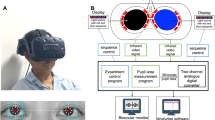
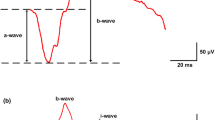
Abstract
Glaucoma is a multifactorial neurodegenerative disease of the optic nerve currently considered a severe health problem because of its high prevalence, being the primary cause of irreversible blindness worldwide. The most common type corresponds to Primary Open-Angle Glaucoma. Glaucoma produces, among other alterations, a progressive loss of retinal ganglion cells (RGC) and its axons which are the key contributors to generate action potentials that reach the visual cortex to create the visual image. Glaucoma is characterized by Visual Field loss whose main feature is to be painless and therefore makes early detection difficult, causing a late diagnosis and a delayed treatment indication that slows down its progression. Intrinsically photosensitive retinal ganglion cells, which represent a subgroup of RGCs are characterized by their response to short-wave light stimulation close to 480 nm, their non-visual function, and their role in the generation of the pupillary reflex. Currently, the sensitivity of clinical examinations correlates to RGC damage; however, the need for an early damage biomarker is still relevant. It is an urgent task to create new diagnostic approaches to detect an early stage of glaucoma in a prompt, quick, and economical manner. We summarize the pathology of glaucoma and its current clinical detection methods, and we suggest evaluating the pupillary response to chromatic light as a potential biomarker of disease, due to its diagnostic benefit and its cost-effectiveness in clinical practice in order to reduce irreversible damage caused by glaucoma.


Similar content being viewed by others
Availability of data and materials
Not applicable.
References
Guglielmi P, Carradori S, Campestre C, Poce G (2019) Novel therapies for glaucoma: a patent review (2013–2019). Expert Opin Ther Pat 29:769–780. https://doi.org/10.1080/13543776.2019.1653279
Tehrani S (2015) Gender difference in the pathophysiology and treatment of glaucoma. Curr Eye Res 40:191–200. https://doi.org/10.3109/02713683.2014.968935
Weinreb RN, Aung T, Medeiros FA (2014) The pathophysiology and treatment of glaucoma: a review. JAMA 311:1901–1911. https://doi.org/10.1001/jama.2014.3192
Tham Y-C, Li X, Wong TY et al (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121:2081–2090. https://doi.org/10.1016/j.ophtha.2014.05.013
Quigley HA, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90:262–267. https://doi.org/10.1136/bjo.2005.081224
Kelbsch C, Maeda F, Strasser T et al (2016) Pupillary responses driven by ipRGCs and classical photoreceptors are impaired in glaucoma. Graefe’s Arch Clin Exp Ophthalmol = Albr von Graefes Arch fur Klin und Exp Ophthalmol 254:1361–1370. https://doi.org/10.1007/s00417-016-3351-9
Mantravadi AV, Vadhar N (2015) Glaucoma. Prim Care 42:437–449. https://doi.org/10.1016/j.pop.2015.05.008
Jonas JB, Aung T, Bourne RR et al (2017) Glaucoma. Lancet Lond Engl 390:2183–2193. https://doi.org/10.1016/S0140-6736(17)31469-1
Evangelho K, Mogilevskaya M, Losada-Barragan M, Vargas-Sanchez JK (2019) Pathophysiology of primary open-angle glaucoma from a neuroinflammatory and neurotoxicity perspective: a review of the literature. Int Ophthalmol 39:259–271. https://doi.org/10.1007/s10792-017-0795-9
Weinreb RN, Khaw PT (2004) Primary open-angle glaucoma. Lancet Lond Engl 363:1711–1720. https://doi.org/10.1016/S0140-6736(04)16257-0
Conlon R, Saheb H, Ahmed IIK (2017) Glaucoma treatment trends: a review. Can J Ophthalmol 52:114–124. https://doi.org/10.1016/j.jcjo.2016.07.013
