Abstract
Conspecific animal groups often compete for access to fitness-enhancing resources. The more valuable the resource at stake is, the greater the costs groups can afford to outcompete their neighbours, leading to between-group conflicts. We investigated what factors affected intergroup encounter outcome (win, loss, or draw) and intensity (level of aggression and duration) in wild, crested macaques (Macaca nigra). We collected data on 158 dyadic intergroup encounters among three groups of crested macaques in Tangoko Nature Reserve (Indonesia) between November 2015 and July 2016. Intergroup encounters were more likely to have a clear winner the larger the group size difference was between the opposing groups and when both groups rarely used the intergroup encounter location. Groups tended to win in specific parts of their home range, regardless of the numerical advantage, the frequency of use of the intergroup encounter location and its distance to the closest core area. Most encounters involved aggression, but contact aggression was rare. None of our candidate predictors helped to explain the differences in intergroup encounter escalation. Male intergroup aggression was more common than female intergroup aggression. The probability of female and male participation in intergroup aggression increased with the participation of the other sex. Males chased and attacked females in their group (i.e., herded them) in most encounters. Our study suggests that intragroup sexual conflict occurs during intergroup encounters in crested macaques. More detailed and longer studies on intergroup participation may help to understand the factors behind crested macaque intergroup encounter outcome and intensity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conspecific animal groups often compete for limited resources, such as food (Brown & Crofoot, 2013; Harris, 2006; Williams et al., 2004), mates (Cooper et al., 2004; Fashing, 2001), shelter (Markham et al., 2016; Strong et al., 2018), or water (Markham et al., 2012). During intergroup encounters (IGEs), competition for key resources can lead to intergroup conflicts, in which members of the opposing groups behave aggressively towards one another. The outcome and escalation of IGEs have successfully been modelled by using dyadic conflict theory (Cassidy et al., 2015; Crofoot et al., 2008; Green et al., 2021; Koch et al., 2016a; Radford & Du Plessis, 2004; Roth & Cords, 2016). Dyadic conflict theory predicts that the outcome and intensity of conflicts reflect asymmetries in resource holding potential (RHP) and resource value between opponents (Kokko, 2013). RHP is the fighting capacity of an individual/group and influences its ability to acquire or retain access to resources (Green et al., 2021; Parker, 1974). The number of group members (i.e., group size) often is used as a proxy for a group’s maximum RHP (Cassidy et al., 2015; Crofoot et al., 2008; Furrer et al., 2011; Green et al., 2021; Radford & Du Plessis, 2004). Bigger groups tend to displace and win IGEs against smaller groups (Majolo et al., 2020). Suppose opposing groups differ in size, but other factors are equal (e.g., the fighting ability of the two groups). In that case, the smallest group is expected to retreat quickly, leading to short encounters with minimal or no aggression (Roth & Cords, 2016). In contrast, aggressive escalation is expected when group sizes are similar between opponents, especially if resources at stake are valuable (Kokko, 2013; Roth & Cords, 2016; Smith & Parker, 1976). Nevertheless, if numerical imbalances between groups are extreme, such as when an isolated individual faces multiple attackers, aggression can also escalate and even be lethal (Gros-Louis et al., 2003; Martínez-Íñigo et al., 2021; Rosenbaum et al., 2016; Wilson et al., 2014; Wrangham, 1999). Thus, numerical imbalance and aggressive escalation in intergroup conflicts may follow a U-shaped relationship.
Resource value (RV) is the worth of the contested resource as perceived by the contesting groups (Kokko, 2013). Food resources are often patchily distributed across groups’ home ranges (Brown, 2013), and food-rich areas are visited more frequently (Di Bitetti, 2001; Harris, 2006; Seiler et al., 2017). Consequently, models of IGE outcome and intensity have employed the frequency of use of an IGE location (e.g., frequency of occupation) (Roth & Cords, 2016; Strong et al., 2018), its distance to the edge (Roth & Cords, 2016), or to the centre of the home range of each group (Crofoot et al., 2008) as a proxy of the RV for that location and group. Groups may assign greater value to the centre of their home range and thus spend more time there than in peripheral areas if the home range centre has higher resource density and/or have resources (e.g., shelter) that are not found anywhere else in the home range (Crofoot et al., 2008; Giraldeau & Ydenberg, 1987). For these reasons, when RHP is similar between contesting groups, the group using the IGE area more often (Roth & Cords, 2016; Strong et al., 2018) or which is closer to its home range centre (Crofoot et al., 2008; Koch et al., 2016a) tends to win.
