Abstract
Graves ophthalmopathy (GO), which occurs in autoimmune thyroid disease, can reduce patients’ quality of life due to its impact on visual function, physical appearance, and emotional health. Corticosteroids have been the first-line treatment for GO. More recently, the pathogenesis of GO has made significant progress. Various targeting biological agents and immunosuppressive agents make GO management more promising. Fully understanding GO pathogenesis and precise clinical management are beneficial for the prognosis of patients. Therefore, we conducted a comprehensive review of the medical management of GO and summarized research developments to highlight future research issues.
Similar content being viewed by others
Introduction
Graves ophthalmopathy (GO) is also known as thyroid-associated ophthalmopathy (TAO), thyroid eye disease (TED), or Graves orbitopathy. GO is mostly a thyroid-related organ autoimmune disease. It mainly occurs in patients with hyperthyroidsm and can also be accompanied by euthyroidism, subclinical or overt hypothyroidism [1]. The prevalence rate of GO in Europe is around 90–155/100,000 [2]. The proportion of Graves’ patients with all grades of GO, particularly those with severe disease, appears to decline over time [3]. Women are more prone to the GO than men. All ages could suffer from GO, but the incidence peaks between 30 and 50 years old [4]. The histopathologic changes such as immune cell infiltration, hyaluronan deposition, and lipogenesis result in orbital tissue expansion and muscle hypertrophy. The characteristic manifestations, including eyelid contracture, proptosis, and even sight loss, burden society and families and seriously endanger people’s health and quality of life [5]. This review provides a concise overview of GO, focusing on the causes and recent advances in drug therapy.
Methods
We conducted structured searches on GO in PubMed and Web of Science. The search strategy included keywords, i.e., Graves ophthalmopathy, thyroid-associated ophthalmopathy, thyroid eye disease, Graves orbitopathy, diagnosis, and treatment. The related references until July 2022 are also traced. Since some diagnoses were published in the 1960s, the past 40 years’ studies are all included. Most of the literature cited for GO medical management has been published in recent five years. Only English literature has been included.
Pathogenesis
Immune pathway
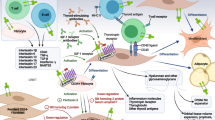
GO can be divided into progressive and stable phases, including orbital tissue inflammation, expansion, remodeling, and fibrosis in succession. The GO pathogenesis is shown in Fig. 1. The progressive phase involves inflammation and infiltration of orbital tissue by Type 1 T helper (Th 1) cells, B-lymphocytes, mast cells, and macrophages. B‑cells migrate to the orbit and are activated by recognizing TSHR or IGF‑1R and bound by the T‑cells. B‑cells are further activated by interleukin (IL) 4 and differentiate into plasma cells that produce autoantibodies. The infiltrated immune cells interact with the orbital fibroblasts (OFs) either via cellular interactions, e.g., CD40-CD154 ligation, or via various cytokines and growth factors, e.g., cytokines, chemokines, and stimulatory autoantibodies (GD-IgG) [6]. This leads to the activation of OFs, which express relatively high levels of functional thyrotropin receptor (TSHR) and insulin-like growth factor 1 receptor (IGF-1R) [7]. OFs can be divided into two main subgroups according to the expression of cell surface marker Thy-1 (CD90): CD90 + OFs, which are prone to differentiate into myofibroblasts, and CD90‑ OFs, which are mostly transformed into adipocytes [6]. When stimulated by different types of cytokines, the balance of CD90‑ OFs and CD90 + OFs determines whether the pathological changes are adipogenesis or fibrosis, leading to orbital fat expansion or fibrosis, and the symptoms, signs, and disease course of patients will also be different [8]. The extracellular matrix synthesis (especially hyaluronan) also contributes to tissue expansion and remodeling [6].
Graves Ophthalmopathy pathogenesis. Autoreactive T cells interact with orbital fibroblasts (OFs) via CD40:CD154 ligation. B cells differentiate into plasma cells, which synthesize and secrete TRAb. TRAb can mediate the autoimmune response of GO by recognizing TSHR. Activated OFs then secrete various inflammatory cytokines. TSHR and IGF-1R are expressed in the common area of the OFs membrane and exist in the form of a complex which plays a synergistic role in the functional regulation of orbital fibroblasts. Activated OFs can differentiate into either myofibroblasts or adipocytes and promote hyaluronan synthesis. As a result, orbital soft tissue expansion and congestion in GO. Created with BioRender.com
Oxidative stress
Reactive oxygen species (ROS) are produced during cell metabolism, including superoxide and hydrogen peroxide (H2O2). The over-formed ROS will be eliminated through enzymatic and non-enzymatic mechanisms during transferring one electron from the redox donor to molecular oxygen (O2) [9]. Oxidative stress occurs when the balance between ROS production and elimination is disrupted, resulting in peroxide and free radicals’ accumulation, subsequent causing remarkable damage to proteins, lipids, membranes, and nucleic acids in cells, and ultimately leading to mitochondrial dysfunction and a loss of enzymatic activity [10].
GO is in a status of high oxidative stress, which promotes the occurrence and development of diseases through its effect on orbital fibroblasts. Oxidative stress can enhance the patients’ inflammatory response by promoting the proliferation of orbital fibroblasts and the expression of inflammatory mediators [11]. The inflammatory response will further aggravate oxidative stress and keep periorbital tissues in a state of inflammation. The oxidative stress response of GO will accelerate the exhaustion of the enzymatic antioxidant and further reduce the ability to resist oxidative stress. In addition, the thyroid hormone overproduction of GD also leads to a hypermetabolic state with increased oxygen and intracellular adenosine triphosphate (ATP) consumption and ROS generation, which in turn damages thyrocytes that exacerbate autoimmune reactions as well as extrathyroidal tissues [12, 13].
Risk factors
The risk factors of GO can be divided into endogenous (unmodifiable) and exogenous (modifiable). Endogenous factors include age, race, gender, and genetics. Exogenous factors include thyroid dysfunction, smoking, radioactive iodine (RAI), hypercholesterolemia, oxidative stress, and diabetes mellitus (DM). The multigenic conditions, gene–gene, and gene-environment interactions should be considered to affect the development of GO [14].
