Abstract
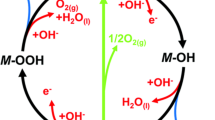
Three new organic–inorganic hybrid phosphites salts, namely, (enH2)[M(H2O)6](HPO3)2, with [M = Co (1), Ni (2) and Mg (3), and “en” refers to ethylenediamine C2N2H8] have been synthesized at room temperature by the slow evaporation method. The solid-state structures were solved from single crystal X-ray diffraction data. These compounds are isostructural, all crystallizing in the orthorhombic system, space group, Pbca (no 61). The FTIR spectroscopy shows the expected bands of ethylenediamine (en), water molecules, and hydrogen-phosphite oxoanion groups. The thermal stability until 100 °C of the three compounds was confirmed using combined analyses (TGA/DTA, powder X-ray diffraction and Raman spectroscopy). Two catalytic activity performances were investigated: the catalytic efficiency on the water decontamination of the three compounds by the reduction of three nitrophenol isomers and the Hydrogen Evolution Reaction (HER) in an alkaline environment. The three hybrid compounds turned out to be very efficient new catalysts for reducing the three nitrophenol isomers. The fastest electron transport and the most favorable HER reaction kinetics are displayed by (enH2)[Ni(H2O)6](HPO3)2, while the highest current density with the lowest overpotential was obtained for (enH2)[Co(H2O)6](HPO3)2.
Graphical abstract













Similar content being viewed by others
References
Davis ME (2002) Ordered porous materials for emerging applications. Nature 417:813–821. https://doi.org/10.1038/nature00785
Parnham ER, Morris RE (2007) Ionothermal synthesis of zeolites, metal–organic frameworks, and inorganic-organic hybrids. Acc Chem Res 40:1005–1013. https://doi.org/10.1021/ar700025k
Jiang J, Yu J, Corma A (2010) Extra-large-pore zeolites: bridging the gap between micro and mesoporous structures. Angew Chem Int Ed 49:3120–3145. https://doi.org/10.1002/anie.200904016
Natarajan S, Mandal S (2008) Open-framework structures of transition-metal compounds. Angew Chem Int Ed 47:4798–4828. https://doi.org/10.1002/anie.200701404
Guillou N, Gao Q, Foster PM, Chang J-S, Nogues M, Park S-E, Ferey G, Cheetham AK (2001) Nickel (II) phosphate VSB-5: a magnetic nanoporous hydrogenation catalyst with 24-ring tunnels. Angew Chem Int Ed 40:2831–2834. https://doi.org/10.1002/1521-3773(20010803)40:15%3c2831::AID-ANIE2831%3e3.0.CO;2-Z
Wang P, Wang G-M, Li J-H, Wang Z-H, Wang Y-X, Lin J-H (2012) Syntheses and structures of two open-framework zinc phosphites with extra-large 24-ring channels. Solid State Sci 14:1030–1035. https://doi.org/10.1016/j.solidstatesciences.2012.05.007
Lin H-Y, Chin C-Y, Huang H-L, Huang W-Y, Sie M-J, Huang L-H, Lee Y-H, Lin C-H, Lii K-H, Bu X, Wang S-L (2013) Crystalline inorganic frameworks with 56-ring, 64-ring, and 72-ring channels. Science 339:811–813. https://doi.org/10.1126/science.1232097
Teofilo R, Jose Luis M, Jorge L, Begona B, Pizarro JL, Isabel Arriortuab JL (2009) Organically templated open-framework phosphites. J Mater Chem 19:3793–3818. https://doi.org/10.1039/B808795B
Yang Y, Zhao Y, Yu J, Wu S, Wang R (2008) Doping-induced structure variation of 1,3-cyclohexane-bis(methylamine)-templated zinc-phosphorus open structures. Inorg Chem 47:769–771. https://doi.org/10.1021/ic701825m
