Abstract
Objectives
To examine the effectiveness of Janus-kinase inhibitors (JAKis) or abatacept (ABA) in patients with rheumatoid arthritis-interstitial lung disease (RA-ILD).
Methods
Patients with RA-ILD receiving JAKis or ABA were retrospectively evaluated at baseline and after 18 months of treatment. A computer-aided method (CaM) was used to assess the extent of high-resolution computed tomography (HRCT) fibrosis percentage. According to HRCT fibrosis changes, patients were classified as “worsened” (progression of 15% or more), “stable” (changes within 15%) or “improved” (reduction of 15% or more). Correlations between RA characteristics and JAKis or ABA responses were studied using a multivariate regression model.
Results
Seventy-five patients (69.3% women) were evaluated, 31 received a JAKi while 44 received ABA. In the JAKis group, five patients (16.1%) showed RA-ILD progression, 20 patients (64.5%) were considered stable, and six patients (19.4%) demonstrated RA-ILD improvement. In the ABA group, five patients (11.3%) showed RA-ILD progression, 32 patients (72.7%) were stable, and seven patients (16.0%) demonstrated RA-ILD improvement. In both groups, the percentage of current smokers was different between those classified as "worsened" and those classified as "improved/stable" (p = 0.01). In multivariate regression analysis, current smoking habit (p = 0.0051) and concomitant methotrexate treatment (p = 0.0078) were the two variables related to RA-ILD progression in ABA-treated patients, whereas in JAKis-treated patients, the only RA-ILD progression-related variable was disease duration of RA (p < 0.001).
Conclusions
Treatment with JAKis or ABA was related to stability or improvement of RA-ILD in 83.9% and 88.6% of patients, respectively. RA duration is the only variable associated with worsening RA-ILD in JAKis-treated patients.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by joint inflammation and destruction, with an incidence of 0.5% of the adult population in Western countries (Salaffi et al. 2005). In addition to joint involvement, extra-articular manifestations of RA can affect several organs and systems. Pulmonary involvement in RA has been the subject of increasing interest in recent years, particularly interstitial lung disease (ILD) as one of the most severe extra-articular manifestation, leading to progressive respiratory failure (Salaffi et al. 2019). ILD occurs in 1.8% to 67% of RA patients, determining a worse prognosis and mortality (Fazeli et al. 2021; Spagnolo et al. 2018; Raimundo et al. 2019). The management and treatment of RA-ILD are challenging because there is still little information available and there are no clinical trials dedicated on the topic, although this aspect is increasingly considered by guidelines (Holroyd et al. 2019). Current therapies for RA treatment are worldwide extensively evaluated to determine their effect on lung and RA-ILD patients. Since few years the efficacy of anti-fibrotic drugs used in idiopathic pulmonary fibrosis has been evaluated in RA-ILD patients (Redente et al. 2018). Many authors discuss the lung toxicity of disease modifying anti-rheumatic drugs (DMARDs), particularly methotrexate (England and Hershberger 2020; Roubille and Haraoui 2014). If until recently methotrexate was thought to be toxic for patients with RA-ILD because it could lead to pulmonary toxicity, now the most recent data show that it could slow down the progression of lung damage (Wells 2021; Rojas-Serrano et al. 2017).
The introduction of biotechnological DMARDs (bDMARDs) and, more recently, of Janus-kinase inhibitors (JAKis) has changed the course of RA, markedly improving the control of synovitis and, consequently, reducing joint destruction and physical disability (Nash et al. 2021; Fraenkel et al. 2021). We have recently underlined the “protective” effect of abatacept (ABA), a T lymphocyte co-stimulation antagonist, in patients with RA-ILD, showing reasonable efficacy in slowing the progression of RA-ILD in about 88% of cases (Tardella et al. 2021). Other bDMARDs are suggested for RA-ILD therapy, such as rituximab and tocilizumab, but in addition to their higher infectious risk compared with ABA, cases of onset or worsening of ILD have been reported in patients taking these drugs (Manfredi et al. 2020; Soubrier et al. 2008; Wendling et al. 2013; Hadjinicolaou et al. 2011).