Endogenous factors
GO is known to be a multifactorial and polygenic disease. Currently, thyroid-specific genes (e.g., the TSH-R gene) and sensitivity genes, e.g., human leucocyte antigen (HLA) and cytotoxic T-lymphocyte antigen-4 (CTLA-4), are closely related to the occurrence and progression of GO. Lahooti et al. also found that the CASQ1 gene polymorphism is related to GO, and the CASQ1 gene expression is significantly up-regulated in the thyroid tissue of GO patients [15]. Several genes were discovered to be differentially expressed in OFs from GO patients, but the relative importance of each gene has yet to be determined [16].
Exogenous factors
Thyroid dysfunction
Both hyper and hypothyroidism can influence the severity of GO [17, 18]. Euthyroidism should be promptly restored and stably maintained in all GO patients [19]. Thyrotropin receptor antibody (TRAb) level is an independent risk factor of GO. TRAb is divided into three groups according to its interaction with the TSH-R [20], and current evidence indicates that thyroid-stimulating antibodies (TSAb) are more closely associated with GO [21, 22]. TSAb enters the bloodstream and induces differentiation of OFs and secretion of hydrophilic glycosaminoglycans (GAG), leading to edema and later fibrosis [23]. Accordingly, great attention should be paid to ocular changes in GD patients with high titers of TSAb. TRAb is significantly higher in GO patients than in non-GO patients. Eckstein et al. followed 159 GO patients for 12–24 months and described that the TRAb trigger and constantly maintain the autoimmune process in orbit [24]. The continuous measurement of TRAb could prevent more severe clinical outcomes.
Smoking
Smoking is considered to be the most significant exogenous risk factor for GO. Hägg and Asplund et al. firstly proposed a direct relationship between smoking and severe GO in 1987 [25]. In addition, the amount of tobacco, even past smoking, is related to the severity of the disease in GO patients [26]. Compared to nonsmokers, active smokers suffer from a higher degree of GO severity and higher rates of poor response to therapies [27, 28]. Smoking might induce tissue remodeling through hypoxia, oxygen-free radical generation, and stimulation of adipogenesis [29].
Radioiodine treatment
RAI is used alone or with other medications or surgery to treat GO. However, hypothyroidism may occur after radiation exposure by autoimmune thyroiditis and thyroid atrophy [30]. RAI was verified to be associated with progression or worsening of GO [31], but these are often transient [32] and can be prevented with prophylactic glucocorticoids [33]. The 2021 EUGOGO guidelines recommended 0.3–0.5 mg/kg/body weight as starting dose, tapered, and withdrawn after three months for RAI-treated patients at high risk of progression or de novo development; 0.1–0.2 mg/kg/bodyweight, tapered and withdrawn after six weeks for low-risk patients [19]. Steroid cover is unnecessary for the inactive GO if hypothyroidism is avoided and other GO progression risk factors, particularly smoking and high serum TSHR-Ab titers, are absent.
Hypercholesterolemia
Recent studies showed that the CAS was higher in patients with high cholesterol, suggesting a role of cholesterol itself in the development of GO [34,35,36]. Hypercholesterolemia is related to oxidative stress and the release of proinflammatory cytokines, resulting in systemic proinflammatory actions [35]. In addition, hypercholesterolemia can also lead to tissue inflammation by activating Toll-Like Receptor 4-Myeloid Differentiation Factor 2 (TLR4–MD2) [37].
Clinical manifestation and diagnosis
General clinical manifestation of GO includes exophthalmos, lid retractions, diplopia, and decreased visual acuity. In severe conditions, pain, cornea exposure, dysthyroid optic neuropathy (DON), and vision loss can also be found [38]. DON may also result in characteristic changes in color vision, contrast sensitivity, pupillary examination, and automated visual field perimetry [39]. For GO diagnosis, visual acuity, slit lamp, ultrasound, CT, and MRI scan can be helpful. In addition, the high concentration of proteins in tear fluid makes tear sampling and testing an important non-invasive technique to investigate the mechanism and diagnose GO [40]. Tear levels of some proteins were significantly elevated in GD patients with moderate/ severe GO compared to GD without GO [41, 42]. Numerous studies have also proposed potential protein biomarkers for GO, allowing the early diagnosis and progress prediction of the GO [43, 44]. After diagnosing, physicians should assess the clinical disease activity by Clinical Activity Score (CAS) [19, 45] and severity by NOSPECS [46, 47], VISA [19, 48], and European Group on Graves Orbitopathy (EUGOGO) classification [49, 50] (Table 1).
Management
The management of GO depends on the patients’ symptoms and severity, consisting of fundamental, medical, and surgical treatments. The ideal treatment goal is to decrease the risk of visual complications, minimize side effects, eliminate the demand for surgical interventions, restore thyroid function and avoid progression or recurrence of GO.
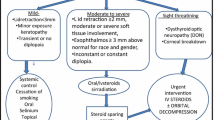
The early diagnosis and preventive management, i.e., removal of modifiable risk factors, may reduce the prevalence and change the clinical manifestations of GO. Generally, fundamental treatments are recommended regardless of the severity, i.e., artificial tears (ointment are needed when corneal exposure occurs), dark glasses, parasol, raising the pillow at night, and controlling risk factors [51]. Most mild GO patients have no progressive development of the affected extraocular muscles, and a wait-and-see strategy is feasible. A minority of mild GO patients may be suggested for low-dose immunomodulation if GO is active. Moderate-to-severe and active GO patients with obvious symptoms and active disease are mainly treated with medical or orbital irradiation therapy. For DON, it is necessary to be treated with a high dose of IVMP immediately. If patients are non-responsive or intolerant within two weeks, urgent orbital decompression is recommended to be performed. If DON is improved or resolved after two weeks, weekly IVMP should be continued to manage moderate-to-severe and active GO.
In 20–30% of GO patients, rehabilitative surgeries are needed to correct severe signs [52]. Elective rehabilitative surgery is suitable for improving the visual function and QoL after GO has been inactive for at least six months. When more than one surgical procedure is required, orbital decompression surgery, eye muscle, and eyelid surgery should be performed in the given order.