Bertaud S, Aragno V, Baudouin C, Labbé A (2019) Primary open-angle glaucoma. La Rev Med Interne 40:445–452. https://doi.org/10.1016/j.revmed.2018.12.001
Davis BM, Crawley L, Pahlitzsch M et al (2016) Glaucoma: the retina and beyond. Acta Neuropathol 132:807–826. https://doi.org/10.1007/s00401-016-1609-2
Berson DM, Dunn FA, Takao M (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science 295:1070–1073. https://doi.org/10.1126/science.1067262
Hannibal J, Christiansen AT, Heegaard S et al (2017) Melanopsin expressing human retinal ganglion cells: subtypes, distribution, and intraretinal connectivity. J Comp Neurol 525:1934–1961. https://doi.org/10.1002/cne.24181
Ba-Ali S, Lund-Andersen H (2017) Pupillometric evaluation of the melanopsin containing retinal ganglion cells in mitochondrial and non-mitochondrial optic neuropathies. Mitochondrion 36:124–129. https://doi.org/10.1016/j.mito.2017.07.003
Kuze M, Morita T, Fukuda Y et al (2017) Electrophysiological responses from intrinsically photosensitive retinal ganglion cells are diminished in glaucoma patients. J Optom 10:226–232. https://doi.org/10.1016/j.optom.2016.07.004
Feigl B, Zele AJ (2014) Melanopsin-expressing intrinsically photosensitive retinal ganglion cells in retinal disease. Optom Vis Sci Off Publ Am Acad Optom 91:894–903. https://doi.org/10.1097/OPX.0000000000000284
Spitschan M (2019) Melanopsin contributions to non-visual and visual function. Curr Opin Behav Sci 30:67–72. https://doi.org/10.1016/j.cobeha.2019.06.004
Bouffard MA (2019) The pupil. Continuum (Minneap Minn) 25:1194–1214. https://doi.org/10.1212/CON.0000000000000771
Gamlin PDR, McDougal DH, Pokorny J et al (2007) Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vis Res 47:946–954. https://doi.org/10.1016/j.visres.2006.12.015
Markwell EL, Feigl B, Zele AJ (2010) Intrinsically photosensitive melanopsin retinal ganglion cell contributions to the pupillary light reflex and circadian rhythm. Clin Exp Optom 93:137–149. https://doi.org/10.1111/j.1444-0938.2010.00479.x
McDougal DH, Gamlin PD (2010) The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vis Res 50:72–87. https://doi.org/10.1016/j.visres.2009.10.012
Nissen C, Sander B, Milea D et al (2014) Monochromatic pupillometry in unilateral glaucoma discloses no adaptive changes subserved by the ipRGCs. Front Neurol 5:15. https://doi.org/10.3389/fneur.2014.00015
Münch M, Léon L, Collomb S, Kawasaki A (2015) Comparison of acute non-visual bright light responses in patients with optic nerve disease, glaucoma and healthy controls. Sci Rep 5:15185. https://doi.org/10.1038/srep15185
Chang DS, Xu L, Boland MV, Friedman DS (2013) Accuracy of pupil assessment for the detection of glaucoma: a systematic review and meta-analysis. Ophthalmology 120:2217–2225. https://doi.org/10.1016/j.ophtha.2013.04.012
Rukmini AV, Milea D, Gooley JJ (2019) Chromatic pupillometry methods for assessing photoreceptor health in retinal and optic nerve diseases. Front Neurol 10:76. https://doi.org/10.3389/fneur.2019.00076
Rukmini AV, Milea D, Baskaran M et al (2015) Pupillary responses to high-irradiance blue light correlate with glaucoma severity. Ophthalmology 122:1777–1785. https://doi.org/10.1016/j.ophtha.2015.06.002
Wride N, Habib M, Morris K et al (2009) Clinical evaluation of a rapid, pupil-based assessment of retinal damage associated with glaucoma. Clin Ophthalmol 3:123–128
Chen Y, Wyatt HJ, Swanson WH, Dul MW (2008) Rapid pupil-based assessment of glaucomatous damage. Optom Vis Sci Off Publ Am Acad Optom 85:471–481. https://doi.org/10.1097/OPX.0b013e318177ec02