JAKis is a group of drugs classified as "small molecules" or "targeted synthetic DMARDs" to differentiate themselves from bDMARDS by the different mechanism of action. They are medications that are taken orally every day and have demonstrated efficacy in the treatment of RA, either alone or in combination with methotrexate (Fleischmann et al. 2017; Lee and Bae 2016). Little is known about the safety and tolerability of JAKis in patients with RA-ILD (Khoo et al. 2020; Salvarani et al. 2021; Citera et al. 2021). New data are emerging in favor of tofacitinib (TFN), the longest used JAKi, to slowing the progression of ILD associated to connective tissue diseases (Romero-Bueno et al. 2020; Chen et al. 2019; Pineton De Chambrun et al. 2020). Sendo and colleagues showed the effect of TFN on mice that developed arthritis and ILD, revealing a significant slowdown in ILD progression (Sendo et al. 2019). Lescoat and colleagues tested the effect of ruxolitinib, a JAKi used in the treatment of myelofibrosis, on mice with scleroderma-like ILD, revealing improved skin and lung involvement (Lescoat et al. 2020). These preliminary data prompt testing of the combined anti-inflammatory and anti-fibrotic properties of JAKis also in patients with RA-ILD.
The primary outcome of interest of this study is the evaluation of the evolution of pulmonary fibrosis in patients receiving JAKis compared to those receiving ABA, using computerized HRCT assessment. A secondary objective is to identify predictors of an unfavorable treatment outcome of RA-ILD during JAKis therapy.
Materials and methods
Study population and assessment
Study data were extracted from a database dedicated to RA patients referred to the Rheumatology Clinic, Università Politecnica delle Marche (Italy). Patients were included if aged > 18 years, met the American College of Rheumatology (ACR)/EUropean League Against Rheumatism (EULAR) classification criteria for RA diagnosis and, concomitantly, the American Thoracic Society/ATS/ERS 2015 criteria for ILD diagnosis (Aletaha et al. 2010; Travis et al. 2013). Moreover, patients included in the study were: RA-ILD patients who took continuous JAKi therapy (TFN at an oral dose of 5 mg BID or baricitinib at an oral dose of 4 mg daily) or ABA therapy at a standard dose of 125 mg/week subcutaneously for at least 18 months, who performed a rheumatologic evaluation [tender joint count (TJC) and swollen joint count (SJC), Clinical Disease Activity Index (CDAI), Health Assessment Questionnaire-Disability Index (HAQ-DI)] and a pulmonary investigation [high-resolution computed tomography of the lung (HRCT), pulmonary function test (PFT), single-breath diffusion lung capacity of carbon monoxide (DLco, % predicted, corrected for hemoglobin), Borg's dyspnea index (BDI)] within two weeks the beginning of a JAKi or ABA (time 0) and after 18 months (time 18). We included patients with ≥ 10% extent of fibrosis on HRCT. We considered the group of patients treated with ABA as a control group because it is one of the recommended treatments in RA-ILD. Patients concomitantly taking methotrexate (MTX) or other conventional synthetic DMARDs (csDMARDs) and/or glucocorticoids at a dose of less than 10 mg daily prednisone or equivalent were included. Patients with a history of lung diseases, other than ILD, and/or NYHA stage II-IV heart failure and patients who had been previously treated with ABA or JAKis were excluded.
Baseline data included demographic variables, smoking habits, duration of disease (defined as time elapsed since diagnosis), and the presence or absence of rheumatoid factor (RF) and anti-citrullinated peptide antibodies (ACPA).
The evaluation of the ILD extension was performed with a computer-aided method (CaM) able to estimate the percentage of fibrosis at HRCT, based on what has been described in detail in previous works (Salaffi et al. 2020a; Ariani et al. 2014; Salaffi et al. 2020b). The CaM was performed with OsiriX MD 7, a DICOM visualization software (OsiriX MD version 7, 64-bit format) on a Mac Mini (2.8 GHz Intel Core 2 Duo Desktop Computer, 16 GB random access memory; Apple Computer, Cupertino, CA, USA) with Mac OSX 10.12.2 operating system. Two independent radiologists, blind to the clinical data, evaluated HRCT lung abnormalities and the CaM quantification process.