Nonspecific therapies
Antioxidants
Selenium (Se), a common antioxidant, can decompose excessively generated ROS and play a protective role against large-scale oxidative stress. Se is crucial in the antioxidant defense system and maintains a stable redox state. Se could prevent necrosis and apoptosis by counteracting the proliferation of orbital fibroblasts induced by H2O2 and inhibiting the release of proinflammatory cytokines and hyaluronic acid [53]. Se supplementation is shown to reduce the impact of high oxidative stress levels in GO patients by reducing the level of TRAb, thyroglobulin antibody (TGAb), and thyroid peroxidase antibody (TPOAb) [54]. A multicenter, double-blind clinical trial proved that the course of GO could be improved after six months’ continuous Se supplementation (100mcg twice a day), and the treatment effects were still maintained after six months [55]. However, the patients included are all in the selenium-deficient area. Se status was rarely evaluated in the clinic before the Se supplementation [56]. The action of selenium for the long-term outcome and whether the same beneficial effects can be found in regions without deficient selenium remains to be well cleared [57].
In addition to Se, many antioxidants are available in nature, such as quercetin, vitamin C, N-acetyl-l-cysteine, melatonin, and β-carotene, and exert functions in reducing the level of oxidative stress. Quercetin has anti-proliferative properties and can reduce orbital fibroblasts proliferation via necrosis and hyaluronic acid (HA) release. However, quercetin may sustain and worsen the autoimmune reaction because further exposure to endogenous antigens worsens the prognosis [58]. Vitamin C and N-acetyl-l-cysteine reduced cell proliferation in GO. N-Acetyl-l-cysteine can also reduce the release of HA, IFNγ, and IL1β, but TNFα is unaffected [59]. Melatonin may not be a strong candidate, considering it cannot affect fibroblast proliferation, but it lacks toxicity and affects fibroblast functions, i.e., HA and cytokines release. It may incorporate other antioxidants in a mixture to be administered to GO patients [59]. β-carotene, displaying antioxidant, anti-inflammatory, and antiproliferation effects, seems promising [60] but needs to be further analyzed in clinical practice.
Corticosteroids
Corticosteroids have been the mainstay treatment since the 1950s but are often less effective and associated with side effects, e.g., cushingoid features, weight gain, palpitation, myalgia, hypertension, liver damage, and cardiovascular and cerebrovascular events [61, 62]. Ocular steroid injection avoids systemic complications but can cause local complications, e.g., globe perforation, toxic ON, lower eyelid herniation of orbital fat, or arterial occlusion [63]. IVMP has the advantages of better efficacy and lower incidence of adverse reactions than OGC. The side effects of oral treatment can be minimized by IVMP [64]. Stiebel-Kalish and coworkers evaluated 1367 patients from 33 trials and concluded that IVMP has a small but statistically significant advantage in treatment effects and causes fewer adverse events over oral glucocorticoids (OGC) [65]. Gao et al. also reported that intravenous glucocorticoid pulse (IVGC) was markedly more effective than OGC, and IVGC + orbital radiation was more effective than OGC + orbital radiation [66].
As already recommended in the 2021EUGOGO guideline [19], the combination of a moderate cumulative dose of IVMP (0.5 g/week/6 weeks) and a moderate daily dose of mycophenolate sodium(0.72 g/day/6 weeks)is recommended as the first line of treatment. Alternative treatment is a high single dose of IVMP (0.75 g/week/6 weeks). If the response is poor or absent, second-line treatments can be planned, i.e., the second course of GCs, orbital radiotherapy with oral or intravenous glucocorticoids, the combination of azathioprine, or cyclosporine with oral prednisone, teprotumumab, tocilizumab, or rituximab (RTX). In addition, monthly monitoring during the treatment is necessary. Patients should also be screened for recent hepatitis, liver function, severe hypertension, inadequately managed diabetes, cardiovascular morbidity, and glaucoma before IVMP.
Mycophenolate mofetil
Mycophenolic acid (MPA), a selective immunosuppressant, inhibits inosine monophosphate dehydrogenase, blocks DNA synthesis, and has cytostatic effects on B and T cells [67]. MPA can inhibit the differentiation and generation of T lymphocytes and the synthesis of immune markers on the surface of immune cells. If the cell metabolism and proliferation are active, the inhibitory effect of MPA will be vital. Hence, it has a strong target for the treatment of GO. Ye and collaborators evaluated and compared the efficacy and safety of GCs to MMF in 174 patients with active moderate-to-severe GO. Patients are treated with IVMP at 0.5 g per day for three consecutive days a week for two weeks, then oral prednisone at 60 mg daily for eight weeks, followed by a gradual reduction plan of 5 mg per week for 14 weeks. The mycophenolate mofetil (MMF) group found a more significant overall response rate (91.3% vs. 67.9%, p < 0.001), a better CAS response (92.5% vs. 70.5% improvement, p < 0.05), and a significant improvement rate of diplopia and proptosis (90.4% and 68.8% improvement, respectively), indicating that MMF has a particular effect on active GO [68]. The add-on mycophenolate benefit was also detected in ophthalmic symptoms and signs at 24 weeks and 36 weeks by post-hoc analysis in patients with active and moderate-to-severe GO in a multicenter, observer-masked trial [69]. However, its widespread use in clinics is restricted because the cost of MMF oral capsules is relatively high (an oral capsule of 250 mg is around $51). In addition, MMF cause dose-dependent gastrointestinal (GI) side effects, including ulcers, diarrhea, and nausea [70]. Enteric-coated mycophenolate sodium (EC-MPS), a delayed-release formulation, improves GI tolerability [71, 72].
Cyclosporine
Cyclosporine can inhibit the activation of cytotoxic T lymphocytes. It also can reduce the antigen expression of monocytes and macrophages, activating inhibitory T lymphocytes and further inhibiting the production of cytokines. Furthermore, cyclosporine has a specific synergistic effect combined with oral GCs, reducing hormone resistance, and continuing its anti-inflammatory effects after the cessation of glucocorticoids. Concerning ocular outcome and recurrence rate, cyclosporine combined with GCs can achieve a better curative rate than cyclosporine alone [73]. Since the adverse effects of cyclosporine (dose-dependent liver and renal toxicities, lung damage, and hypertension, among others) cannot be overlooked [74], cyclosporine is generally recommended to be administered in combination with oral GCs rather than itself alone.
