Abstract
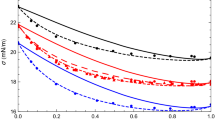
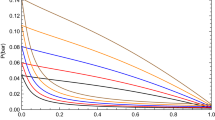
This work has been dedicated to modeling the interfacial tension of the heptane + alcohol mixtures in the temperature range of 288.15 K to 333.15 K. The cubic plus association equation of state is applied to the liquid–vapor phase equilibrium calculations. The binary interaction parameters are obtained according to the experimental isothermal and isobaric phase equilibrium data. For the binary interaction parameters, correlations have been obtained as a function of temperature for isothermal phase equilibrium, and a constant value for isobaric phase equilibrium. The linear gradient theory is used as a predictive and adjustment approach to describe the interfacial tension of the heptane + alcohol mixtures. The influence parameters of the pure components were constant and the symmetric parameters of the binary mixtures were correlated with the temperature. The results of this work show that the cubic plus association equation of state is capable of simultaneously representing the phase equilibrium and interfacial tension of the mixtures studied. The results obtained in the interfacial tension are in agreement with those published in the literature.











Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- a :
-
Attractive parameter in CPA-EOS
- \(a_0\) :
-
Adjustable parameter of the CPA-EOS
- AAD:
-
statistical deviation
- b :
-
Covolume parameter in the CPA-EOS
- C :
-
Number of carbons in the alcohol
- c :
-
Influence parameter
- c 1 :
-
Adjustable parameter of the CPA EOS
- f 0 :
-
Helmholtz energy density
- g :
-
The radial distribution function
- \(k_{ij}\) :
-
Interaction parameter in the CPA-EOS
- n :
-
Number of points
- \(n_c\) :
-
Number of components
- P :
-
Absolute pressure
- R :
-
Universal gas constant
- T :
-
Absolute temperature
- x :
-
Mole fraction of the liquid phase
- \(X_{A_i}\) :
-
The mole fraction of the molecule i not bonded at site A
- z :
-
Position in the interface
- \(\beta ^{A_i B_i}\) :
-
The association volume
- \(\beta _{ij}\) :
-
Symmetric parameter
- \(\Delta ^{A_i B_j}\) :
-
The association strength
- \(\epsilon ^{A_i B_i}\) :
-
The association energy
- \(\eta\) :
-
Reduced density
- \(\mu\) :
-
Chemical potential
- \(\Omega\) :
-
Grand thermodynamic potential
- \(\rho\) :
-
Molar concentration
- \(\sigma\) :
-
Interfacial tension
- c :
-
Critical condition
- i, j, s :
-
Species
- 0:
-
Equilibrium condition
- exp :
-
Experimental
- L :
-
Liquid phase
- theo :
-
Theoretical
- V :
-
Vapor phase
References
I.V. Yakoumis, G.M. Kontogeorgis, E.C. Voutsas, D.P. Tassios, Vapor-liquid equilibria for alcoholhydrocarbon systems using the CPA equation of state. Fluid Phase Equilib. 130, 31–47 (1997)
L. Segade, J. Jiménez de Llano, M. Domínguez-Pérez, O. Cabeza, M. Cabanas, E. Jiménez, Density, surface tension, and refractive index of octane+ 1-alkanol mixtures at t= 298.15 k. J. Chem. Eng. Data 48, 1251–1255 (2003)
A. Hernández, Modeling interfacial tension of hexane + alcohol mixtures at different temperatures using linear gradient theory with cubic plus association equation of state. Int. J. Thermophys. 41, 1–18 (2020)
J.D. van der Waals, The thermodynamik theory of capillarity under the hypothesis of a continuous variation of density. Zeit Phys. Chem. 13, 675–725 (1894)
