Abstract
Purpose
To determine the prognostic value of optical coherence tomography (OCT) measurement of the peripapillary retinal nerve fiber layer (RNFL) thickness in visual recovery after orbital decompression of patients with dysthyroid optic neuropathy (DON).
Methods
A total of 52 eyes of 37 patients who underwent orbital decompression for DON between 2013 and 2019 were retrospectively reviewed. We examined peripapillary RNFL thickness, best-corrected visual acuity (BCVA), visual field (VF) for mean deviation (MD) and pattern standard deviation (PSD), and pattern-reversed visual evoked potential (PVEP) for P100 latency and amplitude before and after surgery. Black and white checkerboard square sizes of PVEP were 15 and 60 arcmin (arcminute and minute of angle). Changes in RNFL overall thickness and by quadrant and interocular differences were evaluated and studied regarding changes in BCVA, VF and PVEP.
Results
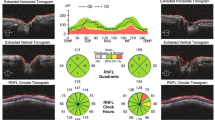
There was a significant improvement in BCVA, VF, and PVEP, whereas a dramatic reduction in RNFL thickness of all DON patients in global average, temporal, superior, and inferior quadrants (P = 0.005, P = 0.024, P = 0.016, and P = 0.001, respectively) after decompression surgery, except for nasal quadrant (P = 0.057). The preoperative RNFL thickness in each quadrant was negatively correlated with postoperative changes of BCVA and PSD and positively correlated with changes of MD and P100 amplitude at 60 arcmin (all P < 0.05). Except for temporal quadrant (P = 0.125), the preoperative RNFL thickness in other quadrants was positively correlated with postoperative changes of P100 amplitude at 15 arcmin (all P < 0.05). The nasal RNFL thickness was an excellent predictor for improvement in BCVA by 20/25 or better and in MD by 10 dB or more after surgery, whose cutoff value was 73.50 μm, while the inferior and superior RNFL thickness could act as a predictor for improvement in P100 amplitude by 5 μV or more at 60 arcmin and at 15 arcmin, respectively, whose cutoff value was, respectively, 143.00 μm and 130.50 μm (all P < 0.05).
Conclusion
RNFL thickness measured by OCT was correlated with visual function recovery after decompression surgery in patients with DON, which could also act as a predictor for better visual prognosis.




Similar content being viewed by others
References
McKeag D, Lane C, Lazarus JH, Baldeschi L, Boboridis K, Dickinson AJ, Hullo AI, Kahaly G, Krassas G, Marcocci C, Marino M, Mourits MP, Nardi M, Neoh C, Orgiazzi J, Perros P, Pinchera A, Pitz S, Prummel MF, Sartini MS, Wiersinga WM (2007) Clinical features of dysthyroid optic neuropathy: a European Group on Graves’ Orbitopathy (EUGOGO) survey. Br J Ophthalmol 91(4):455–458
Trobe JD, Glaser JS, Laflamme P (1978) Dysthyroid optic neuropathy. Clinical profile and rationale for management. Arch Ophthalmol 96(7):1199–1209
Hutchison BM, Kyle PM (1995) Long-term visual outcome following orbital decompression for dysthyroid eye disease. Eye (Lond) 9(Pt 5):578–581
Samantha J, Chai R, Foroozan, (2007) Decreased retinal nerve fibre layer thickness detected by optical coherence tomography in patients with ethambutol-induced optic neuropathy. Br J Ophthalmol 91(7):895–897
Rajabi MT, Ojani M, Riazi Esfahani H, Tabatabaei SZ, Rajabi MB, Hosseini SS (2019) Correlation of peripapillary nerve fiber layer thickness with visual outcomes after decompression surgery in subclinical and clinical thyroid-related compressive optic neuropathy. J Curr Ophthalmol 31(1):86–91
Danesh-Meyer HV, Papchenko T, Savino PJ, Law A, Evans J, Gamble GD (2008) In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Invest Ophthalmol Vis Sci 49(5):1879–1885