Wang G-M, Li J-H, Zhang X, Jiang W-W, Bao Z-Z, Zhao X-M, Wang Y-X, Lin J-H (2014) (C5H6N)4[Be6(HPO3)8]·H2O: a low-density open-framework beryllium phosphite with multidirectional 12-ring channels. Solid State Sci 33:53–57. https://doi.org/10.1016/j.solidstatesciences.2014.04.013
Bonavia G, De Bord J, Haushalter RC, Rose D, Zubieta J (1995) Hydrothermal synthesis and characterization of two- and three-dimensional solids of the oxovanadium(IV)-phosphite system. the structures of [HN(Me)(CH2CH2)2N(Me)H][(VO)4(OH)2(HPO3)4], [H2N(CH2CH2)2NH2][(VO)3(HPO3)4(H2O)2], and [VO(HPO3)(H2O)]. Chem Mater 7:1995–1998. https://doi.org/10.1021/cm00059a002
Zhao L, Li J, Chen P, Li G, Yu J, Xu R (2008) [Co8 (HPO3)9(CH3OH)3]·2H2O: an open-framework cobalt phosphite containing extra-large 18-ring channels. Chem Mater 20:17–19. https://doi.org/10.1021/cm7021906
Liang J, Li J, Yu J, Chen P, Li L, Xu R (2006) [Ni(C6N2H14)2][Zn4 (H2O)(HPO3)5]: a new open-framework zinc phosphite with intersecting 8-, 12- and 16-ring channels. J Solid-State Chem 179:1977–1983. https://doi.org/10.1016/j.jssc.2006.03.034
Chen W, Li N, Xiang S (2004) Zn3(HPO3)4·Zn(H2O)6: a purely inorganic open-framework zincophosphite with octahedral zinc complex [Zn(H2O)6]2+ encapsulating in the channels. J Solid-State Chem 177:3229–3234. https://doi.org/10.1016/j.jssc.2004.04.060
Li J, Li L, Liang J, Chen P, Yu J, Xu Y, Xu R (2008) Template-designed syntheses of open-framework zinc phosphites with extra-large 24-ring channels. Cryst Growth Des 8:2318–2323. https://doi.org/10.1021/cg701080u
Luo X, Luo D, Zeng H, Gong M, Chen Y, Lin Z (2011) A 3,4-connected beryllium phosphite framework containing 24-ring channels with a very low density. Inorg Chem 50:8697–8699. https://doi.org/10.1021/ic2014539
Lai Y-L, Lii K-H, Wang S-L (2007) 26-ring-channel structure constructed from bimetal phosphite helical chains. J Am Chem Soc 129:5350–5351. https://doi.org/10.1021/ja070733k
Fernandez S, Mesa JL, Pizarro JL, Chung U-C, Arriortua MI, Rojo T (2005) Two new two-dimensional organically templated phosphite compounds:(C6H16N2)0.5[M (HPO3)F], M= Fe(II) and Co(II): solvothermal synthesis, crystal structures, thermal, spectroscopic, and magnetic properties. J Solid-State Chem 178:3554–3562. https://doi.org/10.1016/j.jssc.2005.09.032
Wang KC, Bian YX, Li J, Xu DG, Lin ZE (2016) Amine-ligated approach for the synthesis of extra-large-pore zinc phosphites with qtz-h and bnn topologies. Inorg Chem 55:3727–3729. https://doi.org/10.1021/acs.inorgchem.6b00589
Fernandez S, Mesa JL, Pizarro JL, Lezama L, Arriortua MI, Olazcuaga R, Rojo T (2000) A new layered inorganic-organic hybrid manganese (II) phosphite:(C2H10N2)[Mn3 (HPO3)4]. Hydrothermal synthesis, crystal structure, and spectroscopic and magnetic properties. Chem Mater 12:2092–2098. https://doi.org/10.1021/cm001026s
Fernandez S, Mesa JL, Pizarro JL, Lezama L, Arriortua MI, Rojo T (2001) Hydrothermal synthesis of a new layered inorganic-organic hybrid cobalt (II) phosphite:(C2H10N2)[Co3(HPO3)4]: crystal structure and spectroscopic and magnetic properties. Int J Inorg Mater 3:331–336. https://doi.org/10.1016/S1466-6049(01)00031-9