J.W. Cahn, J.E. Hilliard, Free energy of a nonuniform system. I. Interfacial free energy. J. Chem. Phys. 28, 258–267 (1958)
A. Mejía, J.C. Pàies, D. Duque, H. Segura, L.F. Vega, Phase and interface behaviors in type-i and type-v Lennard–Jones mixtures Theory and simulations. J. Chem. Phys. 123, 034505 (2005)
X. Liang, M.L. Michelsen, G.M. Kontogeorgis, A density gradient theory based method for surface tension calculations. Fluid Phase Equilib. 428, 153–163 (2016)
A. Hernández, Interfacial behavior prediction of alcohol+ glycerol mixtures using gradient theory. Chem. Phys. 534, 110747 (2020)
Y.-X. Zuo, E.H. Stenby, Calculation of surface tensions of polar mixtures with a simplified gradient theory model. J. Chem. Eng. Jpn. 29, 159–165 (1996)
K.A.G. Schmidt, G.K. Folas, B. Kvamme, Calculation of the interfacial tension of the methane-water system with the linear gradient theory. Fluid Phase Equilib. 261, 230–237 (2007)
A. Mejía, H. Segura, L. Vega, J. Wisniak, Simultaneous prediction of interfacial tension and phase equilibria in binary mixtures: an approach based on cubic equations of state with improved mixing rules. Fluid Phase Equilib. 227, 225–238 (2005)
X. Liang, M.L. Michelsen, General approach for solving the density gradient theory in the interfacial tension calculations. Fluid Phase Equilib. 451, 79–90 (2017)
G.M. Kontogeorgis, G.K. Folas, Thermodynamic models for industrial applications: from classical and advanced mixing rules to association theories (Wiley, Hoboken, 2009)
G.M. Kontogeorgis, E.C. Voutsas, I.V. Yakoumis, D.P. Tassios, An equation of state for associating fluids. Ind. Eng. Chem. Res. 35, 4310–4318 (1996)
X. Liang, M.L. Michelsen, G.M. Kontogeorgis, Pitfalls of using the geometric-mean combining rule in the density gradient theory. Fluid Phase Equilib. 415, 75–83 (2016)
G.K. Folas, J. Gabrielsen, M.L. Michelsen, E.H. Stenby, G.M. Kontogeorgis, Application of the cubic-plus-association (CPA) equation of state to cross-associating systems. Ind. Eng. Chem. Res. 44, 3823–3833 (2005)
E.C. Voutsas, I.V. Yakoumis, D.P. Tassios, Prediction of phase equilibria in water/alcohol/alkane systems. Fluid Phase Equilib. 158, 151–163 (1999)
G. Soave, Equilibrium constants from a modified Redlich-Kwong equation of state. Chem. Eng. Sci. 27, 1197–1203 (1972)
S.H. Huang, M. Radosz, Equation of state for small, large, polydisperse, and associating molecules. Ind. Eng. Chem. Res. 29, 2284–2294 (1990)
G.M. Kontogeorgis, I.V. Yakoumis, H. Meijer, E. Hendriks, T. Moorwood, Multicomponent phase equilibrium calculations for water-methanol-alkane mixtures. Fluid Phase Equilib. 158, 201–209 (1999)
T.Y. Kwak, G.A. Mansoori, Van der Waals mixing rules for cubic equations of state. applications for supercritical fluid extraction modelling. Chem. Eng. Sci. 41, 1303–1309 (1986)
Y.-X. Zuo, E.H. Stenby, A linear gradient theory model for calculating interfacial tensions of mixtures. J. Colloid Interface Sci. 182, 126–132 (1996)
Y.-X. Zuo, E.H. Stenby et al., Prediction of interfacial tensions of reservoir crude oil and gas condensate systems. SPE J. 3, 134–145 (1998)
M.B. Oliveira, I.M. Marrucho, J.A.P. Coutinho, A.J. Queimada, Surface tension of chain molecules through a combination of the gradient theory with the CPA EoS. Fluid Phase Equilib. 267, 83–91 (2008)