Martucci A, Cesareo M, Napoli D et al (2014) Evaluation of pupillary response to light in patients with glaucoma: a study using computerized pupillometry. Int Ophthalmol 34:1241–1247. https://doi.org/10.1007/s10792-014-9920-1
Kankipati L, Girkin CA, Gamlin PD (2011) The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci 52:2287–2292. https://doi.org/10.1167/iovs.10-6023
Pradhan ZS, Rao HL, Puttaiah NK et al (2017) Predicting the magnitude of functional and structural damage in glaucoma from monocular pupillary light responses using automated pupillography. J Glaucoma 26:409–414. https://doi.org/10.1097/IJG.0000000000000634
Pillai MR, Sinha S, Aggarwal P et al (2019) Quantification of RAPD by an automated pupillometer in asymmetric glaucoma and its correlation with manual pupillary assessment. Indian J Ophthalmol 67:227–232. https://doi.org/10.4103/ijo.IJO_648_18
Feigl B, Mattes D, Thomas R, Zele AJ (2011) Intrinsically photosensitive (melanopsin) retinal ganglion cell function in glaucoma. Invest Ophthalmol Vis Sci 52:4362–4367. https://doi.org/10.1167/iovs.10-7069
Chang DS, Arora K, Boland MV, Friedman DS (2019) The relationship between quantitative pupillometry and estimated ganglion cell counts in patients with glaucoma. J Glaucoma 28:238–242. https://doi.org/10.1097/IJG.0000000000001183
Gracitelli CPB, Duque-Chica GL, Moura AL et al (2014) A positive association between intrinsically photosensitive retinal ganglion cells and retinal nerve fiber layer thinning in glaucoma. Invest Ophthalmol Vis Sci 55:7997–8005. https://doi.org/10.1167/iovs.14-15146
Gracitelli CPB, Duque-Chica GL, Roizenblatt M et al (2015) Intrinsically photosensitive retinal ganglion cell activity is associated with decreased sleep quality in patients with glaucoma. Ophthalmology 122:1139–1148. https://doi.org/10.1016/j.ophtha.2015.02.030
Adhikari P, Zele AJ, Thomas R, Feigl B (2016) Quadrant field pupillometry detects melanopsin dysfunction in glaucoma suspects and early glaucoma. Sci Rep 6:33373. https://doi.org/10.1038/srep33373
Najjar RP, Sharma S, Atalay E et al (2018) Pupillary responses to full-field chromatic stimuli are reduced in patients with early-stage primary open-angle glaucoma. Ophthalmology 125:1362–1371. https://doi.org/10.1016/j.ophtha.2018.02.024
Duque-Chica GL, Gracitelli CPB, Moura ALA et al (2018) Inner and outer retinal contributions to pupillary light response: correlation to functional and morphologic parameters in glaucoma. J Glaucoma 27:723–732. https://doi.org/10.1097/IJG.0000000000001003
Carle CF, James AC, Kolic M et al (2015) Blue multifocal pupillographic objective perimetry in glaucoma. Invest Ophthalmol Vis Sci 56:6394–6403. https://doi.org/10.1167/iovs.14-16029
Sharts-Hopko NC, Glynn-Milley C (2009) Primary open-angle glaucoma. Am J Nurs 109:40–47. https://doi.org/10.1097/01.NAJ.0000345434.37734.ee
McMonnies CW (2017) Glaucoma history and risk factors. J Optom 10:71–78. https://doi.org/10.1016/j.optom.2016.02.003
Kass MA, Heuer DK, Higginbotham EJ et al (2002) The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol (Chicago, Ill 1960) 120:701–730. https://doi.org/10.1001/archopht.120.6.701
Nucci C, Martucci A, Cesareo M et al (2015) Links among glaucoma, neurodegenerative, and vascular diseases of the central nervous system. Prog Brain Res 221:49–65. https://doi.org/10.1016/bs.pbr.2015.04.010
Schuster AK, Erb C, Hoffmann EM et al (2020) The diagnosis and treatment of glaucoma. Dtsch Arztebl Int 117:225–234. https://doi.org/10.3238/arztebl.2020.0225
Moon J, Park KH, Kim DM, Kim SH (2018) Factors affecting ISNT Rule satisfaction in normal and glaucomatous eyes. Korean J Ophthalmol 32:38–44. https://doi.org/10.3341/kjo.2017.0031