Jacob M, Raverot G, Jouanneau E, Borson-Chazot F, Perrin G, Rabilloud M, Tilikete C, Bernard M, Vighetto A (2009) Predicting visual outcome after treatment of pituitary adenomas with optical coherence tomography. Am J Ophthalmol 147(1):64–70
Drexler W, Sattmann H, Hermann B, Ko TH, Stur M, Unterhuber A, Scholda C, Findl O, Wirtitsch M, Fujimoto JG, Fercher AF (2003) Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch Ophthalmol 121(5):695–706
Rebolleda G, Munoz-Negrete FJ (2009) Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Invest Ophthalmol Vis Sci 50(11):5197–5200
MrLR M, Cunha LP, Costa-Cunha LVF, OlO M, Oyamada MK (2009) Relationship between optical coherence tomography, pattern electroretinogram and automated perimetry in eyes with temporal hemianopia from chiasmal compression. Invest Ophthalmol Vis Sci 50(8):3535
Neigel JM, Rootman J, Belkin RI, Nugent RA, Drance SM, Beattie CW, Spinelli JA (1988) Dysthyroid optic neuropathy. Ophthalmology 95(11):1515–1521
Bendschneider D, Tornow RP, Horn FK, Laemmer R, Roessler CW, Juenemann AG, Kruse FE, Mardin CY (2010) Retinal nerve fiber layer thickness in normals measured by spectral domain OCT. J Glaucoma 19(7):475–482
Hwang YH, Lee SM, Kim YY, Lee JY, Yoo C (2012) Astigmatism and optical coherence tomography measurements. Graefes Arch Clin Exp Ophthalmol 250(2):247–254
Odom JV, Bach M, Barber C, Brigell M, Marmor MF, Tormene AP, Holder GE, Vaegan, (2004) Visual evoked potentials standard (2004). Doc Ophthalmol 108(2):115–123
Schuman JS, Pedut-Kloizman T, Hertzmark E, Hee MR, Wilkins JR, Coker JG, Puliafito CA, Fujimoto JG, Swanson EA (1996) Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography. Ophthalmology 103(11):1889–1898
Ferris FL, Kassoff A, Bresnick GH, Bailey I (1982) New visual acuity charts for clinical research. Am J Ophthalmol 94(1):91–96
Liang QW, Yang H, Luo W, He JF, Du Y (2019) Effect of orbital decompression on dysthyroid optic neuropathy: a retrospective case series. Medicine (Baltimore) 98(3):e14162
Park KA, Kim YD, Woo KI, Kee C, Han JC (2016) Optical coherence tomography measurements in compressive optic neuropathy associated with dysthyroid orbitopathy. Graefes Arch Clin Exp Ophthalmol 254(8):1617–1624
Park KA, Kim YD, Woo KI (2018) Changes in optical coherence tomography measurements after orbital wall decompression in dysthyroid optic neuropathy. Eye (Lond) 32(6):1123–1129
Jefferis JM, Jones RK, Currie ZI, Tan JH, Salvi SM (2018) Orbital decompression for thyroid eye disease: methods, outcomes, and complications. Eye (Lond) 32(3):626–663
Cubuk MO, Konuk O, Unal M (2018) Orbital decompression surgery for the treatment of Graves’ ophthalmopathy: comparison of different techniques and long-term results. Int J Ophthalmol 11(8):1363–1370
Blandford AD, Zhang D, Chundury RV, Perry JD (2017) Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Rev Ophthalmol 12(2):111–121
Miskiewicz P, Rutkowska B, Jablonska A, Krzeski A, Trautsolt-Jeziorska K, Kecik D, Milczarek-Banach J, Pirko-Kotela K, Samsel A, Bednarczuk T (2016) Complete recovery of visual acuity as the main goal of treatment in patients with dysthyroid optic neuropathy. Endokrynol Pol 67(2):166–173
Saeed P, Tavakoli Rad S, Bisschop P (2018) Dysthyroid Optic Neuropathy. Ophthal Plast Reconstr Surg 34(4S):S60–S67
Tagami M, Honda S, Azumi A (2020) Preoperative clinical factors and visual outcomes following orbital decompression with dysthyroid optic neuropathy. BMC Ophthalmol 20(1):30
Razek AA, EI-Hadidy EM, Moawad ME, EI-Metwaly N, Ei-Said AAE (2019) Assessment of lacrimal glands in thyroid eye disease with diffusion-weighted magnetic resonance imaging. Polish J Radiol 84:e142–e146