D. Papaioannou, C.G. Panayiotou, Surface tensions and relative adsorptions in hydrogen-bonded systems. J. Chem. Eng. Data 39, 457–462 (1994)
I.A. McLure, J.T. Sipowska, I.L. Pegg, Surface tensions of (an alkanol+ an alkane)1. Propan-1-ol+ heptane. J. Chem. Thermodyn. 14, 733–741 (1982)
J. Vijande, M.M. Pineiro, J. García, J.L. Valencia, J.L. Legido, Density and surface tension variation with temperature for heptane+ 1-alkanol. J. Chem. Eng. Data 51, 1778–1782 (2006)
C. Berro, M. Rogalski, A. Péneloux, A new ebulliometric technique. Vapour-liquid equilibria in the binary systems ethanol-n-heptane and ethanol-n-nonane. Fluid Phase Equilib. 8, 55–73 (1982)
S.G. Sayegh, J.H. Vera, G.A. Ratcliff, Vapor-liquid equilibria for the ternary system n-heptane/n-propanol/l-chlorobutane and its constituent binaries at 298.15 k. Can. J. Chem. Eng. 57, 513–519 (1979)
A.G. Pradhan, V.R. Bhethanabotla, S.W. Campbell, Vapor-liquid equilibrium data for ethanol-n-heptane-1-propanol and ethanol-n-heptane-2-propanol and their interpretation by a simple association model. Fluid Phase Equilib. 84, 183–206 (1993)
H.C. Van Ness, C.A. Soczek, G.L. Peloquin, R.L. Machado, Thermodynamic excess properties of three alcohol-hydrocarbon systems. J. Chem. Eng. Data 12, 217–224 (1967)
J.R. Powell, M.E.R. McHale, A.M. Kauppila, W.E. Acree, P.H. Flanders, V.G. Varanasi, S.W. Campbell, Prediction of anthracene solubility in alcohol+ alkane solvent mixtures using binary alcohol+ alkane vle data. comparison of kretschmer-wiebe and mobile order models. Fluid Phase Equilib. 134, 185–200 (1997)
A. Belabbaci, R.M. Villamanan, L. Negadi, C.M. Martin, A Ait Kaci, M. Villamanan, Vapor–liquid equilibria of binary mixtures containing 1-butanol and hydrocarbons at 313.15 k. J. Chem. Eng. Data 57, 114–119 (2012)
C.P. Smyth, E.W. Engel, Molecular orientation and the partial vapor pressures of binary liquid mixtures. II. Systems containing an alcohol. J. Am. Chem. Soc. 51, 2660–2670 (1929)
P.R. Rao, C. Chiranjivi, C.J. Dasarao, Vapour-liquid equilibria systems: Hexane-hexylalcohol and heptane-hexylalcohol. J. Appl. Chem. 18, 166–168 (1968)
M. Goral, P. Oracz, A. Skrzecz, A. Bok, A. Maczynski, Recommended vapor-liquid equilibrium data binary n-alkanol-n-alkane systems. J. Phys. Chem. Ref. Data 31, 701–748 (2002)
R.H. Weiland, T. Chakravarty, A.E. Mather, Solubility of carbon dioxide and hydrogen sulfide in aqueous alkanolamines. Ind. Eng. Chem. Res. 32, 1419–1430 (1993)
R.H. Weiland, T. Chakravarty, A.E. Mather, Solubility of carbon dioxide and hydrogen sulfide in aqueous alkanolamines. Ind. Eng. Chem. Res. 34, 3173 (1995)
A. Hernández, M. Cartes, A. Mejía, Measurement and modeling of isobaric vapor-liquid equilibrium and isothermal interfacial tensions of ethanol+ hexane+ 2, 5-dimethylfuran mixture. Fuel 229, 105–115 (2018)
S. Khosharay, F. Varaminian, Modeling interfacial tension of (CH4+ N2)+ H2O and (N2+ Co2)+ H2O systems using linear gradient theory. Korean J. Chem. Eng. 30, 724–732 (2013)
Acknowledgments
A.H acknowledges the economic support given by the UCSC.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hernández, A. Modeling Interfacial Tension of Heptane + Alcohol Mixtures Using Cubic Plus Association Equation of State Plus Simplified Gradient Theory. Int J Thermophys 44, 16 (2023). https://doi.org/10.1007/s10765-022-03126-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-022-03126-6