Abstract
Environmentally friendly and cost-effective inhibitors based on orange peel extract (OPE) have been developed for potential applications in ejector tubes of a multistage desalination plant during the acid cleaning process. After conducting tests under both static and hydrodynamic circumstances, it was determined that the inhibitor formulation was effective against corrosion of stainless steel (SS) in 1 M HCl solution at 30 °C under the conditions tested. Electrochemical methods were used along with electron microscopy to collect information on the corrosion inhibition efficiency of the extract. Experiments were conducted for 1, 2, 3, 4, 6, 12, and 24 h, and the performance of OPE was compared to that of a commercial acid corrosion inhibitor to determine which performed better. The results revealed that OPE demonstrated outstanding corrosion inhibition performance compared to the commercial acid corrosion inhibitor. With remarkable inhibition efficacy for up to 24 h under both static and dynamic settings, 0.4% of OPE displayed typical mixed-type corrosion inhibitor behavior in the tested environment. All of the procedures that were used produced results that were in good agreement. Due to the synergistic action between the two compounds, the corrosion inhibition of OPE on SS was improved in 1 M HCl in the presence of iodide ions. In the absence of KI, OPE was found to inhibit SS corrosion at a concentration-dependent rate, with the concentration of OPE being the most significant factor. A study of the adsorption of OPE onto a SS surface revealed that the Langmuir adsorption isotherm controls the process. Based on the quantity of free energy of adsorption observed, it was determined that there is physical contact between the OPE and the surface of SS in this experiment. It was possible to identify the most effective phytochemicals for corrosion inhibition based on the findings of quantum chemical calculations, which were subsequently evaluated in the laboratory.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Seawater desalination is an alternative to increasing the supply of freshwater around the world. Some desalination facilities, which are now in operation worldwide, produce approximately 95 million m3 of freshwater each day for direct use. Because of the increasing need for drinkable water in recent decades, desalination technologies have improved rapidly. Thermal desalination is one of the most widely used procedures, and involves multiple steps such as multi-stage flashing (MSF) and multi-effect distillation (MED) [1,2,3,4]. It is necessary to provide suitable provisions to safeguard the facilities against corrosion (particularly the corrosion of metallic components) and scale development for a thermal desalination plant to operate sustainably and efficiently [5, 6].
The employment of corrosion inhibitors to protect metallic components of the desalination plant is one of the most acceptable options [7,8,9,10,11]. However, the choice of corrosion inhibitors is the most fundamental challenge because suitable inhibitors for this purpose must be non-toxic, environmentally friendly, cost-effective, and should not interfere with the quality or portability of the produced water. Along the recent years there are various group of plant extracts such as Tunbergia fragrans, Garlic extract Rosmarinus, Dardagan Fruit extract, Hyalomma tick extract, Tea factory solid waste extract, and Ficus racemosa leaf extract that were used in the corrosion inhibition of steel, stainless steel (SS), and copper in HCl [12,13,14,15,16].
Ammonium-based ionic liquid has been applied to mitigate corrosion in desalination plants by Deyab and Mohsen [17]. Obot et al. [18] conducted a review listing some inhibitors that are known since 1989 to date for their role in controlling corrosion in the thermal desalination plant, including cinnamaldehyde + quat-nitrogen salt, 2-mercaptobenzoazole derivative, benzamide, Mannich Bas, Polysaccharide derivatives, and ten others [3, 19]. Little effort is directed toward the employment of the less toxic given the expected sensitivity of corrosion inhibitors for thermal desalination plants; current efforts are concentrated on the use of non-toxic plant extract. For example, Obot et al. [20] reportedly investigated the effect of an extract from Date palm leaves and reported good inhibition capacity. There has been no published research on the efficacy of OPE as a corrosion inhibitor of SS used in thermal desalination plants of water, at least not to our knowledge. The current study aims to look at the efficacy of OPE as a corrosion inhibitor for SS when multistage flash water desalination plants are acid-cleaned and how it can be applied in this context.
2 Materials and methods
2.1 Materials
The test samples were prepared from a SS rod that was readily available on the market. The chemical makeup (in wt%) of SS was: Cr (18.25), Ni (8.5), Mo (1.17), Mn (0.85), Ti (0.06), Al (0.02), V (0.06), S (0.005), C (0.17), Cu (0.11), while Fe constituted the remaining component. A cylindrical sample of SS was fixed into a polytetrafluoroethylene (PTFE, Teflon) rod with an epoxy resin in such a way that no crevice was created, and only one side of a constant surface area (1.281 cm2) was exposed to the solution. A thick copper wire was screwed to the metal specimen and introduced into an insulated glass tube for electrical connection copper. Before using the samples for the relevant experiment, they were completely rinsed with distilled water, degreased with ethanol, rinsed with acetone, and dried in the air. Different strengths of hydrochloric acid solutions were generated by dilution with doubly distilled water from an analytical grade HCl solution (37%).
The orange peel samples were dried to constant weight and blended to a powdered form. 100 g of the orange peel powder sample was dissolved in 0.5 L of double-distilled water in a round bottom flask and refluxed (100 °C) for about two hours under constant stirring. The resulting product was allowed to cold. Vacuum filtration was applied, and the excess solvent was evaporated with the aid of a rotary evaporator. This stock extract was kept in a refrigerator (− 24 °C), and it is ready to be used for FTIR analysis.
2.2 Methodology
2.2.1 Weight loss (WL) measurements
The method of WL was employed to estimate the loss in the weight of the metal as corrosion proceeded. The information obtained from the measurement of WL before and after the corrosion test, the rate of corrosion, and the inhibitor's efficiency were evaluated using the following Eq. (1).