Razek AA, El-Hadidy M, Moawad ME, El-Metwaly N, El-Said AA (2017) Performance of apparent diffusion coefficient of medial and lateral rectus muscles in Graves’ orbitopathy. Neuroradiol J 30(3):230–234
Chan J, Shin JH, Woo KI, Kim YD (2012) Clinical profile and visual outcomes after treatment in patients with dysthyroid optic neuropathy. Korean J Ophthalmol 26(2):73–79
Yaqub M (2012) Visual fields interpretation in glaucoma: a focus on static automated perimetry. Community Eye Health 25(79–80):1–8
Liao SL, Chang TC, Lin LL (2006) Transcaruncular orbital decompression: an alternate procedure for graves ophthalmopathy with compressive optic neuropathy. Am J Ophthalmol 141(5):810–818
Choe CH, Cho RI, Elner VM (2011) Comparison of lateral and medial orbital decompression for the treatment of compressive optic neuropathy in thyroid eye disease. Ophthal Plast Reconstr Surg 27(1):4–11
Perry JD, Kadakia A, Foster JA (2003) Transcaruncular orbital decompression for dysthyroid optic neuropathy. Ophthal Plast Reconstr Surg 19(5):353–358
Korkmaz S, Konuk O (2015) Surgical Treatment of Dysthyroid Optic Neuropathy: Long-Term Visual Outcomes with Comparison of 2-Wall versus 3-Wall Orbital Decompression. Curr Eye Res 41(2):1–6
Iao TWU, Rong SS, Ling AN, Brelen ME, Young AL, Chong KKL (2017) Electrophysiological studies in thyroid associated orbitopathy: a systematic review. Sci Rep 7(1):12108
Setala K, Raitta C, Valimaki M, Katevuo V, Lamberg BA (1992) The value of visual evoked potentials in optic neuropathy of Graves’ disease. J Endocrinol Invest 15(11):821–826
Tsaloumas MD, Good PA, Burdon MA, Misson GP (1994) Flash and pattern visual evoked potentials in the diagnosis and monitoring of dysthyroid optic neuropathy. Eye (Lond) 8(Pt 6):638–645
Salvi M, Spaggiari E, Neri F, Macaluso C, Gardini E, Ferrozzi F, Minelli R, Wall JR, Roti E (1997) The Study of Visual Evoked Potentials in Patients with Thyroid-Associated Ophthalmopathy Identifies Asymptomatic Optic Nerve Involvement. J Clin Endocrinol Metab 82(4):1027–1030
Lipski A, Eckstein A, Esser J, Loesch C, Mann K, Mohr C, Jurklies B (2011) Course of pattern-reversed visual evoked cortical potentials in 30 eyes after bony orbital decompression in dysthyroid optic neuropathy. Br J Ophthalmol 95(2):222–226
Funding
This work was supported by the National Natural Science Foundation of China (No.81900912). The funding organization had no role in the design or conduct of this study.
Author information
Authors and Affiliations
Contributions
XW and FJ contributed to conception and design; SC, YY, JC, and XP performed data collection; XW and SC performed analysis and interpretation of data and literature search; SC carried out statistical analysis and wrote the article; SC, YY, JC, XP, XW, and FJ done critical revision of the article.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the Ethics Committee of Union Hospital affiliated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, and was conducted in strict accordance with the Helsinki Declaration. All participants provided written informed consent.
Consent to participate
All participants provided written informed consent.
Consent for publication
All authors reviewed and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, S., Yu, Y., You, Y. et al. Retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after orbital decompression for dysthyroid optic neuropathy. Int Ophthalmol 41, 3121–3133 (2021). https://doi.org/10.1007/s10792-021-01877-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01877-z