Ouarsal R, Tahiri AA, El Bali B, Lachkar M, Bolte M (2002) Strontium dihydrogenphosphite. Acta Crystallogr Sect E E58:i19–i20. https://doi.org/10.1107/S1600536802000806
Ouarsal R, Tahiri AA, Lachkar M, Slimani Z, El Bali B, Bolte B (2002) Barium dihydrogen phosphate hemihydrates. Acta Crystallogr Sect E E58:i72–i73. https://doi.org/10.1107/S1600536802013569
Chaouche S, Ouarsal R, El Bali B, Lachkar M, Bolte M, Dusek M (2010) Li2HPO3 H2O: crystal structure and IR spectrum. J Chem Crystallogr 40:526–530. https://doi.org/10.1007/s10870-010-9690-1
El Bali B, Massa W (2002) Redetermination of copper(II) hydrogenphosphite dehydrate. Acta Crystallogr E E58:i29–i31. https://doi.org/10.1107/S1600536802003574
Chaouch S, Ouarsal R, Akouibaa M, Rakib S, Lachkar M, El Bali B, Dusek M (2018) Cs2[M(H2O)6]3(HPO3)4, M= Co, Ni: Crystal structures, IR and thermal studies. J Phys Conf Ser 984:12–15. https://doi.org/10.1088/1742-6596/984/1/012015
Ouarsal R, Tahiri AA, El Bali B, Lachkar M, Harrison WTA (2002) Sodium zinc tris(dihydrogenphosphite) hydrate NaZn(H2PO3)3⋅H2O. Acta Crystallogr E58:i23–i25. https://doi.org/10.1107/S1600536802002209
Ouarsal R, Tahiri AA, Lachkar M, Dusek M, Fejfarová K, El Bali B (2003) Sodium magnesium tris(dihydrogenphosphite)monohydratehydrate, NaMg(H2PO3)3⋅H2O. Acta Crystallogr E59:i33–i35. https://doi.org/10.1107/S1600536803002757
Ouarsal R, Essehli R, Lachkar M, Zenkouar M, Dusek M, Fejfarová K, El Bali B (2004) Dipotassium cobalt(II) bis(hydrogenphosphite) dihydrate, K2Co(HPO3)2⋅2H2O. Acta Crystallogr E60:i66–i68. https://doi.org/10.1107/S1600536804007895
Menssouri I, El Bali B, Capitelli F, Piniella JF, Lachkar M, Slimani Z (2005) Diammoniumtris[hexaaquamagnesium(II)]tetrakis[hydrogenphosphate(III)], (NH4)2[Mg(H2O)6]3(HPO3)4. Acta Crystallogr E61:i129–i131. https://doi.org/10.1107/S160053680501723X
Ouarsal R, El Bali B, Lachkar M, Dusek M, Fejfarova K (2005) Diammoniumtris [hexaaquanickel(II)] tetrakis[hydrogenphosphate(III)], (NH4)2[Ni(H2O)6]3(HPO3)4. Acta Crystallogr E61:i171–i173. https://doi.org/10.1107/S1600536805021720
Ouarsal R, El Bali B, Lachkar M, Dusek M, Fejfarova k, (2005) Diammonium tris[hexaaquacobalt(II)] tetrakis[hydrogenphosphate(III)], (NH4)2[Co(H2O)6]3(HPO3)4. Acta Crystallogr E61:i168–i170. https://doi.org/10.1107/S1600536805021719
Ouarsal R, Lachkar M, Dusek M, Albert EB, Castelló GBC, El Bali B (2016) Crystal structure of NaCd(H2PO3)3⋅H2O and spectroscopic study of NaM(H2PO3)3⋅H2O, M = Mn Co, Ni, Zn, Mg and Cd. Polyhedron 106:132–137. https://doi.org/10.1016/j.poly.2016.01.006
Chaouche S, Ouarsal R, Lachkar M, Capitelli F, El Bali B (2010) Crystal structure and IR study of (C6H5NH3)[ZnCl(HPO3)]. J Chem Crystallogr 40:486–490. https://doi.org/10.1007/s10870-009-9682-1
Hamdi N, Chaouch S, da Silva I, Ezahri M, Lachkar M, Alhasan R, Yaman Abdin A, Jacob C, El Bali B (2019) Synthesis, structural characterization, and biological activities of organically templated cobalt phosphite (C4N2H14)[Co(H2PO3)4]·2H2O. Sci 1:41. https://doi.org/10.3390/sci1020041
Hamdi N, Hidaoui S, Hassani HO, Lachkar M, Dusek M, Morley N, El Bali B (2020) Crystal structure, physical and catalytic oxidation studies of a new hybrid phosphate [(N2H5)2Co(HPO4)2]. J Mol Struct. https://doi.org/10.1016/j.molstruc.2020.128317