2.2.2 Electrochemical experiments
Electrochemical measurements were carried out with a multiport glass cell that was equipped with a Gamry Potentiostat/Galvanostat (model Series-G 300) that was controlled by Gamry Fram work version 6.12, DC105, and EIS300 software and a Gamry Potentiostat/Galvanostat (model Series-G 300). When employing alternating current signals, the impedance measurements were carried out in the frequency range of 100 kHz to 10 Hz with peak-to-peak amplitude of 10 mV using SS alloy under open circuit potential (OCP) conditions, and the results were published in the journal Electron Devices. The obtained impedance spectra were checked with the help of an equivalent circuit program using a fixed phase element model. It was necessary to achieve a constant value of equilibrium corrosion potential by immersing the SS working electrode in the test solution before performing EIS and polarization measurements, which were both successful. The polarization curves were developed by the information obtained from the circuit that used at a scan rate of 2 mV s−1 by sweeping the applied over potential starting with (ca. − 250 mV vs. OCP) for cathodic polarization [extending to rest potential (Erest)] before proceeding with the anodic polarization by applying (ca. + 250 mV vs. OCP) above the rest potential.
2.2.3 FTIR and SEM study
The purpose of this Fourier transform infrared (FTIR) study was to identify the functional groups contained in the aqueous extract and the level of interaction between these functional groups and the metal surface following inhibition. The surface characterization of uninhibited and inhibited SS specimens was carried out using an FTIR analysis performed using Bruker VERTEX 70v FTIR Spectrometer connected to a platinum ATR model V-100 in the wavenumber 400–4000 cm−1. For 12 h, the produced SS specimens were immersed in 1 M HCl in the presence of OPE (0.4 mg L−1) to determine their corrosion resistance. The specimens were then removed and allowed to dry. The FTIR spectra of the thin coating that had adhered to the SS surface were obtained after it had been scraped away with care. A scanning electron microscope (SEM) (JEOL-JSM-5300LV, Tokyo, Japan) of secondary electrons (SE) was used to examine the surface morphology in both the absence and presence of OPE and the results were compared.
2.2.4 GC–MS study
GC–MS analysis of the OPE was conducted using Agilent technologies Gas Chromatography model 7890A GC system connected to 5975C inert MSD with triple Axis Detector and Autosampler Model 7593. HP-5 GC Column was used, and Helium gas was used as carrier gas with a 1.2 ml min−1 flow rate. Temperature range (80 to 300 °C) with ramping temperature (5 °C min−1) was applied as GC oven program.
3 Results and discussions
3.1 FTIR and GC–MS analyses of OPE
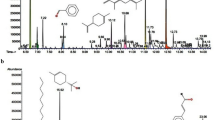
The FTIR spectrum of OPE is shown in Fig. 1. The spectrum reveals a broad peak due to O–H bond stretch at 3286 cm−1. The C–H (CH2) bond stretch vibration is described by the peaks observed at 2921 and 2851 cm−1. Carbonyl C=O stretches were observed at 1622 and 1645 cm−1, while C=O stretch due to carboxylic acid was found at 1417 cm−1. Other functional groups native to the spectrum included C–O bending at 1089 cm−1 and strong C=C bending vibrations at 610 and 606 cm−1. Also, Fig. 2 shows the GC–MS spectrum of the aqueous extract of OPE and the compounds identified through the standard database indicated the presence of 5-hydroxymethylfurfural, Spirost-8-en-11-one, 3-hydroxy-, (3β,5α,14β,20β,22β,25R)-, Spiro[4-methyl-5-oxo-10,13-dioxatricyclo[7.3.1.0(4,9)]tridecane]-2,2′-oxirane, bis(2-ethylhexyl)phthalate, and hexadecanedioic acid and dimethyl ester. These identified compounds are listed in Table 1. From the chemical structures of the detected compounds and associated functional groups, it is evident that the aqueous extract contains compounds that can exhibit good corrosion inhibition efficiency, including the presence of heteroatoms, multiple bonds, and an aromatic system with high molecular weight.
3.2 Weight loss technique
Figure 3 depicts plots of the corrosion rate of the metal as a function of the concentration of HCl and the length of time the metal was in contact with the solution. The observed patterns in the plots confirmed that the corrosion rate of the examined metal increased with an increase in the OPE concentration and with an increase in the amount of time spent in the presence of the acid (Tables 2, 3). As a result, corrosion reaction was slowed down when OPE was present. As a result, the rate of corrosion decreases with an increase in OPE concentration (Fig. 4), which also translates to an increase in the inhibitory efficiency of OPE (for SS in a solution of HCl) with increasing concentration of the aqueous extract of OPE (for SS in a solution of HCl). The most significant inhibition efficiency was reached at a dose of 0.4 g L−1, resulting in a 70% inhibition efficiency (Table 4). It was observed by Ukpe [21] that an ethanol extract of orange peel was effective in inhibiting the corrosion of aluminum in concentrated HCl, and that the fluctuation in inhibitory efficacy with concentration was identical.
3.3 Electrochemical techniques
3.3.1 Potentiodynamic curves
In this work, the effect of OPE concentration on the corrosion behavior of SS working electrode in 1 M HCl solution was investigated using polarization measurements. The recorded OCP and Tafel plots are depicted in Fig. 5a and b, respectively, and the polarization data derived from the plots are presented in Table 5. By analyzing the Tafel plot, it was possible to estimate electrochemical corrosion kinetic characteristics such as corrosion potential (Ecorr), current density (Icorr), corrosion rate (mm year−1), cathodic and anodic Tafel slopes (βc and βa), polarization resistance, and corrosion rate (mm year−1). Table 5 also included the IE (%) and θ (surface coverage) measurements.
To compute the percentage inhibitory efficiency E (%), as well as the degree of surface covering (θ), the following Eqs. (2) and (3), respectively, were used [22]:
where Icorrinhi and Icorr are the current density of corrosion in the presence and the absence of OPE, respectively.