According to HRCT results, performed at time 0 and time 18, patients were divided into three groups based on HRCT-CaM comparison: the "worsened" group included patients with ≥ 15% progression of pulmonary fibrosis, the "improved" group included patients with ≥ 15% reduction of pulmonary fibrosis, and the "stable" group included patients with progression or reduction of fibrosis < 15%. The 15% CaM-change threshold derived from the standard deviation of the change in mean value after 18 months of follow-up.
Statistical analysis
Data were processed with MedCalc 19.0.6 (statistical software packages for Windows XP). Normal distribution was tested using the Kolmogorov–Smirnov test. The median and interquartile ranges (IQR) as well as the mean and standard deviations (SD) were presented wherever appropriate. Continuous variables were compared using the parametric two-sample t test and the one-way analysis of variance (ANOVA) test, while categorical variables among patients were compared using the χ2 test. Values at time 0 and time 18 were compared with the two-sided coupled t test and the non-parametric Wilcoxon signed rank test.
Corrected multivariate regression analysis was used to assess the strength of the association between RA characteristics and HRCT response to ABA or JAKis, considering CaM quantification as a dependent variable. Age, sex, disease duration, disease onset, smoking habit, presence of RF, presence of ACPA, CDAI, and HAQ-DI were the covariates. Results were expressed as multivariate regression coefficient (R) and adjusted quadratic regression coefficient (R2) for the number of variables included in the analysis. Significance was set at p < 0.05.
Results
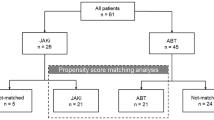
We enrolled 75 patients (69.3% women, mean age 59.5 ± 7.77 and mean disease duration 7.44 ± 3.25 years), of whom 31 received a JAKi (18 patients took baricitinib, 13 patients took TFN) and 44 were treated with ABA. Both treatment groups had similar demographic and clinical features (Table 1). As shown, at time 0 the included patients had on average a mild to moderate lung function impairment (forced vital capacity 81.7% and DLco 59.2%) and the CaM assessment showed a mean fibrosis rate of 19.4 and 18.5% in patients treated with ABA or JAKis, respectively. A fibrosis rate greater than 20% at CaM evaluation occurred in 14/31 (45%) and 18/44 (41%) of patients in the JAKis and ABA groups, respectively. In ABA group 23 (52.3%) patients were ACPA positive and 28 (63.6%) RF positive, all patients were concomitantly treated with csDMARDs, 16 (36.4%) patients were previously treated with a bDMARD and 31 (70.4%) patients took steroids at a mean dose of 3.7 (range 1.25–8.5) mg prednisolone/day equivalent. In JAKis group 16 (51.6%) patients were ACPA positive and 19 (61.3%) RF positive, no patients were simultaneously taking csDMARDs, 11 (35.5%) patients were previously treated with a bDMARD and 21 (67.7%) patients took steroids at a mean dose of 3.3 (range 1.11–8.22) mg prednisolone/day equivalent.
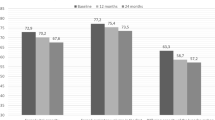
At time 18 the RA articular manifestations had a statistically significant improvement (p < 0.001) both as an estimate of disease activity and as of patients-outcome in both treatment groups (Fig. 1). In contrast, the pulmonary component did not show a statistically significant reduction for both patients-outcomes and instrumental evaluation (Fig. 2). In ABA group 5 (11.4%) patients showed a HRCT deterioration, 32 (72.6%) were considered stable, 7 (16.0%) patients showed an HRCT improvement, while in JAKis group 5 (16.1%) patients showed a HRCT deterioration, 20 (64.5%) were considered stable, 6 (19.4%) patients showed an HRCT improvement at time 18.
Considering the whole cohort, the percentage of current smokers was different between those classified as "worsened" and those classified as "improved/stable" (p = 0.01).
In multivariate regression analysis, current smoking habit (p = 0.005) and concomitant methotrexate treatment (p = 0.0078) were the two variables related to RA-ILD progression in ABA-treated patients. In JAKis-treated patients, the only variable related to RA-ILD deterioration was disease duration (p < 0.001), whereas smoking exposure, immunological profile, lung function, percentage of lung fibrosis at time 0, and disease activity did not show a statistical correlation (Table 2).