Azathioprine
Azathioprine, an anti-proliferative medicine, has been used as a steroid-sparing therapy. A post hoc analysis illustrates that azathioprine might improve 48-week clinical outcomes in combination with glucocorticoids to treat active moderate-to-severe GO [75]. It suggests that azathioprine may potentially prevent the long-term recurrence of GO after steroid discontinuation. Azathioprine-related adverse effects, including bone marrow suppression, nausea, and vomiting, have been extensively studied because of its widely used in many other autoimmune diseases [76, 77].
Statins
The effects of statins could be attributed to their ability to decrease cholesterol, pleiotropic anti-inflammatory actions, interact with methylprednisolone, and influence tissue remodeling [35, 78]. The use of statins is considered to increase the effectiveness of the immunosuppressive medication, decreasing the dosage of GC and thereby reducing their side effects. The results of both basic and clinical studies on the impact of statins on GO are promising [79,80,81]. A large register-based study examined the effect of statin therapy on the incidence of GO among 34,894 newly diagnosed GD and reported that statins, instead of other lipid-lowering agents, may protect against the development of GO [81]. A recent phase 2 randomized clinical trial suggested that the combination of atorvastatin and ivGCs regimen improves the outcome of moderate-to-severe active GO [80]. However, a phase 3 clinical trial with a longer follow-up is still needed before introducing statins into the clinic.
Targeted molecular therapies
Tumor Necrosis Factor α Inhibitors
GO pathogenesis is related to the up-regulation of proinflammatory cytokines, e.g., tumor necrosis factor-α (TNF-α). TNF-α antagonists, e.g., imatinib mesylate, adalimumab, etanercept, thalidomide, and infliximab [82], may be used as steroidal protective drugs to treat GO. Ten mild-to-moderate GO patients were subcutaneously injected with etanercept 25 mg twice weekly. After three months of treatment, the mean CAS decreased from 4 to 1.6 (60%). However, three patients developed recurrence of GO after discontinuation of the etanercept [83]. Van Steensel et al. studied the potential efficacy of imatinib mesylate and adalimumab through a whole orbital tissue culture system [84]. The results showed that imatinib mesylate reduces inflammation and tissue remodeling. Adalimumab can reduce IL-6 production but is ineffective enough to affect orbital hyaluronan production. A case series showed a reduced inflammatory response in 6/12 patients [85]. No improvement of proptosis or motility was reported. Further RCTs are necessary to determine the efficacy of TNF-α inhibitors in GO management.
Rituximab
RTX is a monoclonal antibody against the human CD20 antigen. RTX inhibits B-cell activation and mediates specific decreases in thyroid-peroxidase antibody and IgM levels [86, 87]. RTX is a relatively safe and feasible treatment method in moderate to severe GO. It can significantly reduce the CAS after treatment, especially after previous corticosteroid failure [49]. The minor adverse effects, including fever, throat itching, infusion reaction, and nausea, can be relieved by slowing down RTX infusion or intravenous hydrocortisone. Several studies have investigated the efficacy of RTX in GO management. The outcomes are not entirely consistent. Stan et al. concluded that RTX in the placebo-controlled trial is not efficacious [88]. Salvi et al. found RTX quite effective in patients with moderate to severe GO in better ocular mobility, visual function, and decreased surgery demand [89]. Li et al. also reported the promising prospect of RTX combined with orbital radiation. The noticeable baseline inconsistency related to differences in subjects’ age, gender, and smoking history may contribute to the discrepancy. According to a study by Eid et al., RTX only offered limited and partial improvement in active moderate-to-severe GO patients with a long duration of disease. Response to RTX therapy appears to be significantly influenced by GO duration. Even though it is not advised as a first-line treatment, RTX appears to have the potential to control active moderate-to-severe GO, especially when applied in the early active stage of the disease [90]. RTX can be used as one of several options for patients who are not sensitive to or do not respond to IVMP.
Tocilizumab
Tocilizumab, a recombinant humanized monoclonal anti-IL-6 receptor inhibitor, is used to treat inflammatory and autoimmune conditions. It was first described in the literature as a humanized anti-interleukin (IL)-6 receptor monoclonal antibody MRA Chugai in 2003 [91]. The first study on tocilizumab was a prospective interventional nonrandomized study with 18 steroid-resistant GO patients in 2014. Improvement occurred after intravenous tocilizumab treatment with a mean CAS score reduction of 5.89 ± 1.41 points, and the mean TSI (thyroid-stimulating immunoglobulin) levels were significantly lower. All adverse events reported were mild, with mostly minor events such as tiredness, neutropenia, upper respiratory tract infection, and slight elevation of liver enzymes [92]. The first RCT with tocilizumab, in 2018, double-masked, showed 93.3% of tocilizumab-treated patients improved at least 2 points by CAS, and greater improvement in EUGOGO score and a more considerable reduction of exophthalmos compared to placebo. One patient experienced a moderately raised transaminase, and another experienced acute pyelonephritis [93]. Tocilizumab can also be injected subcutaneously, which improves patient compliance and convenience [94]. More large sample size clinical trials are needed to verify the efficacy of tocilizumab in active, moderate-to-severe GO patients.