The concentration of 0.4 mg L−1 of OPE resulted in inhibitory effectiveness of around 75%. It is clear from the recorded data that increasing the concentration of OPE reduces the corrosion current density while simultaneously increasing the current suppression. Anodic dissolution of SS was delayed when OPE was added to the acid solution. Cathodic hydrogen evolution processes were also delayed when OPE was added. It was discovered that, in the presence of OPE, the Tafel constants (βa and βc) were not significantly affected in an inhibited solution when compared to an uninhibited solution, thus encouraging the adsorption of the inhibitor's molecules on a metal surface through blockages of the active sites that would otherwise have aided corrosion. Suppose the displacement in the corrosion potential (Ecorr) for the uninhibited system is greater than 85 mV for the inhibited system. In that case, the inhibitor is considered to be of the cathodic or anodic type, respectively. The mixed kind of inhibitor can be identified when the displacement is less than 85 mV [23, 24].
The data in Table 5 show that the variations in Ecorr values between the inhibited and uninhibited systems are often less than 85 mV, indicating that the inhibitor (OPE) under investigation is a mixed-type inhibitor in general. The extent to which organic compounds included in OPE extract are adsorbed and coated on the metal surface is correlated to the efficacy of the organic compounds. Corrosion inhibitors are highly effective in preventing metal oxidation and so eliminating the undesirable destructive effect.
When OPE was used at a concentration of 0.4 mg L−1, the inhibitory efficiency was estimated to be around 75%. By looking at the results obtained, it is evident that raising the concentration of OPE lowers the corrosion current density, while concurrently increasing the suppression of the corrosion current. When OPE was added to the acid solution, the anodic breakdown of SS was significantly slowed. The addition of OPE also resulted in a delay in the cathodic hydrogen evolution processes. As a result of this discovery, it was found that, in the presence of OPE, the Tafel constants (βa and βc) in an inhibited solution were not significantly affected when compared to an uninhibited solution, thereby promoting the adsorption of the inhibitor's molecules on a metal surface by blocking the active sites that would otherwise have aided corrosion. If the corrosion potential (Ecorr) displacement for the unfettered system is larger than 85 mV for the inhibited system, the inhibitor is classified as either cathodic or anodic. When the displacement is less than 85 mV, the presence of a mixed kind of inhibitor can be determined [23, 24].
Data in Table 5 demonstrate that the variation in Ecorr values between the inhibited and uninhibited systems is frequently less than 85 mV, indicating that the OPE inhibitor under research is generally a mixed-type inhibitor. A relationship exists between the extent to which organic compounds present in OPE extract are adsorbed and coated on the metal surface and the effectiveness of the organic compounds. When it comes to preventing metal oxidation and reducing the negative, destructive effect, corrosion inhibitors are quite effective.
3.3.2 Electrochemical impedance spectroscopy (EIS) studies
It was determined that SS exhibits EIS in 1 M HCl in the absence and presence of different concentrations of an OPE inhibitor when heated to 30 °C in the absence and presence of the inhibitor. Figure 6a, b show the findings of Nyquist plots and Bode plots, which were both performed at 35 degrees Celsius. Other temperatures were studied, and the plots of a similar sort were made for each of those values. The high-frequency (HF) section of all of these graphs was dominated by a massive semi-circular capacitive loop, which was followed by a moderate inductive loop in the low-frequency (LF) area. Inductive loops are assumed to be responsible for the relaxation process caused by adsorbed species such as Clads− and Hads+ on the electrode surface. In contrast, capacitive loops are thought to be responsible for the charge transfer between the alloy and the electrolyte. Additional factors that contribute to this phenomenon include the adsorption of the inhibitor or corrosion intermediates on the electrode surface, as well as the redissolution of the passivated steel surface when the frequency is dropped to a low level of operation [25]. It is believed that the corrosion process is under activation control (charge-transfer regulation) when capacitor loops with clearly defined boundaries are observed [26]. In addition, because the current plots produced in the presence and absence of the OPE inhibitor have similar shapes, the results indicate that the inhibitor does not affect on the mechanism of SS corrosion, as evidenced by the findings. When seen as a depressed graph, the capacitive loops in the Nyquist plots had their centers below the real axis of the chart, indicating that they were depressed. This could be due to the frequency dispersion often associated with porous/heterogeneous electrode surfaces, which results in a divergence from ideal capacitive behavior compared to a uniform electrode surface, which could explain the observed difference. Gamry Echem analyzer 6.0 was used to fit the impedance data into the CPE 2 repetition equivalent circuit (shown in Fig. 7) utilizing the impedance data (Table 6).
3.3.2.1 Synergistic study
It was decided to undertake a synergistic investigation by combining different amounts of OPE with 0.0001 M KI. It was discovered that the interaction of the adsorbed KI with the examined OPE inhibitors increases θ and, consequently, increases the effectiveness of the inhibitors (Fig. 8). Figure 8 illustrates the impedance of SS submerged in 1 M HCl in the absence and presence of 0.0001 M KI with 0.4 g L–1 of OPE at a temperature of 25 °C. Figure 8 shows that the corrosion rate of SS was considerably reduced in the presence of iodide ions, which provides further evidence of this. According to Table 6 and Fig. 8, the action of OPE is enhanced synergistically by adding 0.1 M iodide ions to the solution. The most significant percentage is 74%, corresponding to an OPE concentration of 0.4 g L−1. With the addition of 0.0001 M KI, this efficiency rises to 89%, a significant improvement.
