta). Biol Res 34:23–30
Gómez P, González M (2004) Genetic variation among seven strains of Dunaliella salina (Chlorophyta) with industrial potential based on RAPD banding patterns and on nuclear ITS rDNA sequences. Aquaculture 233:149–162
Gómez P, González M (2005) The effect of temperature and irradiance on the growth and carotenogenic capacity of seven strains of Dunaliella salina (Chlorophyta) cultivated under laboratory conditions. Biol Res 38:151–162
Gómez PI, Inostroza I, Pizarro M, Pérez J (2013) From genetic improvement to commercial-scale mass culture of a Chilean strain of the green microalga Haematococcus pluvialis with enhanced productivity of the red ketocarotenoid astaxanthin. AoB Plants 5:plt026
González MA, Cifuentes AS, Gómez PI (2009) Growth and total carotenoid content in four Chilean strains of Haematococcus pluvialis Flotow, under laboratory conditions. Gayana Bot 66:58–70
Guillard RRL (1973) Division rates. In: Stein JR (ed) Handbook of phycological methods. Culture Methods and Growth Measurements. Cambridge University Press, New York, pp 290–311
Gutiérrez CL, Escobar C, Marshall SH (2012) Chloroplast genetic tool for the green microalgae Haematococcus pluvialis (Chlorophyceae, Volvocales). J Phycol 48:976–983
Hallmann A (2007) Algal transgenics and biotechnology. Transgenic Plant J 1:81–98
Hoshaw RW, Rosowski JR (1973) Methods for microscopic algae. In: Stein JR (ed) Handbook of phycological methods. Culture Methods and Growth Measurements. Cambridge University Press, New York, pp 53–68
Izquierdo A (1906) Ensayo sobre los Protozoos de las aguas dulce de Chile. Imprenta Cervantes, Santiago de Chile, p 228, 10 plates
Kang CD, Lee JS, Park TH, Sim SJ (2005) Comparison of heterotrophic and photoautotrophic induction on astaxanthin production by Haematococcus pluvialis. Appl Microbiol Biot 68:237–241
Kim ZH, Kim SH, Lee HS, Lee CG (2006) Enhanced production of astaxanthin by flashing light using Haematococcus pluvialis. Enzyme Microb Tech 39:414–419
Kobayashi M, Kakizono T, Nishio N, Nagai S, Kurimura Y, Tsuji Y (1997) Antioxidant role of astaxanthin in the green alga Haematococcus pluvialis. Appl Microbiol Biot 48:351–356
León-Bañares R, González D, Galván A, Fernández E (2004) Transgenic microalgae as green cell-factories. Trends Biotechnol 22:45–52
Li J, Zhu D, Niu J, Shen S, Wang G (2011) An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotech Adv 29:568–574
Liang Y, Mai K, Sun S (2005) Differences in growth, total lipid content and fatty acid composition among 60 clones of Cylindrotheca fusiformis. J Appl Phycol 17:61–65
López Alonso D, Molina Grima E, Sánchez Pérez JA, García Sánchez JL, García Camacho F (1992a) Fatty acid variation among different isolates of a single strain of Isochrysis galbana. Phytochemistry 31:3901–3904
López Alonso D, Molina Grima E, Sánchez Pérez JA, García Sánchez JL, García Camacho F (1992b) Isolation of clones of Isochrysis galbana rich in eicosapentaenoic acid. Aquaculture 102:363–371
López Alonso D, Segura del Castillo CI, García Sánchez JL, Sánchez Pérez JA, Camacho FG (1994) Quantitative genetics of fatty acid variation in Isochrysis galbana (Prymnesiophyceae) and Phaeodactylum tricornutum (Bacillariophyceae). J Phycol 30:553–558
Lorenz RT, Cysewski GR (2000) Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol 18:160–167
Mendes-Pinto M, Raposo M, Bowen J, Young A, Morais R (2001) Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis: effects on astaxanthin recovery and implications for bio-availability. J Appl Phycol 13:19–24
Mostafa N, Omar H, Tan SG, Napis S (2011) Studies on the genetic variation of the green unicellular alga Haematococcus pluvialis (Chlorophyceae) obtained from different geographical locations using ISSR and RAPD molecular marker. Molecules 16:2598–2608
Park E, Lee CG (2001) Astaxanthin production by Haematococcus pluvialis under various light intensities and wavelengths. J Microbiol Biotech 11:1024–1030
Proctor VW (1957) Some controlling factors in the distribution of Haematococcus pluvialis. Ecology 38:457–462
Sarada R, Tripathi U, Ravishankar GA (2002a) Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem 37:623–627
Sarada R, Bhattacharya S, Ravishankar GA (2002b) Optimization of culture conditions for growth of the green alga Haematococcus pluvialis. World J Microb Biot 18:517–521
Sharon-Gojman R, Maimon E, Leu S, Zarka A, Boussiba S (2015) Advanced methods for genetic engineering of Haematococcus pluvialis (Chlorophyceae, Volvocales). Algal Res 10:8–15
Shaw PM, Jones GJ, Smith JD, Johns RB (1989) Intraspecific variations in the fatty acids of the diatom Skeletonema costatum. Phytochemistry 28:811–815
Starr RC, Zeikus JA (1993) UTEX-The culture collection of algae at the University of Texas at Austin. J Phycol 29(Suppl):1–106
Sun H, Komg Q, Geng Z, Duan L, Yang M, Guan B (2015) Enhancement of cell biomass and cell activity of astaxanthin-rich Haematococcus pluvialis. Bioresource Technol 186:67–73
Torzillo G, Goksan T, Faraloni C, Kopecky J, Masojídek J (2003) Interplay between photochemical activities and pigment composition in an outdoor culture of Haematococcus pluvialis during the shift from the green to red stage. J Appl Phycol 15:127–136
Tripathi U, Venkateshwaran G, Sarada R, Ravishankar GA (2001) Studies on Haemococcus pluvialis for improve production of astaxanthin by mutagenesis. World J Microb Biot 17:143–148
Wegmann K, Metzner H (1971) Synchronization of Dunaliella salina cultures. Arch Mikrobiol 78:360–367
Yong YYR, Lee KK (1991) Do carotenoids play a photoprotective role in the cytoplasm of Haematococcus lacustris (Chlorophyta)? Phycologia 30:257–261
Zhang BY, Geng YH, Li ZK, Hu HJ, Li YG (2009) Production of astaxanthin from Haematococcus in open pond by two-stage growth one step process. Aquaculture 295:275–281
Acknowledgments
This investigation was supported by grants from the Dirección de Investigación, Universidad de Concepción, Chile (Project DIUC No. 204.111.040-1.0), and Fundación Andes, Chile (Project No. C-14055-7).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez, P.I., Haro, P., Lagos, P. et al. Intraspecific variability among Chilean strains of the astaxanthin-producing microalga Haematococcus pluvialis (Chlorophyta): an opportunity for its genetic improvement by simple selection. J Appl Phycol 28, 2115–2122 (2016). https://doi.org/10.1007/s10811-015-0777-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0777-0