Teprotumumab
Teprotumumab, an insulin-like growth factor-1 receptor inhibitor, reduces the TSH-stimulated proinflammatory cytokines secretion of isolated fibrocytes [95] and significantly decreases the expression of TSH-R and IGF-1R in fibrocytes [96]. Teprotumumab significantly improved ptosis, diplopia, CAS score, and quality of life in moderate-to-severe GO and showed a favorable safety profile [97, 98]. A randomized, double-blind, placebo-controlled trial was conducted in 22 centers worldwide in 2017. The results showed that teprotumumab could improve proptosis and reduce CAS in moderately to severely active GO patients with only 5% complications incidence [97]. A placebo-controlled teprotumumab phase III clinical trial showed exophthalmos has decreased by ≥ 2 mm in 82.9% moderately to severely active GO at the 24th week [98]. Due to disease-modifying properties, teprotumumab was approved “breakthrough therapy” designation by the U.S. Food and Drug Administration (FDA) in 2020, which marks a significant milestone for GO management. Although the novel drug teprotumumab is promising, we still don’t entirely understand its indications and limitations [99]. The problems related to the high price [100], the durability of the favorable response [101], and the safety of teprotumumab should be solved. Complete recording and reporting of the actual incidence of the adverse event, including muscle spasms, nausea [98], hyperglycemia, and hearing impairment [102, 103], are warranted. Accordingly, it is not recommended to expand the therapeutic indications without rigorous clinical trial evidence demonstrating the efficacy and safety [104].
Conclusions
Early intervention of GO may alter the disease course and significantly improve long-term outcomes. Only by fully understanding the complex cytokine network of GO pathogenesis and cross-integrating with immunology and pharmacology can we break through the bottleneck and benefit patients. Researchers have made headway in GO mechanisms and immunosuppressants in recent years, bringing new hope for GO management. The clinical research process of GO medicine is different, and further studies are merited for their safety and efficacy.
Availability of data and materials
Not applicable.
References
Ponto KA et al (2015) Prevalence, Phenotype, and Psychosocial WellBeing in Euthyroid/Hypothyroid Thyroid-Associated Orbitopathy. Thyroid 5(8):942–948. https://doi.org/10.1089/thy.2015.0031
Perros P et al (2017) Graves’ orbitopathy as a rare disease in Europe: a European Group on Graves’ Orbitopathy (EUGOGO) position statement. Orphanet J Rare Dis 12(1):72. https://doi.org/10.1186/s13023-017-0625-1
Piantanida E et al (2013) Prevalence and natural history of Graves’ orbitopathy in the XXI century. J Endocrinol Invest 36(6):444–449. https://doi.org/10.3275/8937
Smith TJ, Hegedüs L (2016) Graves’ Disease. N Engl J Med 375(16):1552–1565. https://doi.org/10.1056/NEJMra1510030
Ponto KA et al (2013) Public health relevance of Graves’ orbitopathy. J Clin Endocrinol Metab 98(1):145–152. https://doi.org/10.1210/jc.2012-3119
Dik WA, Virakul S, van Steensel L (2016) Current perspectives on the role of orbital fibroblasts in the pathogenesis of graves’ ophthalmopathy. Exp Eye Res 142:83–91. https://doi.org/10.1016/j.exer.2015.02.007
Krieger CC et al (2016) TSH/IGF-1 receptor cross talk in graves’ ophthalmopathy pathogenesis. J Clin Endocrinol Metab 101(6):2340–2347. https://doi.org/10.1210/jc.2016-1315
Wang Y, Smith TJ (2014) Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci 55(3):1735–1748. https://doi.org/10.1167/iovs.14-14002
Shadel GS, Horvath TL (2015) Mitochondrial ROS signaling in organismal homeostasis. Cell 163(3):560–569. https://doi.org/10.1016/j.cell.2015.10.001
Diana T et al (2018) Stimulatory TSH-receptor antibodies and oxidative stress in graves disease. J Clin Endocrinol Metab 103(10):3668–3677. https://doi.org/10.1210/jc.2018-00509
Van Regemorter E et al (2021) Downregulation of Caveolin-1 and upregulation of deiodinase 3, associated with hypoxia-inducible factor-1α increase, are involved in the oxidative stress of graves’ orbital adipocytes. Thyroid 31(4):627–637. https://doi.org/10.1089/thy.2020.0238
Hou TY et al (2021) The role of oxidative stress and therapeutic potential of antioxidants in graves’ ophthalmopathy. Biomedicines. https://doi.org/10.3390/biomedicines9121871
Lanzolla G, Marcocci C, Marinò M (2020) Oxidative Stress in Graves Disease and Graves Orbitopathy. Eur Thyroid J 9(Suppl 1):40–50. https://doi.org/10.1159/000509615
Du P, Ma X, Wang C (2014) Associations of CTLA4 gene polymorphisms with graves’ ophthalmopathy: a meta-analysis. Int J Genomics 2014:537969. https://doi.org/10.1155/2014/537969
Lahooti H et al (2015) Novel single-nucleotide polymorphisms in the calsequestrin-1 gene are associated with Graves’ ophthalmopathy and Hashimoto’s thyroiditis. Clin Ophthalmol 9:1731–1740. https://doi.org/10.2147/opth.S87972
RotondoDottore G et al (2021) Genetic profiling of orbital fibroblasts from patients with graves’ orbitopathy. J Clin Endocrinol Metab 106(5):e2176–e2190. https://doi.org/10.1210/clinem/dgab035
Prummel MF et al (1990) Effect of abnormal thyroid function on the severity of Graves’ ophthalmopathy. Arch Intern Med 150(5):1098–1101
Karlsson F et al (1989) Ophthalmopathy and thyroid stimulation. Lancet 2(8664):691. https://doi.org/10.1016/s0140-6736(89)90945-8
Bartalena L et al (2021) The 2021 European group on graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of graves’ orbitopathy. Eur J Endocrinol 185(4):G43–G67. https://doi.org/10.1530/eje-21-0479