Discussion
ILD is an insidious condition in terms of morbidity and quality of life in patients with connective tissue diseases. Our study highlights the efficacy of JAKis in patients with RA-ILD, in relation also to the results obtained in a similar cohort treated with ABA. To the best of our knowledge, this is the first study evaluating the efficacy of JAKis treatment in patients with RA-ILD by assessing the evolution of lung fibrosis rate with a HRCT-CaM. In 83.9% of RA-ILD patients on JAKis therapy there was no deterioration of the lung disease. This finding is like that obtained in RA-ILD patients treated with ABA (88.6%), representing another step in the knowledge of the complex RA-ILD therapy.
As mentioned, there are encouraging efficacy data of JAKis in studies performed in RA-ILD mice and, especially, in patients with RA-ILD-related myositis (Chen et al. 2019; Citera et al. 2021; Khoo et al 2020; Romero-Bueno et al. 2020; Salvarani et al. 2021). Data on the efficacy of JAKis in the treatment of RA-ILD have not yet been published, except for a few case reports or studies with small groups of patients. Saldarriaga-River and colleagues reported the use of tofacitinib in three RA patients, two with ILD and one with chronic obstructive pulmonary disease. After 8–12 months, they had no lung disease exacerbations and hospitalizations, achieving good control of joint disease (Saldarriaga-Rivera and López-Villegas 2019). A recent Italian monocentric study investigated the efficacy of baricitinib in 11 RA and 4 RA-ILD patients for 6 months, analyzing the trend of serum values of proinflammatory cytokine and lung function test parameters. They found an improvement in DLco and diffusion coefficient (KCO) percentages during baricitinib treatment in the entire population. They also noted a reduction in KL-6, a molecule produced predominantly in the lung by damaged type II pneumocytes, but they did not evaluate the evolution of pulmonary fibrosis at HRCT (d’Alessandro et al. 2020). Another case of a patient with progressive RA-ILD treated with TFN was recently published, showing stabilization of ILD and improvement of respiratory symptoms. The authors emphasized the advantage of managing JAKis in case of infectious complications due to its short half-life (Vacchi et al. 2021). This aspect is not minor because respiratory tract infections in patients treated with JAKis are frequent and insidious. We were unable to study safety data because there was a lack of available data. Salvarani and colleagues recently estimated the incidence of infectious and non-infectious ILD in baricitinib-treated RA patients. They performed a review of 3770 patients with RA from eight randomized clinical trials and one long-term extension study on baricitinib, concluding that RA patients treated with baricitinib were at low risk of developing ILD (Salvarani et al. 2021). A similar analysis was performed on 21 tofacitinib clinical trials, in which 42 (0.16%) ILD events were identified among 7061 RA patients, confirming the low risk of developing ILD in JAKis-treated patients. The authors found also that age 65 years or older, current smokers, and a high disease activity index are the risk factors associated with the development of RA-ILD (Citera et al. 2021). In our analysis, the only variable related to ILD progression in JAKis group was RA disease duration while the remaining covariates did not show correlation, and this could be due to the small number of patients enrolled or a bias in recruitment. This aspect is of non-unique interpretation and in many studies on the topic the results are not always concordant (Dawson et al. 2002; Zamora-Legoff et al. 2017). However, disease duration is one of the already known risk factors to be considered in the management of RA patients.