Escobal J, Pizarro JL, Mesa JL, Lezama L, Olazcuaga R, Arriortua MI, Rojo T (2000) A new manganese (II) phosphate templated by ethylenediamine: (C2H10N2)[Mn2(HPO4)3(H2O)]. Hydrothermal synthesis, crystal structure, and spectroscopic and magnetic properties. Chem Mater 12:376–382. https://doi.org/10.1021/cm9910815
Fernández S, Mesa JL, Pizarro JL, Lezama L, Arriortua MI, Olazcuaga R, Rojo T (2000) A new layered inorganic-organic hybrid manganese (II) phosphite: (C2H10N2)[Mn3(HPO3)4]. Hydrothermal synthesis, crystal structure, and spectroscopic and magnetic properties. Chem Mater 12:2092–2098. https://doi.org/10.1021/cm001026s
Ramaswamy P, Mandal S, Natarajan S (2009) Synthesis, structure and magnetic behavior of a new three-dimensional manganese phosphite-oxalate: [C2N2H10][Mn2II(H2O)2(HPO3)2(C2O4)]. J Solid-State Chem 182:2491–2496. https://doi.org/10.1016/j.jssc.2009.06.040
Jeffrey RD, De Bord J, Reiff WM, Haushalter RC, Zubieta J (1996) The first organically templated layered iron phosphate: hydrothermal synthesis, structure, magnetic properties, and the Mössbauer spectrum of [H3NCH2CH2NH3]0.5[Fe(OH)(PO4)]. J Solid-State Chem 125:186–191. https://doi.org/10.1006/jssc.1996.0284
Fernández S, Mesa JL, Pizarro JL, Lezama L, Arriortua MI, Rojo T (2002) Two new three-dimensional vanadium (III) and iron (III) phosphites templated by ethylenediamine: (C2H10N2)0.5[M(HPO3)2]. ab initio structure determination, spectroscopic, and magnetic properties. Chem Mater 14:2300–2307. https://doi.org/10.1021/cm0112845
Fernández S, Mesa JL, Pizarro JL, Lezama L, Arriortua MI, Rojo T (2001) Hydrothermal synthesis of a new layered inorganic-organic hybrid cobalt (II) phosphite: (C2H10N2)[Co3(HPO3)4]: crystal structure and spectroscopic and magnetic properties. Int J Inorg Mater 3:331–336. https://doi.org/10.1016/S1466-6049(01)00031-9
Chung U-C, Mesa J, Pizarro J, Lezama L, Garitaonandia J, Chapman JP, Arriortua MI (2004) A new layered organically templated iron(II) phosphite, (C2H10N2)Fe3(HPO3)4: hydrothermal synthesis, crystal structure and spectroscopic and magnetic properties. J Solid-State Chem 177:2705–2713. https://doi.org/10.1016/j.jssc.2004.04.020
Yeşilel OZ, Ölmez H, Arici C (2007) The first bis (orotato-N, O) cadmium complex with monodentate protonated ethylenediamine ligands: synthesis, spectrothermal properties of a cadmium (II)-orotato complex with ethylenediamine–crystal structure of trans-[Cd(HOr)2(enH)2]·2H2O and cis-[Cd(H2O)2(phen)2](H2Or)2·2H2O. Polyhedron 26:3669–3674. https://doi.org/10.1016/j.poly.2007.03.060
Yang G-Y, Sevov SC (2001) [Co(en)3][B2P3O11(OH)2]: a novel borophosphate templated by a transition-metal complex. Inorg Chem 40:2214–2215. https://doi.org/10.1021/ic001397a
Pham DNK, Roy M, Golen JA, Manke DR (2017) The first-row transition-metal series of tris (ethylenediamine) diacetate complexes [M(en)3](OAc)2 (M is Mn, Fe Co, Ni, Cu, and Zn). Acta Crystallogr C 73:442–446. https://doi.org/10.1107/S2053229617006738
Dardar F, Day CS, El Jazouli A, Sebti S, Lachgar A (2019) Synthesis and characterization of a new layered gallium phosphonate oxalate [C2H10N2]0.5[Ga3 (PO3CH3)4(C2O4)]⋅H2O. J Chem Crystallogr 49:44–51. https://doi.org/10.1007/s10870-018-00762-5