3.3.3 Impact of self-assembled monolayer application of OPE
SAM (self-assembled monolayer) technology has tremendous potential for a wide range of applications in various fields. For example, corrosion prevention in materials, where it is used as ultra-thin layers of protection [27], is an application for which it is appropriate. The production and characterization of self-assembled OPE monolayers on a SS surface were investigated. To explore the efficacy of these layers to protect SS from corrosion when exposed to a 1 M HCl solution, it was chosen to utilize the EIS technique. As a result of this investigation, it was discovered that layers of OPE can successfully protect SS against corrosion. Experimental evidence (Table 7) indicated that the protective power of OPEs is strongly influenced by the amount of time that samples are exposed to an aqueous solution that contains 0.04 g L−1 of OPE (0.4 g L−1). Figures 9 and 10 depict EIS in 1 M HCl for SS electrodes covered with self-assembled OPE monolayers, similar to those described in the preceding portion of this paper.
It can be noticed in this figure that the Rp of the steel electrodes coated with OPE grew dramatically with an increase in the period of contact time during the experiment. Nyquist diagram analysis revealed that the production of OPE films could successfully protect SS against corrosion when exposed to concentrated HCl solution.
3.4 Surface analysis technique
3.4.1 FTIR spectra
Figure 11a shows the FTIR spectra of pure OPE, while Fig. 11b, c the scrapped samples obtained from the SS surfaces after exposure to 0.4 g L−1 OPE + 0.1 M KI in 1 M HCl. It was found that most peaks in the spectrum of pure OPE were almost adsorbed in case of synergism, but the spectrum of the scrapped metal without synergism, revealed that some functional groups were not adsorbed. The OH group stretching frequency for pure OPE and the scrapped samples after synergism were shifted to 3252 cm−1, carbonyl stretching frequencies were adjusted 1622 cm−1, The carboxylic group was moved to 1417 cm−1, bending C–O vibration for the ether was shifted to 1089 cm−1, while the C=C bending vibrations were shifted to 610 and 606 cm−1, respectively. For the scrapped samples without synergism, only OH, C–O, and C=C appear in the FTIR spectrum. The shift in frequency of adsorption can be ascribed to the existence of interaction, while the formation of new bonds as well as the disappearance of old bonds suggests their involvement in the adsorption process [28].
3.4.2 Scanning electron microscope (SEM)
The SEM displayed in Fig. 12a–d is for the blank before HCL, after immersion for four days in 1 M HCl, after inhibition by 0.4 g L−1 of (OPE) and after synergistic combination with NaCl, respectively. Comparing the micrographs of the metal in the blank (1 M HCl) with others suggests enhanced smoothness of the metal surface that can be linked to inhibition or the formation of protective film.
3.5 Adsorption isotherm
To better understand the mechanism of metal corrosion, adsorption isotherm models are used [29]. The method by which the inhibitor exerts its effect in an aggressive solution is believed to be adsorption at the metal/inhibitor solution contact. The OPE adsorption from aqueous solution is a quasi-substitution process involving the OPE inhibitor in the aqueous phase [denoted as Extract(sol) and water molecules, which are adsorbed on the metal surface H2O(ads)]. This represents the adsorption of the OPE at the surface of the SS/solvent mixture.
The adsorption of OPE on the metal surface demonstrated high adherence to the Langmuir adsorption model, which may be represented by the Eq. 4 [30]:
Cinh is the inhibitor concentration, Kads denotes the adsorption equilibrium constant and the area covered by the inhibitor. On the plot of Cinh/θ versus Cinh, Fig. 13 shows the straight line with the best fit, which was produced by fitting the data. According to this result, the adsorption of OPE onto the surface of the SS occurs following the Langmuir isotherm model. In order to calculate Kads's value, the intercepts of the straight line on the Cinh/θ—axis must first be determined. The free energy of adsorption (G°ads) is the most significant parameter in thermodynamic adsorption calculations. This relationship between Kads and standard free energy of sorption (G°ads) was established by the use of the following Eq. (5):
Concerning water, R is the gas constant (which is 8.314 J K−1 mol−1), T is the absolute temperature (in degrees Kelvin), and Ksol is the molar concentration of the solvent, which in this case is 55.5 mol L−1. According to this findings, the unit of Kads is L g−1, which suggests that the unit of Ksol is g L−1, with a value of roughly 1.0 × 103. At 298 °C, the thermodynamic characteristics of OPE adsorption on SS in 1 M HCl are computed, and the results are presented in Table 8.
The values of ΔG°ads up to − 20 kJ mol−1 are congruent with the concept of electrostatic attraction between the charged OPE and the charged SS (physical adsorption), whereas those greater than − 40 kJ mol−1 have been shown to be consistent with the sharing or transfer of electrons from inhibitor molecules to the electrode surface, resulting in the formation of a coordinate type of bond (chemisorption) [23].
In this work, the value of free energy of adsorption of (OPE) on SS surface, ΔG°ads, is − 18.19 kJ mol−1. The calculated value for the adsorption of (OPE) on the electrode SS is consistent with physiosorption mechanism. The adsorption of (OPE) obeys the Langmuir isotherm, and the negative sign of ΔG°ads indicates a strong contact between inhibitor molecules and metal surfaces. The high efficiency of adsorption is evidenced by the results of this experiment. The applicability of the Kinetic-thermodynamic model suggests that the corrosion reactions have both kinetic and thermodynamic implications (Fig. 14).
The value of the free energy of adsorption of (OPE) on SS surface, ΔG°ads, is determined to be − 18.19 kJ mol−1. This is the first time that this value has been reported. Adsorption of OPE on SS electrodes has been estimated and found to be following the physisorption mechanism. When OPE is adsorbed on SS surface, the Langmuir isotherm is obeyed, and the negative value of ΔG°ads implies that the inhibitor molecules have strong contact with the metal surface and that the adsorption is highly effective. This model's applicability implies that corrosion reactions have both kinetic and thermodynamic repercussions, which is consistent with the previous study's findings (Fig. 14).