George A et al (2020) Stimulatory thyrotropin receptor antibodies are a biomarker for graves’ orbitopathy. Front Endocrinol (Lausanne) 11:629925. https://doi.org/10.3389/fendo.2020.629925
Kampmann E et al (2015) Thyroid stimulating but not blocking autoantibodies are highly prevalent in severe and active thyroid-associated orbitopathy: a prospective study. Int J Endocrinol 2015:678194. https://doi.org/10.1155/2015/678194
Kahaly GJ et al (2019) High titers of thyrotropin receptor antibodies are associated with orbitopathy in patients with graves disease. J Clin Endocrinol Metab 104(7):2561–2568. https://doi.org/10.1210/jc.2018-02705
Fatourechi V (2012) Thyroid dermopathy and acropachy. Best Pract Res Clin Endocrinol Metab 26(4):553–565. https://doi.org/10.1016/j.beem.2011.10.001
Eckstein AK et al (2006) Thyrotropin receptor autoantibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab 91(9):3464–3470. https://doi.org/10.1210/jc.2005-2813
Hägg E, Asplund K (1987) Is endocrine ophthalmopathy related to smoking? Br Med J (Clin Res Ed) 295(6599):634–635. https://doi.org/10.1136/bmj.295.6599.634
Xing L et al (2015) Smoking was associated with poor response to intravenous steroids therapy in Graves’ ophthalmopathy. Br J Ophthalmol 99(12):1686–1691. https://doi.org/10.1136/bjophthalmol-2014-306463
Tanda ML et al (2013) Prevalence and natural history of Graves’ orbitopathy in a large series of patients with newly diagnosed graves’ hyperthyroidism seen at a single center. J Clin Endocrinol Metab 98(4):1443–1449. https://doi.org/10.1210/jc.2012-3873
Pfeilschifter J, Ziegler R (1996) Smoking and endocrine ophthalmopathy: impact of smoking severity and current vs lifetime cigarette consumption. Clin Endocrinol (Oxf) 45(4):477–481. https://doi.org/10.1046/j.1365-2265.1996.8220832.x
Görtz GE et al (2016) Hypoxia-dependent HIF-1 activation impacts on tissue remodeling in graves’ ophthalmopathy-implications for smoking. J Clin Endocrinol Metab 101(12):4834–4842. https://doi.org/10.1210/jc.2016-1279
Reiners C, Drozd V, Yamashita S (2020) Hypothyroidism after radiation exposure: brief narrative review. J Neural Transm (Vienna) 127(11):1455–1466. https://doi.org/10.1007/s00702-020-02260-5
Träisk F et al (2009) Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab 94(10):3700–3707. https://doi.org/10.1210/jc.2009-0747
Bartalena L et al (1998) Relation between therapy for hyperthyroidism and the course of graves’ ophthalmopathy. N Engl J Med 338(2):73–78. https://doi.org/10.1056/nejm199801083380201
Perros P et al (2005) A prospective study of the effects of radioiodine therapy for hyperthyroidism in patients with minimally active graves’ ophthalmopathy. J Clin Endocrinol Metab 90(9):5321–5323. https://doi.org/10.1210/jc.2005-0507
Sabini E et al (2018) High serum cholesterol is a novel risk factor for graves’ orbitopathy: results of a cross-sectional study. Thyroid 28(3):386–394. https://doi.org/10.1089/thy.2017.0430
Lanzolla G et al (2019) Cholesterol serum levels and use of statins in graves’ orbitopathy: a new starting point for the therapy. Front Endocrinol (Lausanne) 10:933. https://doi.org/10.3389/fendo.2019.00933
Lanzolla G et al (2018) Relationship between serum cholesterol and Graves’ orbitopathy (GO): a confirmatory study. J Endocrinol Invest 41(12):1417–1423. https://doi.org/10.1007/s40618-018-0915-z
Tall AR, Yvan-Charvet L (2015) Cholesterol, inflammation and innate immunity. Nat Rev Immunol 15(2):104–116. https://doi.org/10.1038/nri3793
Menconi F, Marcocci C, Marinò M (2014) Diagnosis and classification of Graves’ disease. Autoimmun Rev 13(4–5):398–402. https://doi.org/10.1016/j.autrev.2014.01.013
Blandford AD et al (2017) Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Rev Ophthalmol 12(2):111–121. https://doi.org/10.1080/17469899.2017.1276444
Khazaei H et al (2021) The potential of tear proteomics for diagnosis and management of orbital inflammatory disorders including Graves’ ophthalmopathy. Exp Eye Res 213:108813. https://doi.org/10.1016/j.exer.2021.108813
Aass C et al (2016) Comparative proteomic analysis of tear fluid in Graves’ disease with and without orbitopathy. Clin Endocrinol (Oxf) 85(5):805–812. https://doi.org/10.1111/cen.13122
Aass C et al (2017) Establishment of a tear protein biomarker panel differentiating between Graves’ disease with or without orbitopathy. PLoS ONE 12(4):e0175274. https://doi.org/10.1371/journal.pone.0175274
Kishazi E et al (2018) Thyroid-associated orbitopathy and tears: a proteomics study. J Proteomics 170:110–116. https://doi.org/10.1016/j.jprot.2017.09.001
Chng CL et al (2018) Tear proteins calcium binding protein A4 (S100A4) and prolactin induced protein (PIP) are potential biomarkers for thyroid eye disease. Sci Rep 8(1):16936. https://doi.org/10.1038/s41598-018-35096-x
Oeverhaus M et al (2021) Graves’ orbitopathy: current concepts for medical treatment. Klin Monbl Augenheilkd 238(1):24–32. https://doi.org/10.1055/a-1328-2884
Werner SC (1969) Classification of the eye changes of Graves’ disease. Am J Ophthalmol 68(4):646–648. https://doi.org/10.1016/0002-9394(69)91246-x
Werner SC (1977) Modification of the classification of the eye changes of graves’ disease: recommendations of the Ad Hoc committee of the American thyroid association. J Clin Endocrinol Metab 44(1):203–204. https://doi.org/10.1210/jcem-44-1-203
Dolman PJ, Rootman J (2006) VISA classification for graves orbitopathy. Ophthalmic Plast Reconstr Surg 22(5):319–324. https://doi.org/10.1097/01.iop.0000235499.34867.85
Bartalena L et al (2008) Consensus statement of the European group on graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol 158(3):273–285. https://doi.org/10.1530/eje-07-0666