Hashimoto S, Matsumoto C, Eura M et al (2018) Distribution and progression of visual field defects with binocular vision in glaucoma. J Glaucoma 27:519–524. https://doi.org/10.1097/IJG.0000000000000949
Bussel II, Wollstein G, Schuman JS (2014) OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol 98(Suppl 2):ii15-9. https://doi.org/10.1136/bjophthalmol-2013-304326
Neto A, Camera J, Oliveira S et al (2022) Optic disc and cup segmentations for glaucoma assessment using cup-to-disc ratio. Procedia Comput Sci 196:485–492. https://doi.org/10.1016/j.procs.2021.12.040
Qureshi I, Khan MA, Sharif M et al (2020) Detection of glaucoma based on cup-To-disc ratio using fundus images. Int J Intell Syst Technol Appl 19:1–16. https://doi.org/10.1504/IJISTA.2020.105172
Bočková M, Veselý P, Synek S et al (2019) Sensitivity and specificity of spectral OCT in patients with early glaucoma. Ces a Slov Oftalmol Cas Ces Oftalmol Spol a Slov Oftalmol Spol 75:260–264. https://doi.org/10.31348/2019/5/3
Budenz DL, Rhee P, Feuer WJ et al (2002) Sensitivity and specificity of the Swedish interactive threshold algorithm for glaucomatous visual field defects. Ophthalmology 109:1052–1058. https://doi.org/10.1016/s0161-6420(02)01047-3
Wang H, Li M, Zhang Z et al (2019) Physiological function of myocilin and its role in the pathogenesis of glaucoma in the trabecular meshwork (Review). Int J Mol Med 43:671–681. https://doi.org/10.3892/ijmm.2018.3992
Feng J, Xu J (2019) Identification of pathogenic genes and transcription factors in glaucoma. Mol Med Rep 20:216–224. https://doi.org/10.3892/mmr.2019.10236
Liu Y, Wang Y, Chen Y et al (2019) Discovery and validation of circulating Hsa-miR-210-3p as a potential biomarker for primary open-angle glaucoma. Invest Ophthalmol Vis Sci 60:2925–2934. https://doi.org/10.1167/iovs.19-26663
Romano GL, Platania CBM, Forte S et al (2015) MicroRNA target prediction in glaucoma. Prog Brain Res 220:217–240. https://doi.org/10.1016/bs.pbr.2015.04.013
Singh LN, Crowston JG, Lopez Sanchez MIG et al (2018) Mitochondrial DNA variation and disease susceptibility in primary open-angle glaucoma. Invest Ophthalmol Vis Sci 59:4598–4602. https://doi.org/10.1167/iovs.18-25085
Benoist d’Azy C, Pereira B, Chiambaretta F, Dutheil F (2016) Oxidative and anti-oxidative stress markers in chronic glaucoma: a systematic review and meta-analysis. PLoS ONE 11:e0166915. https://doi.org/10.1371/journal.pone.0166915
Yang X, Zeng Q, Göktas E et al (2019) T-lymphocyte subset distribution and activity in patients with glaucoma. Invest Ophthalmol Vis Sci 60:877–888. https://doi.org/10.1167/iovs.18-26129
Mastropasqua R, Agnifili L, Mastropasqua L (2019) Structural and molecular tear film changes in glaucoma. Curr Med Chem 26:4225–4240. https://doi.org/10.2174/0929867325666181009153212
Yap TE, Davis BM, Guo L et al (2018) Annexins in glaucoma. Int J Mol Sci. https://doi.org/10.3390/ijms19041218
Bua S, Supuran CT (2019) Diagnostic markers for glaucoma: a patent and literature review (2013–2019). Expert Opin Ther Pat 29:829–839. https://doi.org/10.1080/13543776.2019.1667336
Beykin G, Norcia AM, Srinivasan VJ et al (2021) Discovery and clinical translation of novel glaucoma biomarkers. Prog Retin Eye Res 80:100875. https://doi.org/10.1016/j.preteyeres.2020.100875
Barbosa-Breda J, Himmelreich U, Ghesquière B et al (2018) Clinical metabolomics and glaucoma. Ophthalmic Res 59:1–6. https://doi.org/10.1159/000479158
Colligris B, Crooke A, Gasull X et al (2012) Recent patents and developments in glaucoma biomarkers. Recent Pat Endocr Metab Immune Drug Discov 6:224–234. https://doi.org/10.2174/187221412802481739