3.6 The compounds responsible for corrosion inhibition
According to previous studies on pure synthetic or other organic compounds extracted from natural resources, it was possible to identify the natural organic compounds mentioned in Table 1 which have inhibition efficiency on metal corrosion. These pure compounds or their derivatives greatly affect corrosion inhibition on some metals, as reported in Table 9. Different glucosamine derivatives are reported as an environmentally friendly corrosion inhibitor of mild steel in 1 M HCl can cover up to 96.52% of the metal's surface, greatly reducing corrosion [31]. Hydroxymethylfurfural and its derivatives were investigated as corrosion inhibitors in LiN (SO2CF3)2 solution to protect the surface of copper, aluminum and mild steel in HCl [32,33,34]. The inhibiting activity of N-methyllaurotetanine derivatives on steel and aluminum corrosion inhibition in HCl solutions was 91 and 96.2% and investigated. In a 1 M HCl solution, the inhibition activity of 2-methoxy-4-vinylphenol derivatives as an environmentally friendly corrosion inhibitor for aluminum alloy was investigated with inhibition efficiency 60% [35]. Inhibition efficiency of (alkylsulfanyl)-N-(pyridin-2-yl) acetamide, N-[(4-methoxyphenyl)(morpholin-4-yl) methyl] acetamide, and N-[morpholin-4-yl (phenyl) methyl] derivatives on the corrosion of aluminum alloy, steel, and mild steel in HCl was 91.6, 94.10, and 76%, respectively [36,37,38]. The role of Sinigrin extract and Natural oil on steel and copper in NaOH and orthophosphoric acid was 71.11 and 83%, respectively [39, 40]. The corrosion inhibition of steel in seawater in the presence of olive leaf extract was 85.2% [41]. The corrosion inhibition of SS in 0.5 M H2SO4 in the presence of benzoic acid and salicylic acid derivatives was investigated at different temperatures 343 and 323. The inhibition efficiency increased by raising the temperature by 68 and 85%, respectively [42]. Another study on aluminum, copper, and SS corrosion behavior in both petroleum diesel and palm biodiesel by gravimetric analysis at 80 °C for 1200 h [43]. Other synthetic inhibitor derivatives for aluminum corrosion for mild steel in HCl was studied by electrochemical method, and mass loss resulted in maximum efficiency above 90% [44]. With an efficiency of more than 90%, the potentio-dynamics (PD) and electrochemical frequency modulation (EFM) methods were used to investigate the synthesis and description of a new organic corrosion inhibitor, 4-((((5-ethyl-1,3,4-thiadiazol-2-yl) imino) methyl), for mild steel in HCl. A green chemical product, specifically for mild steel corrosion inhibition in 1 M of hydrochloric acid that is environmentally friendly [45].
3.7 Quantum chemical study
Table 10 presents calculated quantum chemical parameters for the chemical compounds present in orange peel (Table 1). It is a fact that corrosion IE% varies with frontier molecular orbital energies [46, 47]. Consequently, an increase in the energy of the highest occupied molecular orbital (EHOMO) and a decrease in the energy of the lowest occupied molecules orbital (ELUMO) and that of the energy gap (ΔE) are associated with better adsorption and hence corrosion inhibition. The pattern of variation must be assessed by carefully considering the tendency toward the donation of electrons (signified by the EHOMO), the tendency toward the acceptance of electrons (i.e., the ELUMO) and the degree of softness of the molecule, which is a function of the energy gap (i.e., ΔE) [48]. Also outlined in Table 10 are favorable parameters of the phytochemicals, that can enhance corrosion inhibition, such as possession of heteroatoms, π-electron and conjugated systems, aromatic system, multiple bonds and long carbon chain [49]. A comprehensive ranking of the phytochemicals’ inhibition potentials based on the calculated quantum chemical parameters and the theoretical requirement suggests that the most corrosion active components of the phytochemicals are pyrimidin-2,4-dione, 1,2,3,4-tetrahydro-5-methyl-1-[[2-hydroxymethyl-3-dimethylamino]tetrahydrofur-5-yl, 5-hydroxymethylfurfural, N-methyllaurotetanine, benzoic acid, 3,4,5-tris[(trimethylsilyl)oxy]-, propyl ester, desulphosinigrin and Bis(2-ethylhexyl) phthalate. Therefore, other components contributed little toward the adsorption of the inhibitor. It is also a fact that the adsorption of orange peel extract (OPE) on the metal surface occurred through the synergistic action of the various phytochemicals [50].
3.8 Mechanism of adsorption and inhibition
Simple explanation of how positively charged SS surfaces react with OPE molecules; interactions between organic compounds in the extract and steel surfaces entail donor–acceptor processes in an acidic media (1 M HCl). Because of the hostile environment, which makes the metal surface of steel positively charged, steel oxidizes. This favors the fixation of negatively charged counter chloride ions, resulting in a negative metallic surface as depicted in Fig. 15. In the acidic solution, the inhibitor molecules 5-hydroxymethylfurfural, N-methyl-N-[4-(3-hydroxypyrrolidinyl)-2-butynyl)-acetamide, and hexadecanedioic acid, dimethyl ester, which are the most abundant chemicals in OPE, undergo attractive electrostatic protonation [52].
Parallel to this, the vacant d-orbitals of the steel atoms could be supplied with electrons by the lone pair electrons of the nitrogen and oxygen atoms of the non-protonated pyrazole ring as well as the π-electrons of the benzene ring. This results in the phenomena of chemisorption and retro-donation, respectively. Inter-electron repulsions are however produced as a result of the accumulation of electrons in the d-orbitals of the metal atoms due to this form of electron transfer. Retro-donation is the process of transferring electrons from the vacant molecular orbitals of the inhibitor molecules to the d-orbitals of the surface metal atoms in order to prevent this repulsive occurrence. This reinforces the adsorption of the inhibitor molecules on the metal surface. As a result, it is reasonable to assume that three different types of phenomena-physisorption, chemisorption, and retro-donation will be involved in the adsorption of various inhibitor molecules on the metal surface of SS in an aggressive acidic solution (1 M HCl). Additionally, the theoretical research reported here has a strong connection with electrochemical investigations, which demonstrate the great metal corrosion inhibition ability of these inhibitors [53].