In 2014 two new anti-fibrotic drugs (nintedanib and pirfenidone) were licensed to treat idiopathic pulmonary fibrosis. Data on their efficacy in the treatment of nonidiopathic ILD are emerging. Data from the INBUILD trial show the efficacy of nintedanib, a small molecule protein kinase inhibitor, in patients with ILD in connective tissue diseases. Eighty-nine RA patients, 44 patients with systemic sclerosis and 20 patients with mixed connective tissue disease were recruited in the study (Wells et al. 2020). The drug reduced the annual rate of decline in forced vital capacity by 57% compared to placebo, promoting drug approval by the US Food and Drug Administration for progressive-ILD, including connective tissue disease-ILD. Additional data are emerging from real-life studies (Vacchi et al. 2020). Narvaez and colleagues used nintedanib in combination with immunosuppressants for at least 6 months in six RA-ILD patients. They showed a reversal of the decline in lung function parameters, achieving a stabilization of forced vital capacity and DLco (Narváez et al. 2020). In RA-ILD mice models pirfenidone has demonstrated an inhibitory effect on transition from fibroblast to myofibroblast in the lungs (Wu et al. 2019). At present a trial on safety and efficacy of pirfenidone on RA-ILD patients is ongoing (Solomon et al. 2019). Certainly, anti-fibrotic drugs offer a very important therapeutic opportunity in progressive RA-ILD as adjunctive therapy to DMARDs. However, it could lead to additional side effects (liver toxicity and diarrhea) reducing adherence to treatment. Therefore, it would be advisable using a drug that can be effective on both articular and extra-articular manifestations and JAKis might be the best choice.
In our study the progression of pulmonary fibrosis at HRCT is used to assess response to treatment. In literature, the most widely used method for this purpose is the trend of PFT parameters, particularly forced vital capacity, and of DLco. We use HRCT-CaM in daily practice because it is a reliable, reproducible and easily comparable parameter, so it might also be used in clinical research (Tardella et al. 2021, Salaffi et al. 2020b). PFT and DLco could be influenced by many factors, both pulmonary and extra-pulmonary, and are operator- and patient-dependent. This aspect is a distinctive feature of our research and is a hot topic in rheumatology investigation because of the clear advantages demonstrated, particularly the rapidity of estimating fibrosis percentage (Salaffi et al. 2020b).
This study has some limitations. It is a retrospective, single-center study with a small number of patients enrolled. We do not know how many patients had severe infectious complications that had to be permanently discontinued JAKis or ABA treatment. Furthermore, we do not know the onset of ILD so we do not know if they are progressive ILDs and the rate at which they are advancing. In addition, lung fibrosis in our cohort is mild to moderate with less than 20% fibrosis in 43/75 (57.3%) cases, so our results cannot be generalized to patients with severe forms of RA-ILD, which are the most difficult to manage in clinical practice, as they can lead to respiratory failure or death.
Conclusions
Our study showed that JAKis-treated patients worsened in only 16% of cases. Therefore, it can be stated that JAKis are effective in slowing down fibrosis in RA-ILD, and they should be considered as a first-choice therapy in RA patients with active synovitis and ILD before a stage of extensive fibrosis. From these data, prospective studies with a larger cohort are mandatory to consolidate these promising results.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aletaha D, Neogi T, Silman AJ et al (2010) 2010 Rheumatoid arthritis classification criteria: an american college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum 62:2569–2581
Ariani A, Carotti M, Gutierrez M et al (2014) Utility of an open-source DICOM viewer software (OsiriX) to assess pulmonary fibrosis in systemic sclerosis: preliminary results. Rheumatol Int 34:511–516
Chen Z, Wang X, Ye S (2019) Tofacitinib in amyopathic dermatomyositis-associated interstitial lung disease. New Engl J Med 381:291–293
Citera G, Mysler E, Madariaga H et al (2021) Incidence rates of interstitial lung disease events in tofacitinib-treated rheumatoid arthritis patients: post hoc analysis from 21 clinical trials. J Clin Rheumaol 27:e482–e490