Beykin G, Goldberg JL (2019) Molecular biomarkers for glaucoma. Curr Ophthalmol Rep 7:171–176. https://doi.org/10.1007/s40135-019-00213-0
Obara EA, Hannibal J, Heegaard S, Fahrenkrug J (2016) Loss of Melanopsin-expressing retinal ganglion cells in severely staged glaucoma patients. Invest Ophthalmol Vis Sci 57:4661–4667. https://doi.org/10.1167/iovs.16-19997
Mure LS, Vinberg F, Hanneken A, Panda S (2019) Functional diversity of human intrinsically photosensitive retinal ganglion cells. Science 366:1251–1255. https://doi.org/10.1126/science.aaz0898
Sexton T, Buhr E, Van Gelder RN (2012) Melanopsin and mechanisms of non-visual ocular photoreception. J Biol Chem 287:1649–1656. https://doi.org/10.1074/jbc.R111.301226
Chen S-K, Badea TC, Hattar S (2011) Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature 476:92–95. https://doi.org/10.1038/nature10206
Do MTH (2019) Melanopsin and the intrinsically photosensitive retinal ganglion cells: biophysics to behavior. Neuron 104:205–226. https://doi.org/10.1016/j.neuron.2019.07.016
Do MTH, Yau K-W (2010) Intrinsically photosensitive retinal ganglion cells. Physiol Rev 90:1547–1581. https://doi.org/10.1152/physrev.00013.2010
Sliney DH (2016) What is light? The visible spectrum and beyond. Eye (Lond) 30:222–229. https://doi.org/10.1038/eye.2015.252
Cui Q, Ren C, Sollars PJ et al (2015) The injury resistant ability of melanopsin-expressing intrinsically photosensitive retinal ganglion cells. Neuroscience 284:845–853. https://doi.org/10.1016/j.neuroscience.2014.11.002
Fogo GM, Shuboni-Mulligan DD, Gall AJ (2019) Melanopsin-containing ipRGCs are resistant to excitotoxic injury and maintain functional non-image forming behaviors after insult in a diurnal rodent model. Neuroscience 412:105–115. https://doi.org/10.1016/j.neuroscience.2019.05.058
Wang S, Gu D, Zhang P et al (2018) Melanopsin-expressing retinal ganglion cells are relatively resistant to excitotoxicity induced by N-methyl-d-aspartate. Neurosci Lett 662:368–373. https://doi.org/10.1016/j.neulet.2017.10.055
DeParis S, Caprara C, Grimm C (2012) Intrinsically photosensitive retinal ganglion cells are resistant to N-methyl-D-aspartic acid excitotoxicity. Mol Vis 18:2814–2827
Li RS, Chen B-Y, Tay DK et al (2006) Melanopsin-expressing retinal ganglion cells are more injury-resistant in a chronic ocular hypertension model. Invest Ophthalmol Vis Sci 47:2951–2958. https://doi.org/10.1167/iovs.05-1295
Daniel S, Clark AF, McDowell CM (2018) Subtype-specific response of retinal ganglion cells to optic nerve crush. Cell death Discov 4:7. https://doi.org/10.1038/s41420-018-0069-y
Honda S, Namekata K, Kimura A et al (2019) Survival of alpha and intrinsically photosensitive retinal ganglion cells in NMDA-induced neurotoxicity and a mouse model of normal tension glaucoma. Invest Ophthalmol Vis Sci 60:3696–3707. https://doi.org/10.1167/iovs.19-27145
Vidal-Villegas B, Di Pierdomenico J, Miralles de Imperial-Ollero JA et al (2019) Melanopsin(+)RGCs are fully resistant to NMDA-induced excitotoxicity. Int J Mol Sci. https://doi.org/10.3390/ijms20123012
Wang H, Lu Q, Wang N et al (2008) Loss of melanopsin-containing retinal ganglion cells in a rat glaucoma model. Chin Med J (Engl) 121:1015–1019
Wilhelm H (2011) Disorders of the pupil. Handb Clin Neurol 102:427–466. https://doi.org/10.1016/B978-0-444-52903-9.00022-4
Muñoz Negrete FJ, Rebolleda G (2013) Automated evaluation of the pupil. Arch Soc Esp Oftalmol 88:125–126
La Morgia C, Carelli V, Carbonelli M (2018) Melanopsin retinal ganglion cells and pupil: clinical implications for neuro-ophthalmology. Front Neurol 9:1047. https://doi.org/10.3389/fneur.2018.01047