4 Conclusion
To evaluate the ability of OPE to suppress the corrosion of SS in 1 M HCl solution, electrochemical tests and surface analysis techniques were used in the current study. The following are the most important conclusions reached:
-
(1)
In general, the rate at which SS corrodes increases as the concentration of OPE in the solution rises.
-
(2)
Increasing the OPE concentration leads to a decrease in the corrosion current density values. In addition, the polarization resistance was found to be relevant when the OPE was added.
-
(3)
The polarization curve showed that OPE operated as a mixed-type inhibitor.
-
(4)
The IE% rises as the concentration of OPE increases, and the effect is synergistically amplified by adding KI to the solution.
-
(5)
The application of SAMs of OPE can provide efficient corrosion protection for SS.
-
(6)
In accordance with the Langmuir isotherm model, it was discovered that OPE was physically adsorbed on the SS surface.
-
(7)
The surface analysis approach verifies OPE's capacity to preserve SS while retarding its corrosion in a concentrated HCl solution.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Hao J, Wang M, Lababidi HM, Al-Adwani H, Gleason KK (2018) A review of heterogeneous nucleation of calcium carbonate and control strategies for scale formation in multi-stage flash (MSF) desalination plants. Desalination 442:75–88. https://doi.org/10.1016/j.desal.2018.05.008
Rahman F, Amjad Z (2010) Scale formation and control in thermal desalination systems. Sci Technol Ind Water Treat Chapter 14:272–297. https://doi.org/10.1201/9781420071450-c14
Obot IB, Solomon MM, Onyeachu IB, Umoren SA, Meroufel A, Alenazi A, Sorour AA (2020) Development of a green corrosion inhibitor for use in acid cleaning of MSF desalination plant. Desalination 495:114675. https://doi.org/10.1016/j.desal.2020.114675
Warsinger DM, Swaminathan J, Guillen-Burrieza E, Arafat HA (2015) Scaling and fouling in membrane distillation for desalination applications: a review. Desalination 356:294–313. https://doi.org/10.1016/j.desal.2014.06.031
Deyab MA (2018) Corrosion inhibition of heat exchanger tubing material (titanium) in MSF desalination plants in acid cleaning solution using aromatic nitro compounds. Desalination 439:73–79. https://doi.org/10.1016/j.desal.2018.04.005
El Nemr A (2007) New developments in electrodeposition and pitting research. Research Signpost Publishers, Transworld Research Network, Trivandrum. ISBN 978-81-7895-304-5
El Ashry ESH, El Nemr A, Essawy SA, Ragab S (2006) Corrosion inhibitors Part II: Quantum chemical studies on the corrosion inhibitions of steel in acidic medium by some triazole, oxadiazole and thiadiazole derivatives. Electrochim Acta 51(19):3957–3968. https://doi.org/10.1016/j.electacta.2005.11.010
El Ashry ESH, El Nemr A, Essawy SA, Ragab S (2006) Corrosion inhibitors Part IV: quantum chemical studies on the corrosion inhibitions of steel in acidic medium by some aniline derivatives. Chem Phys Indian J 1(2–3):41–62
El Ashry ESH, El Nemr A, Essawy SA, Ragab S (2006) Corrosion inhibitors Part III: quantum chemical studies on the efficiencies of some aromatic hydrazides and Schiff bases as corrosion inhibitors of steel in acidic medium. ARKIVOC xi:205–220. https://doi.org/10.3998/ark.5550190.0007.b21
El Ashry ESH, El Nemr A, Essawy SA, Ragab S (2008) Corrosion inhibitors Part V: QSAR of benzimidazole and 2-substituted derivatives as corrosion inhibitors by using the quantum chemical parameters. Prog Org Coat 61:11–20. https://doi.org/10.1016/j.porgcoat.2007.08.009
Eddy NO, Ebenso EE, El Nemr A, El Ashry ESH (2009) Quantum chemical study of the inhibition of the corrosion of mild steel in H2SO4 by some antibiotics. J Mol Model 15:1085–1092. https://doi.org/10.1007/s00894-009-0472-7
Alrefaee SH, Rhee KY, Verma C, Quraishi MA, Ebenso EE (2021) Challenges and advantages of using plant extract as inhibitors in modern corrosion inhibition systems: recent advancements. J Mol Liq 321:114666. https://doi.org/10.1016/j.molliq.2020.114666
El Nemr A, Moneer AA, Khaled A, El Sikaily A, Elsayed GF (2014) Modeling of synergistic halide additives effect on the corrosion of aluminum in basic solution containing dye. Mater Chem Phys 144:139–154. https://doi.org/10.1016/j.matchemphys.2013.12.034
El Nemr A, El Said GF, Khaled A, El Sikaily A, Moneer AA, Abd-El-Khalek DE (2014) Differences in corrosion inhibition of water extract of Cassia fistula L. pods and o-phenanthroline on steel in acidic solutions in the presence and absence of chloride ions. Desalin Water Treat 52(28–30):5187–5198. https://doi.org/10.1080/19443994.2013.807473
Elhebshi A, El-Deab MS, El Nemr A, Ashour I (2019) Corrosion inhibition efficiency of cysteine-metal ions blends on low carbon steel in chloride-containing acidic media. Int J Electrochem Sci 14:3897–3915. https://doi.org/10.20964/2019.03.51