Espinosa Bosch M, Ruiz Sánchez AJ, Sánchez Rojas F, Bosch Ojeda C (2006) Determination of paracetamol: historical evolution. J Pharm Biomed 42:291–321. https://doi.org/10.1016/j.jpba.2006.04.007
Yang P, Xua A, Xiab J, Hea J, Xinga H, Zhanga X, Weia S, Wanga N (2014) Facile synthesis of highly catalytic activity Ni-Co-Pd-P composite for reduction of the p-Nitrophenol. Appl Catal Gen 470:89–96. https://doi.org/10.1016/j.apcata.2013.10.043
Akouibaa M, Hassani HO, Ouarsal R, Rakib S, Lachkar M, Poupon M, Dusek M, Morley N, El Bali B (2022) (H3dien)[Ni(NO3)(C2O4)2]⋅2H2O: synthesis, crystal structure, catalytic activity and magnetic study. Chem Data Collect 42:100969. https://doi.org/10.1016/j.cdc.2022.100969
Hidaoui S, Hamdi N, Akouibaa M, Benali-Cherif R, Vaclav E, Dusek M, Lachkar M, El Bali B (2022) Synthesis, crystal structure and catalytic activity of the new hybrid phosphate (C4H12N2)[Co(H2O)6](HPO4)2. J Mol Struct 1265:133296. https://doi.org/10.1016/j.molstruc.2022.133296
Akouibaa M, Kadiri M, Driouch M, Tanji K, Ouarsal R, Rakib S, Sfaira M, Morley N, Lachkar M, El Bali B, Zarrouk A, Bendeif E (2023) Synthesis, catalytic activity, magnetic study and anticorrosive activity of mild steel in HCl 1M medium of (H3dien)[Cu(NO3)(C2O4)2]⋅2H2O. A redetermination at 100 K. Mater Chem Phys 307:128130. https://doi.org/10.1016/j.matchemphys.2023.128130
Ramírez-Rave S, Hernández-Gordillo A, Calderón HA, Galano A, García-Mendoza C, Gómez R (2015) Synthesis of new ZnS–Bipy based hybrid organic–inorganic materials for photocatalytic reduction of 4-nitrophenol. New J Chem 39:2188–2194. https://doi.org/10.1039/C4NJ01891E
Li N, Sun Q, Zhang P, Jing P (2021) Hydrothermal synthesis of 1T-MoS2/pelagic clay composite and its application in the catalytic reduction of 4-nitrophenol. Materials 14:7020. https://doi.org/10.3390/ma14227020
Hassani HO, Akouibaa M, Rakass S, Abboudi M, El Bali B, Lachkar M, Al Wadaani F (2021) A simple and cost-effective new synthesis method of copper molybdate CuMoO4 nanoparticles and their catalytic performance. J Sci Adv Mater Devices 6:501–507. https://doi.org/10.1016/j.jsamd.2021.06.003
Mohamed MJS, Shenoy S, Bhat DK (2018) Novel NRGO-CoWO4-Fe2O3 nanocomposite as an efficient catalyst for dye degradation and reduction of 4-nitrophenol. Mater Chem Phys 208:112–122. https://doi.org/10.1016/j.matchemphys.2018.01.012
Dedzo GK, Pameté E, Saheu MRT, Ngnie G, Nanseu-Njiki CP, Detellier C, Ngameni E (2017) Hydrogen evolution reaction at PdNPs decorated 1:1 clay minerals and application to the electrocatalytic determination of p-nitrophenol. J Electroanal Chem 801:49–56. https://doi.org/10.1016/j.jelechem.2017.07.030
Zhao H, Yuan ZY (2020) Insights into transition metal phosphate materials for efficient electrocatalysis. ChemCatChem 12:3797–3810. https://doi.org/10.1002/cctc.202000360
Zhao H, Yuan ZY (2017) Transition metal–phosphorus-based materials for electrocatalytic energy conversion reactions. Catal Sci Technol 7:330–347. https://doi.org/10.1039/C6CY01719C
Agilent (2010) CrysAlis PRO. Agilent Technologies, Yarnton, England
