Abstract
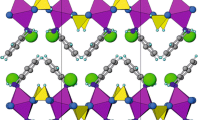
A new layered gallium phosphonate-oxalate hybrid material, [C2H10N2]0.5[Ga3(PO3CH3)4(C2O4)].H2O, denoted as MOP-1, was synthesized under mild hydrothermal conditions (150 °C) in the presence of ethylenediammonium ion (H2en)2+ as structure-directing agent. The compound was structurally characterized by single-crystal X-ray diffraction (SCXRD), Fourier transformed infra-red spectroscopy (FTIR), and thermogravimetric analysis (TGA). Its structure consists of gallium phosphonate double layers formed of GaO6 octahedra, GaO4 tetrahedra, and PO3CH3 groups sharing corners. The double layers are cross-linked by oxalate ligands to form a three-dimensional framework with intersecting channels where the ammonium ions and water molecules are located. The bridging oxalate ligand acts as a bidentate ligand to each Ga in octahedral coordination environment. Crystal data are as follows: Monoclinic P21/n (14), a = 8.7530(5) Å; b = 16.3427(8) Å; c = 14.7522(8) Å; β = 93.284(1)°, V = 2106.8(2) Å3 and Z = 4.
Graphical Abstract

The manuscript describes the synthesis, crystal structure, infrared spectroscopy, and thermal stability of a novel hybrid inorganic-organic gallium phosphonate oxalate two-dimensional material.





Similar content being viewed by others
References
Whittingham MS (1976) Electrical energy storage and intercalation chemistry. Science 192:1126–1127
Lethbridge ZAD, Tiwary SK, Harrison A, Lightfoot P (2001) Synthesis, structural and spectroscopic properties of two new ethylenediamine-templated gallophosphate-oxalate layered materials. J Chem Soc Dalton Trans 1904–1910
Tsai YM, Wang SL, Huang CH, Lii KH (1999) Synthesis and structural characterization of the first organically templated vanadyl (IV) arsenato- and phosphato-oxalates: (C4H12N2)[VO(C2O4)HXO4] (X) As, P). Inorg Chem 38:4183–4187
Chang WJ, Lin HM, Lii KH (2001) Synthesis and characterization of new iron phosphate oxalates: [(S)-C5H14N2] [Fe4(C2O4)3(HPO4)2(H2O)2] and [(S)-C5H14N2] [Fe4(C2O4)3(HPO4)2]. J Solid State Chem 157:233–239
Parise JB (1985) Preparation and structural characterization of two metallophosphate frameworks clathrating diprotonated ethylenediamine: AlPO4-12(en) and GaPO4-12(en). Inorg Chem 24:4312–4316
Parise JB (1985) Some gallium phosphate frameworks related to the aluminium phosphate molecular sieves: X-ray structural characterization of {(PriNH3)[Ga4(PO4)4·OH]}·H2Ot. J Chem Soc Chem Commun 9 606–607
Parise JB (1986) Preparation and structure of a gallium phosphate framework with clathrated isopropylamine. Acta Crystallogr Sect C42:144–147
Yang G, Feng S, Xu R (1987) Crystal structure of the gallophosphate framework: X-ray characterization of Ga9P9O36OH·HNEt3. J Chem Soc Chem Commun 1254
Wang T, Yang G, Feng S, Shang C, Xu R (1989) A novel mixed octahedral–tetrahedral framework: X-ray characterization of a microporous gallophosphate, Ga2P2O8(OH)H2O·NH4·H2O·0.16 PrOH (GaPO4-C7). J Chem Soc Chem Commun 14:948
Lin CH, Wang SL, Lii KH (2001) [Ga2(DETA)(PO4)2]·2H2O (DETA = Diethylenetriamine): A novel Porous gallium-phosphate containing 24-ring channels. J Am Chem Soc 123:4649–4650
Chen CY, Chu PP, Lii KH (1999) Synthesis, crystal structure and 71Ga MAS NMR spectroscopy of a novel gallium phosphatooxalate: [Ga5(OH)2(C10H9N2)(C2O4)(PO4)4]·2H2O. Chem Commun 1473–1474
Lii KH, Chen CY (2000) Synthesis and characterization of (R-C5H14N2)2[Ga4(C2O4)(H2PO4)2(PO4)4]·2H2O, a layered gallium phosphate oxalate containing a chiral amine. Inorg Chem 39:3374–3378
Hung LC, Kao HM, Lii KH (2000) Synthesis, crystal structure and solid-state NMR spectroscopy of K2[Ga4(C2O4)(PO4)4]·2H2O, a new gallium phosphatooxalate with an intersecting tunnel structure. Chem Mater 12:2411–2417
Adair B, Natarajan S, Cheetham AK (1998) Synthesis and structural characterization of a novel tin(II) phosphonate, Sn2(O3PCH3)(C2O4). J Mater Chem 8:1477
Stock N, Stucky GD, Cheetham AK (2000) The hybrid open-framework of tin(II) phosphonopropionate oxalate, Sn4(O3PCH2CH2CO2)2(C2O4). Chem Commun 22:2277
Linand CH, Lii KH (2004) Synthesis and characterization of the first organically templated metal oxalatophosphonate: (C3H12N2)0.5[Ga3(C2O4)(CH3PO3)4]∙0.5H2O. Inorg Chem 43:6403–6407
Shannnon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A 32:751
Smith RW, Holman D, Villa EM (2017) Crystal structure of Li3Ga(BO3)2. Acta Cryst E73:456–458
Mooney RCL (1956) The crystal structure of aluminum phosphate and gallium phosphate, low-cristobalite type. Acta Cryst 9:728
Mooney SRCL (1966) The crystal structure of hydrated gallium phosphate of composition GaPO4.2H20. Acta Cryst 20:526
Achnowledgments
F. Dardar and A. El Jazouli acknowledge the financial support of the Moroccan American Commission for Educational and Cultural Exchanges - Fulbright’s Program (http://www.macece.org). They also acknowledge the support of the Department of Chemistry and the Center for Energy, Environment, and Sustainability at Wake Forest University, during their stay at WFU.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dardar, F., Day, C.S., El Jazouli, A. et al. Synthesis and Characterization of a New Layered Gallium Phosphonate Oxalate [C2H10N2]0.5[Ga3(PO3CH3)4(C2O4)].H2O. J Chem Crystallogr 49, 44–51 (2019). https://doi.org/10.1007/s10870-018-00762-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-018-00762-5