Elhebshi A, El Nemr A, El-Deab MS, Ashour I (2019) CBG-HCl as a green corrosion inhibitor for low carbon steel in 0.5 M H2SO4 with and without 0.1 M NaCl. Desalin Water Treat 164:240–248. https://doi.org/10.5004/dwt.2019.24446
Elhebshi A, El Nemr A, El-Deab MS, Ashour I, Ragab S (2021) Inhibition of copper alloy corrosion using CBG-HCl as a green inhibitor in 0.5 M H2SO4 solution. Desalin Water Treat 242:106–116
Deyab MA, Mohsen Q (2021) Corrosion mitigation in desalination plants by ammonium-based ionic liquid. Sci Rep 11:21435. https://doi.org/10.1038/s41598-021-00925-z
Elhebshi A, El Nemr A, El-Deab MS, Ashour I, Ragab S (2022) Synergistic effect of chitosan biguanidine hydrochloride salt as a green inhibitor for stainless steel alloy corrosion in a 0.5 M H2SO4 solution. Egypt J Chem 65(2):389–398. https://doi.org/10.21608/EJCHEM.2021.87235.4219
Abdullah RS, El Nemr A, El-Sakka SSA, El-Hashash MA, Soliman MH (2021) Synthesis of phthalazinones with amino or hydrazide moiety as corrosion inhibitors of low carbon steel in 0.5 M H2SO4. ChemistrySelect 6:10637–10647. https://doi.org/10.1002/slct.202102513
Obot IB, Meroufel A, Onyeachu IB, Alenaz A, Sorour A (2019) Corrosion inhibitors for acid cleaning of desalination heat exchangers: progress, challenges and future perspectives. J Mol Liq 296:111760. https://doi.org/10.1016/j.molliq.2019.111760
Ukpe RA (2019) Joint effect of ethanol extract of orange peel and halides on the inhibition of the corrosion of aluminum in 0.1 M HCl: an approach to resource recovery. Commun Phys Sci 4(1–2):118–132
Loto RT, Morenikeji T, Charles IA (2020) Data on the corrosion inhibition effect of 2-meracaptobenzothiazole on 316 austenitic stainless steel, low carbon steel and 1060 aluminium in dilute acid media. Chem Data Collect 26:100349. https://doi.org/10.1016/j.cdc.2020.100349
Solmaz R, Şahin EA, Döner A, Kardaş G (2011) The investigation of synergistic inhibition effect of rhodanine and iodide ion on the corrosion of copper in sulphuric acid solution. Corros Sci 53(10):3231–3240. https://doi.org/10.1016/j.corsci.2011.05.067
Perkasa DE, Qonita A (1825) Soegijono B (2021) Influence of heat treatment on crystal structure and corrosion properties of Co–Cr–Mo–Al alloy in simulated body fluid solution. J Phys Conf Ser 1:012055. https://doi.org/10.1088/1742-6596/1825/1/012055
Labjar N, Lebrini M, Bentiss F, Chihib NE, El Hajjaji S, Jama C (2010) Corrosion inhibition of carbon steel and antibacterial properties of aminotris-(methylenephosphonic) acid. Mater Chem Phys 119(1–2):330–336. https://doi.org/10.1016/j.matchemphys.2009.09.006
Okafor PC, Liu X, Zheng YG (2009) Corrosion inhibition of mild steel by ethylamino imidazoline derivative in CO2-saturated solution. Corros Sci 51(4):761–768
Albrimi YA, Addi AA, Douch J, Souto RM, Hamdani M (2015) Inhibition of the pitting corrosion of 304 stainless steel in 0.5 M hydrochloric acid solution by heptamolybdate ions. Corros Sci 90:522–528
Chakravarthy MP, Mohana KN, Kumar CP (2014) Corrosion inhibition effect and adsorption behaviour of nicotinamide derivatives on mild steel in hydrochloric acid solution. Int J Ind Chem 5(2):1–21
Chkirate K, Azgaou K, Elmsellem H, El Ibrahimi B, Sebbar NK, Benmessaoud M, El Hajjaji S, Essassi EM (2021) Corrosion inhibition potential of 2-[(5-methylpyrazol-3-yl) methyl] benzimidazole against carbon steel corrosion in 1 M HCl solution: combining experimental and theoretical studies. J Mol Liq 321:114750
Hamadi L, Kareche A, Mansouri S, Benbouta S (2020) Corrosion inhibition of Fe–19Cr stainless steel by glutamic acid in 1 M HCl. Chem Data Collect 28:100455
Verma C, Olasunkanmi LO, Ebenso EE, Quraishi MA, Obot IB (2016) Adsorption behavior of glucosamine-based, pyrimidine-fused heterocycles as green corrosion inhibitors for mild steel: experimental and theoretical studies. J Phys Chem C 120(21):11598–11611
Faiz M, Zahari A, Awang K, Hussin H (2020) Corrosion inhibition on mild steel in 1 M HCl solution by Cryptocarya nigra extracts and three of its constituents (alkaloids). RSC Adv 10(11):6547–6562
Haque J, Mazumder MAJ, Quraishi MA, Ali SA, Aljeaban NA (2020) Pyrrolidine-based quaternary ammonium salts containing propargyl and hydrophobic C-12 and C-16 alkyl chains as corrosion inhibitors in aqueous acidic media. J Mol Liq 320:114473
Mas Faiz MRH (2019) Investigation of Cryptocarya nigra extracts as a green corrosion inhibitor on mild steel and its phytochemical studies/Mas Faiz Mas Rosemal Hakim. Doctoral Dissertation, University of Malaya
Yurt AYSEL, Ulutas S, Dal H (2006) Electrochemical and theoretical investigation on the corrosion of aluminium in acidic solution containing some Schiff bases. Appl Surf Sci 253(2):919–925. https://doi.org/10.1016/j.apsusc.2006.01.026
Yıldırım A, Cetin M (2008) Synthesis and evaluation of new long alkyl side chain acetamide, isoxazolidine and isoxazoline derivatives as corrosion inhibitors. Corros Sci 50(1):155–165. https://doi.org/10.1016/j.corsci.2007.06.015