Waisbourd M, Lee B, Ali MH et al (2015) Detection of asymmetric glaucomatous damage using automated pupillography, the swinging flashlight method and the magnified-assisted swinging flashlight method. Eye (Lond) 29:1321–1328. https://doi.org/10.1038/eye.2015.106
Suo L, Zhang D, Qin X et al (2020) Evaluating state-of-the-art computerized pupillary assessments for glaucoma detection: a systematic review and meta-analysis. Front Neurol 11:777
Ksendzovsky A, Pomeraniec IJ, Zaghloul KA et al (2017) Clinical implications of the melanopsin-based non-image-forming visual system. Neurology 88:1282–1290. https://doi.org/10.1212/WNL.0000000000003761
Adhikari P, Zele AJ, Feigl B (2015) The post-illumination pupil response (PIPR). Invest Ophthalmol Vis Sci 56:3838–3849. https://doi.org/10.1167/iovs.14-16233
Liao H-W, Ren X, Peterson BB et al (2016) Melanopsin-expressing ganglion cells on macaque and human retinas form two morphologically distinct populations. J Comp Neurol 524:2845–2872. https://doi.org/10.1002/cne.23995
Kim US, Mahroo OA, Mollon JD, Yu-Wai-Man P (2021) Retinal ganglion cells-diversity of cell types and clinical relevance. Front Neurol 12:661938. https://doi.org/10.3389/fneur.2021.661938
Lawlor M, Quartilho A, Bunce C et al (2017) Patients with normal tension glaucoma have relative sparing of the relative afferent pupillary defect compared to those with open angle glaucoma and elevated intraocular pressure. Invest Ophthalmol Vis Sci 58:5237–5241. https://doi.org/10.1167/iovs.17-21688
Fontana L, Coassin M, Iovieno A et al (2017) Cataract surgery in patients with pseudoex-foliation syndrome: current updates. Clin Ophthalmol 11:1377–1383. https://doi.org/10.2147/OPTH.S142870
Nath M, Odayappan A, Tripathy K et al (2021) Predicting zonular strength based on maximum pupillary mydriasis in patients with pseudoexfoliation syndrome. Med Hypotheses 146:110402. https://doi.org/10.1016/j.mehy.2020.110402
Philip SS, John SS, Simha AR et al (2012) Ocular clinical profile of patients with pseudoexfoliation syndrome in a tertiary eye care center in South India. Middle East Afr J Ophthalmol 19:231–236. https://doi.org/10.4103/0974-9233.95259
Rukmini AV, Najjar RP, Atalay E et al (2017) Pupillary responses to light are not affected by narrow irido-corneal angles. Sci Rep 7:10190. https://doi.org/10.1038/s41598-017-10303-3
Kelbsch C, Strasser T, Chen Y et al (2019) Standards in pupillography. Front Neurol 10:129. https://doi.org/10.3389/fneur.2019.00129
Zénon A (2019) Eye pupil signals information gain. Proc Biol Sci 286:20191593. https://doi.org/10.1098/rspb.2019.1593
Kastner A, King AJ (2020) Advanced glaucoma at diagnosis: current perspectives. Eye (Lond) 34:116–128. https://doi.org/10.1038/s41433-019-0637-2
Acknowledgements
Not applicable.
Funding
This research was supported by Fondo Concursable Interno Investigación 2022-DETEM Universidad de Chile and CONICYT-PFCHA/Doctorado Nacional/2020–21200346.
Author information
Authors and Affiliations
Contributions
IPR and CAL designed the study, wrote, and published the manuscript. SM reviewed the first draft of the manuscript. IPR and SG reviewed and edited the manuscript. IPR, CAL and SG prepared the manuscript for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arévalo-López, C., Gleitze, S., Madariaga, S. et al. Pupillary response to chromatic light stimuli as a possible biomarker at the early stage of glaucoma: a review. Int Ophthalmol 43, 343–356 (2023). https://doi.org/10.1007/s10792-022-02381-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-022-02381-8