Altomare A, Burla MC, Camalli M, Cascarano GL, Giacovazzo C, Guagliardi A, Moliterni AGG, Polidori G, Spagna R (1999) a new tool for crystal structure determination and refinement. J Appl Cryst 32:115–119. https://doi.org/10.1107/S0021889898007717
Palatinus L, Chapuis G, (2007) A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J Appl Cryst 40:786–790. https://doi.org/10.1107/S0021889807029238
Petříček V, Palatinus L, Plášil J, Dušek M (2023) Jana 2020–a new version of the crystallographic computing system Jana. Z fur Krist Cryst Mater. https://doi.org/10.1515/zkri-2023-0005
Brandenburg K, Putz KH (2005) DIAMOND. Version 3. Crystal impact GbR, Bonn, Germany
Chen L, Sagar RUR, Chen J, Liu J, Aslam S, Nosheen F, Anwar T, Hussain N, Hou X, Liang T (2021) Cobalt phthalocyanine as an efficient catalyst for hydrogen evolution reaction. Int J Hydrog Energy 46:19338–19346. https://doi.org/10.1016/j.ijhydene.2021.03.075
Escobal J, Pizarro JL, Mesa JL, Arriortua MI, Rojo T (2000) An ionic nickel (II) phosphate with ethylenediamine:(C2H10N2)[Ni(H2O)6](HPO4)2. Hydrothermal synthesis, crystal structure, and spectroscopic properties. J Solid-State Chem 154:460–465. https://doi.org/10.1006/jssc.2000.8865
Shan Y, Huang SD (1999) (C2H10N2)[Co(H2O)6](HPO4)2: a supramolecular three-dimensional hydrogen-bonding network. Acta Crystallogr C 55:921–923. https://doi.org/10.1107/S0108270199001584
Witty M, Dingra NN, Abboud KA, Felts AC, Na Ayudhya TI (2017) Nuclear magnetic resonance and X-ray crystallography to improve struvite determination. Anal Lett 50:2549–2559. https://doi.org/10.1080/00032719.2017.1302459
Steiner T (2002) Die wasserstoffbrücke im festkörper. Angew Chem 114:50–80. https://doi.org/10.1002/1521-3757(20020104)114:1%3c50::AID-ANGE50%3e3.0.CO;2-H
Shreif A, Farag MA, El-Sherbiny MA, Hassaan MY (2023) Effect of Zr ions on the structure and optical properties of lithium borosilicate molybdate glass system. Ceram Int 49:8709–8717
Gharbi A, Jouini A, Averbuch-Pouchot MT, Durif AJ (1994) Ethylenediammonium bis [copper(II) monohydrogendiphosphate ethylenediamine]trihydrate. J Solid-State Chem 111:330–337. https://doi.org/10.1006/jssc.1994.1235
Dolphin D, Wick AE (1977) Tabulation of infrared spectral data. John Wiley and Sons, New York
Nakamoto k, (1997) Infrared and Raman spectra of inorganic and coordination compounds. John Wiley & Sons, New York
Berg RW, Rasmussen K (1971) Vibrational spectra of ethylenediamine salts. I. Tentative assignments of infrared and far infrared spectra. Spectrosc Lett 4:285–293. https://doi.org/10.1080/00387017108064652
John M, Santha N, Nayar VU, Keresztury G (1993) Vibrational spectroscopic studies of Sr[(CH2)2(NH3)2]3P4O12⋅14H2O. Indian J Phys 67:413–420
Angeloni L, Marzocchi CE, MP, (1977) Single crystal vibrational studies of hydrogen bonding in ethylenediammonium chloride. Chem Phys 26:257–265. https://doi.org/10.1016/0301-0104(77)87050-X
Marchewka MK, Drozd M (2012) Ethylenediammonium dication: H-bonded complexes with terephthalate, chloroacetate, phosphite, selenite and sulfamate anions. Detailed vibrational spectroscopic and theoretical studies of ethylenediammonium terephthalate. Spectrochim Acta A 99:223–233. https://doi.org/10.1016/j.saa.2012.09.026
