Abstract
Introduction
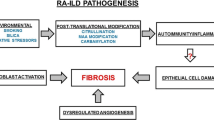
Rheumatoid arthritis (RA) is the most common of the connective tissue diseases (CTD), affecting up to 0.75% of the United States (U.S.) population with an increasing prevalence. Interstitial lung disease is prevalent and morbid condition in RA (RA-ILD), affecting up to 60% of patients with RA, leading to premature death in 10% and accruing an average of US$170,000 in healthcare costs per patient over a 5-year period. Although there have been significant advances in the management of this joint disease, there are no ongoing randomized clinical trials looking at pharmacologic treatments for RA-ILD, and there currently are no U.S. Food and Drug Administration-approved drugs for RA-ILD.
Methods/Design
We describe the Treatment for Rheumatoid Arthritis and Interstitial Lung Disease 1 (TRAIL1) trial, a multicenter randomized, double-blind, placebo-controlled, phase 2 study of the safety, tolerability and efficacy of pirfenidone in patients with RA-ILD. The study will enroll approximately 270 subjects across a network of sites who have RA and ILD as defined by a fibrotic abnormality involving greater than 10% of the lung parenchyma. The primary endpoint of the study is the incidence of the composite endpoint of decline in percent predicted forced vital capacity of 10 or greater or death during the 52-week study period. A number of secondary and exploratory endpoints have been chosen to evaluate the safety and efficacy in different domains.
Discussion
The TRAIL1 trial is designed to evaluate the safety and efficacy of pirfenidone in RA-ILD, a disease with significant impact on patients’ quality of life and outcome. In addition to investigating the safety and efficacy of pirfenidone, this trial looks at a number of exploratory endpoints in an effort to better understand the impact of therapy on areas such as changes in quantitative high-resolution computed tomography scores and a patient’s quality of life. Biospecimens will be collected in order to investigate biomarkers that could potentially predict the subtype of disease, its behavior over time, and its response to therapy. Finally, by creating a network of institutions and clinician investigators with an interest in RA-ILD, this trial will pave the way for future studies of investigational agents in an effort to reduce or eliminate the burden of disease for those suffering from RA-ILD.
Trial Funding
Genentech, a member of the Roche Group.
Trial Registration
Clinicaltrials.gov, identifier NCT02808871.

Similar content being viewed by others
References
Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004-2014. Rheumatol Int. 2017;37(9):1551–7.
Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–22.
Birnbaum H, Pike C, Kaufman R, Marynchenko M, Kidolezi Y, Cifaldi M. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin. 2010;26(1):77–90.
Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;4(5):443–8.
Demoruelle MK, Solomon JJ, Olson AL. The epidemiology of rheumatoid arthritis-associated lung disease. In: Fischer A, Lee JS, editors. Lung disease in rheumatoid arthritis. 1st ed. Totowa: Humana; 2018. p. 45–58.
Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–91.
Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20(120):85–97.
Nagai S, Hamada K, Shigematsu M, Taniyama M, Yamauchi S, Izumi T. Open-label compassionate use one year-treatment with pirfenidone to patients with chronic pulmonary fibrosis. Intern Med. 2002;41(12):1118–23.
Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1061–9.
Gahl WA, Brantly M, Troendle J, et al. Effect of pirfenidone on the pulmonary fibrosis of Hermansky-Pudlak syndrome. Mol Genet Metab. 2002;76(3):234–42.
Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171(9):1040–7.
Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):821–9.
Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–9.
King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–92.
Huang H, Dai HP, Kang J, Chen BY, Sun TY, Xu ZJ. Double-blind randomized trial of pirfenidone in Chinese idiopathic pulmonary fibrosis patients. Medicine (Baltimore). 2015;94(42):e1600.
Costabel U, Albera C, Bradford WZ, et al. Analysis of lung function and survival in RECAP: an open-label extension study of pirfenidone in patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(3):198–205.
Ogura T, Taniguchi H, Azuma A, et al. Safety and pharmacokinetics of nintedanib and pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1382–92.
Behr J, Bendstrup E, Crestani B, et al. Safety and tolerability of acetylcysteine and pirfenidone combination therapy in idiopathic pulmonary fibrosis: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med. 2016;4(6):445–53.
Khanna D, Albera C, Fischer A, et al. An open-label, phase II study of the safety and tolerability of pirfenidone in patients with scleroderma-associated interstitial lung disease: the LOTUSS trial. J Rheumatol. 2016;43(9):1672–9.
Iwata T, Yoshino I, Yoshida S, et al. A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: west Japan Oncology Group 6711 L (PEOPLE Study). Respir Res. 2016;17(1):90.
Vancheri C, Kreuter M, Richeldi L, et al. Nintedanib with add-on pirfenidone in idiopathic pulmonary fibrosis. Results of the INJOURNEY trial. Am J Respir Crit Care Med. 2018;197(3):356–63.
Costabel U, Albera C, Lancaster LH, et al. An open-label study of the long-term safety of pirfenidone in patients with idiopathic pulmonary fibrosis (RECAP). Respiration. 2017;94(5):408–15.
Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81.
Khanna D, Mittoo S, Aggarwal R, et al. Connective tissue disease-associated interstitial lung diseases (CTD-ILD)—report from OMERACT CTD-ILD Working Group. J Rheumatol. 2015;42(11):2168–71.
Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011;183(3):372–8.
Gochuico BR, Avila NA, Chow CK, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med. 2008;168(2):159–66.
Natalini JG, Swigris JJ, Morisset J, et al. Understanding the determinants of health-related quality of life in rheumatoid arthritis-associated interstitial lung disease. Respir Med. 2017;127:1–6.
Raimundo K, Solomon JJ, Olson AL, et al. Rheumatoid arthritis-interstitial lung disease in the United States: prevalence, incidence, and healthcare costs and mortality. J Rheumatol. 2018.
Doyle TJ, Lee JS, Dellaripa PF, et al. A roadmap to promote clinical and translational research in rheumatoid arthritis-associated interstitial lung disease. Chest. 2014;145(3):454–63.
Kim EJ, Collard HR, King TE Jr. Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest. 2009;136(5):1397–405.
Assayag D, Lubin M, Lee JS, King TE, Collard HR, Ryerson CJ. Predictors of mortality in rheumatoid arthritis-related interstitial lung disease. Respirology. 2014;19(4):493–500.
Solomon JJ, Fischer A. Rheumatoid arthritis interstitial lung disease: time to take notice. Respirology. 2014;19(4):463–4.
White ES, Borok Z, Brown KK, et al. An American thoracic society official research statement: future directions in lung fibrosis research. Am J Respir Crit Care Med. 2016;193(7):792–800.
Choi K, Lee K, Ryu SW, Im M, Kook KH, Choi C. Pirfenidone inhibits transforming growth factor-beta1-induced fibrogenesis by blocking nuclear translocation of Smads in human retinal pigment epithelial cell line ARPE-19. Mol Vis. 2012;18:1010–20.
Datta A, Scotton CJ, Chambers RC. Novel therapeutic approaches for pulmonary fibrosis. Br J Pharmacol. 2011;163(1):141–72.
Hostettler K, Papakonstantinou E, Klagas I, et al. Anti-fibrotic effects of pirfenidone in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis. Eur Respir J. 2015;46(suppl 59):PA3040.
Guo J, Yang Z, Jia Q, Bo C, Shao H, Zhang Z. Pirfenidone inhibits epithelial-mesenchymal transition and pulmonary fibrosis in the rat silicosis model. Toxicol Lett. 2018;300:59–66.
Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2010;35(6):1322–8.
Lee HK, Kim DS, Yoo B, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest. 2005;127(6):2019–27.
Park JH, Kim DS, Park IN, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease–related subtypes. Am J Respir Crit Care Med. 2007;175(7):705–11.
Solomon JJ, Ryu JH, Tazelaar HD, et al. Fibrosing interstitial pneumonia predicts survival in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD). Respir Med. 2013;107(8):1247–52.
Solomon JJ, Chung JH, Cosgrove GP, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2016;47(2):588–96.
Collard HR, King TE Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168(5):538–42.
Flaherty KR, Andrei AC, Murray S, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med. 2006;174(7):803–9.
Zappala CJ, Latsi PI, Nicholson AG, et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):830–6.
Acknowledgements
Participants
We would like to thank the participants of this study.
Trial Funding
The trial and article processing charges are funded by Genentech, a member of the Roche Group. The trial sponsor has no role in study design; collection, management, analysis, and interpretation of data.
Trial Registration
The trial is registered with Clinicaltrials.gov, identifier NCT02808871.
Authorship
All named authors meet the International Committee of Medical Journal. Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
JJS, SKD, HJG, CS, SH, PFD and IOS contributed to the conception and design of the study and developed the study protocol, JJS, FW, MK and DCC are responsible for the recruitment of subjects, DD, SHH and EBP are responsible for the management of the trial and DD, CS and SH are responsible for collection and analysis of the data. All authors contributed to modification of the original protocol and all authors read and approved the final manuscript.
Disclosures
JJS receives research support for unrelated studies from Pfizer and Boehringer Ingelheim. MK reports grants from Roche-Genentech, Roche, Boehringer Ingelheim, GSK, Gilead, Actelion, Respivert, Alkermes, Pharmaxis, and Prometic. MK reports personal fees from Roche, Boehringer Ingelheim, GS, Gilead, Genoa, Prometic, Indalo and Third Pole. DCC receives honorarium and grants for research from Roche. PFD receives research support for unrelated studies from Genentech and Bristol-Myers Squibb. SKD, HJG, FW, DD, CS, SHH, SH, EBP and IOR report no conflict of interest.
Compliance with Ethics Guidelines and dissemination
This trial is designed in accordance with the Standard Protocol Items for Clinical Trials (SPIRIT) 2013 statement and will be carried out in compliance with the ethical principles of the Declaration of Helsinki. All documents are initially approved by the institutional review board (IRB) at the sponsor site (BWH) and then by the individual IRBs or competent authorities at the individual sites. Written informed consent will be obtained from all participants before any study-related procedures are implemented. When available, the results will be published in an international peer-reviewed journal.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.9741716.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Solomon, J.J., Danoff, S.K., Goldberg, H.J. et al. The Design and Rationale of the Trail1 Trial: A Randomized Double-Blind Phase 2 Clinical Trial of Pirfenidone in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Adv Ther 36, 3279–3287 (2019). https://doi.org/10.1007/s12325-019-01086-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01086-2