Fayers T, Dolman PJ (2011) Validity and reliability of the TED-QOL: a new three-item questionnaire to assess quality of life in thyroid eye disease. Br J Ophthalmol 95(12):1670–1674. https://doi.org/10.1136/bjophthalmol-2011-300487
Vestergaard P (2002) Smoking and thyroid disorders–a meta-analysis. Eur J Endocrinol 146(2):153–161. https://doi.org/10.1530/eje.0.1460153
Kahaly GJ (2020) Management of graves thyroidal and extrathyroidal disease: an update. J Clin Endocrinol Metab 105(12):3704–3720. https://doi.org/10.1210/clinem/dgaa646
RotondoDottore G et al (2017) Selenium rescues orbital fibroblasts from cell death induced by hydrogen peroxide: another molecular basis for the effects of selenium in graves’ orbitopathy. Endocrine 58(2):386–389. https://doi.org/10.1007/s12020-016-1226-9
Xu B et al (2019) A pilot study on the beneficial effects of additional selenium supplementation to methimazole for treating patients with Graves’ disease. Turk J Med Sci 49(3):715–722. https://doi.org/10.3906/sag-1808-67
Marcocci C et al (2011) Selenium and the course of mild Graves’ orbitopathy. N Engl J Med 364(20):1920–1931. https://doi.org/10.1056/NEJMoa1012985
Negro R et al (2019) A 2018 European thyroid association survey on the use of selenium supplementation in graves’ hyperthyroidism and graves’ orbitopathy. Eur Thyroid J 8(1):7–15. https://doi.org/10.1159/000494837
Lanzolla G, Marinò M, Marcocci C (2020) Selenium in the treatment of graves’ hyperthyroidism and eye disease. Front Endocrinol (Lausanne) 11:608428. https://doi.org/10.3389/fendo.2020.608428
Lisi S et al (2011) Quercetin decreases proliferation of orbital fibroblasts and their release of hyaluronic acid. J Endocrinol Invest 34(7):521–527. https://doi.org/10.3275/7321
RotondoDottore G et al (2018) Action of three bioavailable antioxidants in orbital fibroblasts from patients with Graves’ orbitopathy (GO): a new frontier for GO treatment? J Endocrinol Invest 41(2):193–201. https://doi.org/10.1007/s40618-017-0718-7
RotondoDottore G et al (2018) Antioxidant effects of β-carotene, but not of retinol and vitamin E, in orbital fibroblasts from patients with Graves’ orbitopathy (GO). J Endocrinol Invest 41(7):815–820. https://doi.org/10.1007/s40618-017-0809-5
CORTISONE in exophthalmos: report on a therapeutic trial of cortisone and corticotrophin (A.C.T.H.) in exophthalmos and exophthalmic ophthalmoplegia by a panel appointed by the Medical Research Council. Lancet, 1955. 268(6853):6–9
Marcocci C et al (2012) Fatal and non-fatal adverse events of glucocorticoid therapy for Graves’ orbitopathy: a questionnaire survey among members of the European Thyroid Association. Eur J Endocrinol 166(2):247–253. https://doi.org/10.1530/eje-11-0779
Goldberg RA (2004) Orbital steroid injections. Br J Ophthalmol 88(11):1359–1360
Zang S, Ponto KA, Kahaly GJ (2011) Clinical review: Intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab 96(2):320–332. https://doi.org/10.1210/jc.2010-1962
Stiebel-Kalish H et al (2009) Treatment modalities for Graves’ ophthalmopathy: systematic review and metaanalysis. J Clin Endocrinol Metab 94(8):2708–2716. https://doi.org/10.1210/jc.2009-0376
Gao G et al (2014) Meta-analysis of methylprednisolone pulse therapy for Graves’ ophthalmopathy. Clin Exp Ophthalmol 42(8):769–777. https://doi.org/10.1111/ceo.12317
Allison AC, Eugui EM (2000) Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47(2–3):85–118. https://doi.org/10.1016/s0162-3109(00)00188-0
Ye X et al (2017) Efficacy and safety of mycophenolate mofetil in patients with active moderate-to-severe Graves’ orbitopathy. Clin Endocrinol (Oxf) 86(2):247–255. https://doi.org/10.1111/cen.13170
Kahaly GJ et al (2018) Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol 6(4):287–298. https://doi.org/10.1016/s2213-8587(18)30020-2
Hassan AV, Sinha MD, Waller S (2013) A single-centre retrospective study of the safety and efficacy of mycophenolate mofetil in children and adolescents with nephrotic syndrome. Clin Kidney J 6(4):384–389. https://doi.org/10.1093/ckj/sft071
Arns W et al (2005) Enteric-coated mycophenolate sodium delivers bioequivalent MPA exposure compared with mycophenolate mofetil. Clin Transplant 19(2):199–206. https://doi.org/10.1111/j.1399-0012.2004.00318.x
Jia Y et al (2018) Sites of gastrointestinal lesion induced by mycophenolate mofetil: a comparison with enteric-coated mycophenolate sodium in rats. BMC Pharmacol Toxicol 19(1):39. https://doi.org/10.1186/s40360-018-0234-1
Prummel MF et al (1989) Prednisone and cyclosporine in the treatment of severe Graves’ ophthalmopathy. N Engl J Med 321(20):1353–1359. https://doi.org/10.1056/nejm198911163212002
Kahaly G et al (1986) Ciclosporin and prednisone v. prednisone in treatment of Graves’ ophthalmopathy a controlled, randomized and prospective study. Eur J Clin Invest 16(5):415–422. https://doi.org/10.1111/j.1365-2362.1986.tb01016.x
Rajendram R et al (2018) Combined immunosuppression and radiotherapy in thyroid eye disease (CIRTED): a multicentre, 2 × 2 factorial, double-blind, randomised controlled trial. Lancet Diabetes Endocrinol 6(4):299–309. https://doi.org/10.1016/s2213-8587(18)30021-4
Strianese D (2018) Efficacy and safety of immunosuppressive agents for thyroid eye disease. Ophthalmic Plast Reconstr Surg 34(4S Suppl 1):S56–S59. https://doi.org/10.1097/iop.0000000000001131
Chalvatzis NT et al (2014) Safety and efficacy of combined immunosuppression and orbital radiotherapy in thyroid-related restrictive myopathy: two-center experience. Eur J Ophthalmol 24(6):953–959. https://doi.org/10.5301/ejo.5000463
Shahida B et al (2019) Simvastatin downregulates adipogenesis in 3T3-L1 preadipocytes and orbital fibroblasts from Graves’ ophthalmopathy patients. Endocr Connect 8(9):1230–1239. https://doi.org/10.1530/ec-19-0319