Akouibaa M, Lakkab I, Direm A, Lachkar M, Ouarsal R, Rakib S, Nasif V, Sayin K, Morley N, El Bali B (2023) [Cu2(ox)(dien)2](NO3)3, a precursor for preparation of CuO nanoparticles: synthesis, structural, Hirshfeld surface analyses, and physico-chemical investigations. J Mol Struct 1282:135258. https://doi.org/10.1016/j.molstruc.2023.135258
HighScore Plus | XRD Analysis Software | Malvern Panalytical. https://www.malvernpanalytical.com/en/products/category/software/x-ray-diffraction-software/highscore-with-plus-option (accessed 17 Sept 2020)
Gates-Rector S, Blanton T (2019) The powder diffraction file: a quality materials characterization database. Powder Diffr 34:352–360. https://doi.org/10.1017/S0885715619000812
Dolenko TA, Burikov SA, Dolenko SA, Efitorov AO, Plastinin IV, Yuzhakov VI, Patsaeva SV (2015) Raman spectroscopy of water–ethanol solutions: the estimation of hydrogen bonding energy and the appearance of clathrate-like structures in solutions. J Phys Chem A 119:10806–10815. https://doi.org/10.1021/acs.jpca.5b06678
Ito H, Sone K (1985) On the assignment of band II in the electronic spectrum of [Ni(H2O)6]2+. Bull Chem Soc Jpn 58:2703–2704. https://doi.org/10.1246/bcsj.58.2703
Fernández S, Mesa JL, Pizarro JL, Lezama L, Arriortua ML, Rojo T (2002) Two new three-dimensional vanadium (III) and iron (III) phosphites templated by ethylenediamine:(C2H10N2)0.5[M(HPO3)2]. Ab initio structure determination, spectroscopic, and magnetic properties. Chem Mater 14:2300–2307. https://doi.org/10.1021/cm0112845
Landry-Hum J, Bussière G, Daniel C, Reber C (2001) triplet electronic states in d2 and d8 complexes probed by absorption spectroscopy: a CASSCF/CASPT2 analysis of [V(H2O)6]3+ and [Ni(H2O)6]2+. Inorg Chem 40:2595–2601. https://doi.org/10.1021/ic0010860
Bussière G, Reber C (1998) coupled excited states in nickel (II) complexes probed by polarized absorption spectroscopy. J Am Chem Soc 120:6306–6315. https://doi.org/10.1021/ja9740733
Hassani HO, Al Wadaani FT (2018) Preparation, characterization and catalytic activity of nickel molybdate (NiMoO4) nanoparticles. Molecules 23:273. https://doi.org/10.3390/molecules23020273
Funding
ZE thanks the Scientific and Technological Research Council of Turkey (Grant Number: TUBITAK 2219) for a postdoctoral fellowship. MDS thanks the Royal Society for a University Research Fellowship (150104). This research was supported by the project 20-LM2023051 of the Czech Science Foundation.
Author information
Authors and Affiliations
Contributions
MA performed all the syntheses and descibed the results, helped somewhere by RO under the supervision of ML; MP, VE and MD performed the single crystal X-ray diffraction, JM and JP measured the Raman spectra and TG/TDA measurements, ZE and MDS studied the catalytic performance of the searched materials. BEB suggested the research and validated the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akouibaa, M., El Bali, B., Poupon, M. et al. Catalytic performance on the water decontamination and the water-splitting electrolysis of new phosphite salts (enH2)[M(H2O)6](HPO3)2 (M=Co, Ni and Mg). J Appl Electrochem (2024). https://doi.org/10.1007/s10800-024-02097-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10800-024-02097-w