d’Alessandro M, Perillo F, Metella Refini R et al (2020) Efficacy of baricitinib in treating rheumatoid arthritis: modulatory effects on fibrotic and inflammatory biomarkers in a real-life setting. Int Immunopharmacol 86:106748
Dawson JK, Fewins HE, Desmond J et al (2002) Predictors of progression of HRCT diagnosed fibrosing alveolitis in patients with rheumatoid arthritis. Ann Rheum Dis 61:517–521
England BR, Hershberger D (2020) Management issues in rheumatoid arthritis-associated interstitial lung disease. Curr Opin Rheumatol 32:255–263
Fazeli MS, Khaychuk V, Wittstock K et al (2021) Rheumatoid arthritis-associated interstitial lung disease: epidemiology, risk/prognostic factors, and treatment landscape. Clin Exp Rheumatol 39:1108–1118
Fleischmann R, Mysler E, Hall S et al (2017) Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 390:457–468
Fraenkel L, Bathon JM, England BR et al (2021) 2021 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (hoboken) 73:924–939
Hadjinicolaou AV, Nisar MK, Bhagat S et al (2011) Non-infectious pulmonary complications of newer biological agents for rheumatic diseases-a systematic literature review. Rheumatol (oxford) 50:2297–2305
Holroyd CR, Seth R, Bukhari M et al (2019) The british society for rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatol (oxford) 58:e3–e42
Khoo JK, Barnes H, Key S et al (2020) Pulmonary adverse events of small molecule JAK inhibitors in autoimmune disease: systematic review and meta-analysis. Rheumatol (oxford) 59:2217–2225
Lee YH, Bae SC (2016) Comparative efficacy and safety of tocilizumab, rituximab, abatacept and tofacitinib in patients with active rheumatoid arthritis that inadequately responds to tumor necrosis factor inhibitors: a bayesian network meta-analysis of randomized controlled trials. Int J Rheum Dis 19:1103–1111
Lescoat A, Lelong M, Jeljeli M et al (2020) Combined anti-fibrotic and anti-inflammatory properties of jak-inhibitors on macrophages in vitro and in vivo: perspectives for scleroderma-associated interstitial lung disease. Biochem Pharmacol 178:114103
Manfredi A, Cassone G, Furini F et al (2020) Tocilizumab therapy in rheumatoid arthritis with interstitial lung disease: a multicentre retrospective study. Intern Med J 50:1085–1090
Narváez J, Vicens-Zygmunt V, Alegre JJ et al (2020) Nintedanib for the treatment of refractory progressive rheumatoid arthritis-related interstitial lung disease: a real-life case series. Rheumatology (Oxford) 59:3983–3986
Nash P, Kerschbaumer A, Dörner T et al (2021) Points to consider for the treatment of immune-mediated inflammatory diseases with janus kinase inhibitors: a consensus statement. Ann Rheum Dis 80:71–87
Pineton de Chambrun M, Hervier B, Chauveau S et al (2020) Tofacitinib in antisynthetase syndrome-related rapidly progressive interstitial lung disease. Rheumatol (oxford) 59:e142–e143
Raimundo K, Solomon JJ, Olson AL et al (2019) Rheumatoid arthritis-interstitial lung disease in the united states: prevalence, incidence, and healthcare costs and mortality. J Rheumatol 46(4):360–369
Redente EF, Aguilar MA, Black BP et al (2018) Nintedanib reduces pulmonary fibrosis in a model of rheumatoid arthritis-associated interstitial lung disease. Am J Physiol Lung Cell Mol Physiol 314:L998–L1009
Rojas-Serrano J, Herrera-Bringas D, Pérez-Román DI et al (2017) Rheumatoid arthritis-related interstitial lung disease (RA-ILD): methotrexate and the severity of lung disease are associated to prognosis. Clin Rheumatol 36:1493–1500
Romero-Bueno F, Diaz Del Campo P, Trallero-Araguás E et al (2020) Recommendations for the treatment of anti-melanoma differentiation-associated gene 5-positive dermatomyositis-associated rapidly progressive interstitial lung disease. Semin Arthritis Rheum 50:776–790
Roubille C, Haraoui B (2014) Interstitial lung diseases induced or exacerbated by DMARDS and biologic agents in rheumatoid arthritis: a systematic literature review. Semin Arthritis Rheum 43:613–626
Salaffi F, De Angelis R, Grassi W (2005) Prevalence of musculoskeletal conditions in an italian population sample: results of a regional community-based Study I. MAPP Study Clin Exp Rheumatol 23:819–828
Salaffi F, Carotti M, Di Carlo M et al (2019) High-Resolution Computed tomography of the lung in patients with rheumatoid arthritis: prevalence of interstitial lung disease involvement and determinants of abnormalities. Med (baltim) 98:e17088
Salaffi F, Carotti M, Tardella M et al (2020a) The role of a chest computed tomography severity score in coronavirus disease 2019 pneumonia. Med (baltim) 99:e22433