Marinò M, Lanzolla G, Marcocci C (2021) Statins: a new hope on the horizon of graves orbitopathy? J Clin Endocrinol Metab 106(7):e2819–e2821. https://doi.org/10.1210/clinem/dgab184
Lanzolla G et al (2021) Statins for graves’ orbitopathy (STAGO): a phase 2, open-label, adaptive, single centre, randomised clinical trial. Lancet Diabetes Endocrinol 9(11):733–742. https://doi.org/10.1016/s2213-8587(21)00238-2
Nilsson A, Tsoumani K, Planck T (2021) Statins decrease the risk of orbitopathy in newly diagnosed patients with graves disease. J Clin Endocrinol Metab 106(5):1325–1332. https://doi.org/10.1210/clinem/dgab070
Durrani OM, Reuser TQ, Murray PI (2005) Infliximab: a novel treatment for sight-threatening thyroid associated ophthalmopathy. Orbit 24(2):117–119. https://doi.org/10.1080/01676830590912562
Paridaens D et al (2005) The effect of etanercept on Graves’ ophthalmopathy: a pilot study. Eye (Lond) 19(12):1286–1289. https://doi.org/10.1038/sj.eye.6701768
van Steensel L et al (2011) Whole orbital tissue culture identifies imatinib mesylate and adalimumab as potential therapeutics for Graves’ ophthalmopathy. Br J Ophthalmol 95(5):735–738. https://doi.org/10.1136/bjo.2010.192302
Ayabe R et al (2014) Adalimumab as steroid-sparing treatment of inflammatory-stage thyroid eye disease. Ophthalmic Plast Reconstr Surg 30(5):415–419. https://doi.org/10.1097/iop.0000000000000211
Salvi M et al (2007) Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol 156(1):33–40. https://doi.org/10.1530/eje.1.02325
El Fassi D et al (2009) Treatment of Graves’ disease with rituximab specifically reduces the production of thyroid stimulating autoantibodies. Clin Immunol 130(3):252–258. https://doi.org/10.1016/j.clim.2008.09.007
Stan MN et al (2015) Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab 100(2):432–441. https://doi.org/10.1210/jc.2014-2572
Salvi M et al (2015) Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab 100(2):422–431. https://doi.org/10.1210/jc.2014-3014
Eid L et al (2020) The effects of rituximab on graves’orbitopathy: a retrospective study of 14 patients. Eur J Ophthalmol 30(5):1008–1013. https://doi.org/10.1177/1120672119845224
Ding C, Jones G (2003) Technology evaluation: MRA. Chugai Curr Opin Mol Ther 5(1):64–69
Pérez-Moreiras JV, Alvarez-López A, Gómez EC (2014) Treatment of active corticosteroid-resistant graves’ orbitopathy. Ophthalmic Plast Reconstr Surg 30(2):162–167. https://doi.org/10.1097/iop.0000000000000037
Perez-Moreiras JV et al (2018) Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant graves orbitopathy: a randomized clinical trial. Am J Ophthalmol 195:181–190. https://doi.org/10.1016/j.ajo.2018.07.038
Copperman T et al (2019) Subcutaneous tocilizumab for thyroid eye disease: simplified dosing and delivery. Ophthalmic Plast Reconstr Surg 35(3):e64–e66. https://doi.org/10.1097/iop.0000000000001346
Chen H et al (2015) TSH-Mediated TNFα production in human fibrocytes is inhibited by teprotumumab, an IGF-1R antagonist. PLoS ONE 10(6):e0130322. https://doi.org/10.1371/journal.pone.0130322
Chen H et al (2014) Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J Clin Endocrinol Metab 99(9):El635–El640. https://doi.org/10.1210/jc.2014-1580
Smith TJ et al (2017) Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med 376(18):1748–1761. https://doi.org/10.1056/NEJMoa1614949
Douglas RS et al (2020) Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med 382(4):341–352. https://doi.org/10.1056/NEJMoa1910434
Bartalena L et al (2022) Teprotumumab for Graves’ orbitopathy and ototoxicity: moving problems from eyes to ears? J Endocrinol Invest 45(7):1455–1457. https://doi.org/10.1007/s40618-022-01791-w
Inserro A (2021) The American Journal of Managed Care. Dr Rona Silkiss Details the Use of Biologics to Treat Thyroid Eye Disease. News release of the American Journal of Managed Care. https://www.ajmc.com/view/dr-paul-hahn-highlights-emerging-technologies-in-ophthalmology. Accessed Oct 07 2022
Kahaly GJ et al (2021) Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol 9(6):360–372. https://doi.org/10.1016/s2213-8587(21)00056-5
Belinsky I et al (2022) Teprotumumab and hearing loss: case series and proposal for audiologic monitoring. Ophthalmic Plast Reconstr Surg 38(1):73–78. https://doi.org/10.1097/iop.0000000000001995
Ding AS et al (2022) Sensorineural hearing loss after teprotumumab therapy for thyroid eye disease: a case report. Otol Neurotol 43(2):e148–e152. https://doi.org/10.1097/mao.0000000000003428
Allen RC et al (2021) A perspective on the current role of teprotumumab in treatment of thyroid eye disease. Ophthalmology 128(8):1125–1128. https://doi.org/10.1016/j.ophtha.2021.03.006
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was supported by State Scholarship Fund from China Scholarship Council (Nr. 202006370027 of XL).
Author information
Authors and Affiliations
Contributions
XL and SL: Conception and design. WF, ACR, SJ, and XJ collected data. GY and LMH: Administrative support. XL took the lead in writing the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Human and animal rights
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yongwei Guo and Ludwig M. Heindl these authors have contributed equally to this work and share senior authorship.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Li, S., Fan, W. et al. Recent advances in graves ophthalmopathy medical therapy: a comprehensive literature review. Int Ophthalmol 43, 1437–1449 (2023). https://doi.org/10.1007/s10792-022-02537-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-022-02537-6