Nasser AJA, Sathiq MA (2017) Comparative study of N-[(4-methoxyphenyl)(morpholin-4-yl) methyl] acetamide (MMPA) and N-[morpholin-4-yl (phenyl) methyl] acetamide (MPA) as corrosion inhibitors for mild steel in sulfuric acid solution. Arab J Chem 10:S261–S273. https://doi.org/10.1016/j.arabjc.2012.07.032
Umoren SA (2016) Biomaterials for corrosion protection: evaluation of mustard seed extract as eco-friendly corrosion inhibitor for X60 steel in acid media. J Adhes Sci Technol 30(17):1858–1879. https://doi.org/10.1080/01694243.2016.1168339
Abdallah M, Radwan MA, Shohayeb SM, Abdelhamed S (2010) Use of some natural oils as crude pipeline corrosion inhibitors in sodium hydroxide solutions. Chem Technol Fuels Oils 46(5):354–362. https://doi.org/10.1007/s10553-010-0234-3
Taha AA, Abouzeid FM, Elsadek MM, Habib FM (2020) Effect of methanolic plant extract on copper electro-polishing in ortho-phosphoric acid. Arab J Chem 13(8):6606–6625. https://doi.org/10.1016/j.arabjc.2020.06.017
Ben Harb M, Abubshait S, Etteyeb N, Kamoun M, Dhouib A (2020) Olive leaf extract as a green corrosion inhibitor of reinforced concrete contaminated with seawater. Arab J Chem 13(3):4846–4856. https://doi.org/10.1016/j.arabjc.2020.01.016
Bilgiç S (2002) The inhibition effects of benzoic acid and salicylic acid on the corrosion of steel in sulfuric acid medium. Mater Chem Phys 76(1):52–58. https://doi.org/10.1016/S0254-0584(01)00521-1
Fazal MA, Haseeb ASMA, Masjuki HH (2010) Comparative corrosive characteristics of petroleum diesel and palm biodiesel for automotive materials. Fuel Process Technol 91(10):1308–1315. https://doi.org/10.1016/j.fuproc.2010.04.016
Berchmans LJ, Iyer SV, Sivan V, Quraishi MA (2001) 1,2,4,5 Tetrazo spiro (5, 4) decane-3 thione as a corrosion inhibitor for arsenical aluminium brass in 3.5% NaCl solution. Anti-corros Methods Mater. https://doi.org/10.1108/EUM0000000006259
Junaedi S, Kadhum AAH, Al-Amiery AA, Mohamad AB, Takriff MS (2012) Synthesis and characterization of novel corrosion inhibitor derived from oleic acid: 2-amino 5-oleyl-1,3,4-thiadiazol (AOT). Int J Electrochem Sci 7(4):3543–3554
Abbas MA, Bedair MA, El-Azabay OE, Gad ES (2021) Anticorrosion effect of ethoxylate sulfanilamide compounds on carbon steel in 1 M hydrochloric acid: electrochemical and theoretical studies. ACS Omega 6(23):15089–15102. https://doi.org/10.1021/acsomega.1c01274
El Ashry ESH, El Nemr A, Ragab S (2012) Quantitative structure activity relationships of some pyridine derivatives as corrosion inhibitors of steel in acidic medium. J Mol Model 18:1173–1188. https://doi.org/10.1007/s00894-011-1148-7
Ameh PO, Eddy NO (2018) Theoretical and experimental investigations of the corrosion inhibition action of Piliostigma thonningii extract on mild steel in acidic medium. Commun Phys Sci 3(1):27–42
Eddy NO, Awe F, Ebenso EE (2010) Adsorption and inhibitive properties of ethanol extracts of leaves of Solanum melongena for the corrosion of mild steel in 0.1 M HCl. Int J Electrochem Sci 5:1996–2011
Eddy NO, Ita BI, Dodo SN, Paul ED (2011) Inhibitive and adsorption properties of ethanol extract of Hibiscus sabdariffa calyx for the corrosion of mild steel in 0.1 M HCl. Green Chem Lett Rev 5(1):43–53. https://doi.org/10.1080/17518253.2011.578589
Zhou L et al (2018) Experimental and theoretical investigations of 1,3,5-tris(4-aminophenoxy)benzene as an effective corrosion inhibitor for mild steel in 1 M HCl. J Mol Liq 249:179–187. https://doi.org/10.1016/j.molliq.2017.10.129
Daoud D, Douadi T, Issaadi S, Chafaa S (2014) Adsorption and corrosion inhibition of new synthesized thiophene Schiff base on mild steel X52 in HCl and H2SO4 solutions. Corros Sci 79:50–58. https://doi.org/10.1016/j.corsci.2013.10.025
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors are grateful to Egypt's Science and Technological Development Fund (STDF) for partial financial support for this work (Project No. CB-4874).
Author information
Authors and Affiliations
Contributions
NAB was in charge of conducting the experiment and writing the original manuscript, while AEN offered a topic for investigation, oversaw the research, finalized it, and then submitted it to the journal for publication. Several members of the research team, including BAAEN, HAF and SR, supervised the work and reviewed the manuscript, in addition to assisting with the experimental work, and NOE conducted the quantum research and read the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest regarding the publication of this article.
Ethical approval
No human or animals were used in the present research.
Consent for publication
All authors read and approved the final manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barghout, N.A., El Nemr, A., Abd-El-Nabey, B.A. et al. Use of orange peel extract as an inhibitor of stainless steel corrosion during acid washing in a multistage flash desalination plant. J Appl Electrochem 53, 379–399 (2023). https://doi.org/10.1007/s10800-022-01772-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01772-0