Salaffi F, Carotti M, Tardella M et al (2020b) Computed tomography assessment of evolution of interstitial lung disease in systemic sclerosis: comparison of two scoring systems. Eur J Intern Med 76:71–75
Saldarriaga-Rivera LM, López-Villegas VJ (2019) Janus kinase inhibitors as a therapeutic option in rheumatoid arthritis and associated interstitial lung disease. report of four cases. Rev Colomb Reumatol 26:137–139
Salvarani C, Sebastiani M, Dieude P et al (2021) Baricitinib and the risk of incident interstitial lung disease: a descriptive clinical case report from clinical trials. Rheumatol Ther 8:1435–1441
SendoS SJ, Yamada H et al (2019) Tofacitinib facilitates the expansion of myeloid-derived suppressor cells and ameliorates interstitial lung disease in SKG mice. Arthritis Res Ther 21:184
Solomon JJ, Danoff SK, Goldberg HJ et al (2019) The Design and rationale of the trail1 trial: a randomized double-blind phase 2 clinical trial of pirfenidone in rheumatoid arthritis-associated interstitial lung disease. Adv Ther 36:3279–3287
Soubrier M, Jeannin G, Kemeny JL et al (2008) Organizing pneumonia after rituximab therapy: two cases. Joint Bone Spine 75:362–365
Spagnolo P, Lee JS, Sverzellati N et al (2018) The Lung in rheumatoid arthritis: focus on interstitial lung disease. Arthritis Rheumatol 70:1544–1552
Tardella M, Di Carlo M, Carotti M et al (2021) Abatacept in rheumatoid arthritis-associated interstitial lung disease: short-term outcomes and predictors of progression. Clin Rheumatol 40:4861–4867
Travis WD, Costabel U, Hansell DM et al (2013) An Official american thoracic society/European respiratory society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188:733–748
Vacchi C, Manfredi A, Cassone G et al (2020) Combination therapy with nintedanib and sarilumab for the management of rheumatoid arthritis related interstitial lung disease. Case Rep Med 2020:6390749
Vacchi C, Manfredi A, Cassone G et al (2021) To facitinib for the treatment of severe interstitial lung disease related to rheumatoid arthritis. Case Rep Med 2021:6652845
Wells AU (2021) New insights into the treatment of CTD-ILD. Nat Rev Rheumatol 17:79–80
Wells AU, Flaherty KR, Brown KK et al (2020) Nintedanib in patients with progressive fibrosing interstitial lung diseases—subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med 8:453–460
Wendling D, Vidon C, Godfrin-Valnet M et al (2013) Exacerbation of combined pulmonary fibrosis and emphysema syndrome during tocilizumab therapy for rheumatoid arthritis. Joint Bone Spine 80:670–671
Wu C, Lin H, Zhang X (2019) Inhibitory effects of pirfenidone on fibroblast to myofibroblast transition in rheumatoid arthritis-associated interstitial lung disease via the downregulation of activating transcription factor 3 (ATF3). Int Immunopharmacol 74:105700
Zamora-Legoff JA, Krause ML, Crowson CS et al (2017) Progressive decline of lung function in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol 69:542–549
Funding
Open access funding provided by Università Politecnica delle Marche within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
FS conceived and designed the study and the protocol. MC and AG performed the HRCT examinations and their relative interpretation and were involved in revising the paper for important intellectual content. FS, MT, and MDC carried out data interpretation and analysis. FS, MT, MDC, and MC wrote the paper. MT, AG and MDC were involved in drafting the article or revising it critically for important intellectual content. All authors approved the final version to be submitted for publication.
Corresponding author
Ethics declarations
Conflict of interest
No competing interests to be declared.
Ethical approval
The local Ethics Committee (Comitato Unico Regionale-ASUR Marche) approved the protocol. The study was conducted in accordance with the Helsinki Declaration in its fifth edition (2000).
Consent to participate
All patients signed an informed consent for inclusion of personal records in the local database and for use to scientific research purposes.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tardella, M., Di Carlo, M., Carotti, M. et al. A retrospective study of the efficacy of JAK inhibitors or abatacept on rheumatoid arthritis-interstitial lung disease. Inflammopharmacol 30, 705–712 (2022). https://doi.org/10.1007/s10787-022-00936-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-00936-w