Models of IGE outcome and intensity accounting for group size and location (i.e., group size-location models) can be highly explanatory for species where within-group individual interests are similar, such as in one-male-multi-female groups (e.g., blue monkeys (Cercopithecus mitis stuhlmanni) (Cords, 2007; Roth & Cords, 2016)). However, in multi-male-multi-female groups, females and males may follow different strategies during IGEs (Johnstone et al., 2020; Kitchen & Beehner, 2007; Kitchen et al., 2004; Wilson et al., 2012). Then, group size-location models might explain a small variance of IGE outcome and intensity. In mammals, the most frequent limiting factor for reproduction is access to food for females and mates for males (Trivers, 1972). Therefore, male participation in intergroup conflicts reflects the need to defend access to mates, whereas female participation is linked to the defence of food resources (Fashing, 2001; Kitchen & Beehner, 2007; Nunn & Deaner, 2004).
Female active participation in intergroup conflict is expected whenever the potential fitness benefits of winning an IGE, derived from retaining or gaining access to food resources, are greater than the potential costs (Arseneau-Robar et al., 2017; Cheney & Seyfarth, 1987; Cooper et al., 2004). In this case, the number of adult females and an IGE location-value measure could function as proxies for RHP and RV, respectively. The more valuable the food resource, the more motivation females may have to defend it (Arseneau-Robar et al., 2017; Cheney & Seyfarth, 1987). However, female participation costs may depend on their reproductive status. First, females carrying infants risk losing reproductive investment if their offspring are injured or killed during an intergroup conflict. Thus, females with infants may avoid engaging in IGEs (Arseneau-Robar et al., 2017; Cheney & Seyfarth, 1987; Koch et al., 2016b, but see Lewis et al., 2020), which would reduce the realised RHP of the group (i.e., the actual number of participants). Second, males may attack females from their group to prevent outgroup mating. Such herding behaviour is often directed towards sexually receptive females (Henzi et al., 1998; Kitchen & Beehner, 2007), although indiscriminate female herding also is observed (Brown, 2013; Zhao, 1997, personal observation). Herding increases female participation costs due to larger energy expenditure, potential injuries, and stress (Palombit, 2014). Thus, the proportion of sexually receptive females might decrease the realised RHP. Moreover, the herding effort may vary according to a group’s sex ratio. The relationship between herding and sex ratio is understudied. However, savannah baboons (Papio cynocephalus) are more likely to herd females if there is a female-biased sex ratio in their troop (Henzi et al., 1998). When the number of females per male is low, within-group male competition for females might be so high that many males may not profit from herding since they will unlikely mate within their group. Yet, the number of herders may increase when the number of females is high enough for several males to mate. Nevertheless, if female bias is extreme, herding efforts become ineffective, and males are expected to refrain from doing it. Thus, we might expect an inverted U relationship between the sex ratio (females:male) and herding and, consequently, a U-shaped relationship between the sex ratio and realised RHP.
Two strategies may modulate male participation in intergroup conflicts: direct mate access defence and indirect mate access defence. Males may directly defend access to females through intergroup aggression (i.e., hired guns (Rubenstein, 1986)) and intragroup sexual coercion (i.e., herding). Males also may indirectly defend access to females by defending resources, which would improve female reproductive success and, potentially, mating opportunities (Fashing, 2001; Harris, 2006; Williams et al., 2004). The maximum male RHP can be measured by the number of adult males in the group for these strategies. If males directly defend access to females, they should participate more actively when the proportion of sexually receptive females in their group is greater, and the sex ratio is more biased toward females (Henzi et al., 1998; Kitchen & Beehner, 2007; Kitchen et al., 2004). These two parameters can be used as proxies for RV. If males defend access to mates indirectly, they will participate according to resources useful for ingroup females (Kitchen & Beehner, 2007; Scarry, 2017). Thus, an IGE location-value measure would function as RV. In either case, the number of infants within a group may promote male participation. Males may defend infants, because they might be the father when promiscuity is high (Lewis et al., 2020; Van Belle et al., 2014), but also because they may use infants as social tools to improve within-group relationships (Henkel et al., 2010; Kerhoas et al., 2016).
Crested macaques live in large multi-male-multi-female groups, often ranging from 60 to 80 individuals (Supriatna & Andayani, 2008). There often are two to five females per male (Martínez-Íñigo, 2018; O'Brien & Kinnaird, 1997; Waterman, 2021), with an average of 0.06 sexually receptive females to receptive male (Higham et al., 2021). Males are twice as heavy as females and possess large canines (Thorén et al., 2006). Females are philopatric and socially tolerant (Duboscq et al., 2013), which are features associated with intragroup cooperation to defend food resources in intergroup conflicts (Sterck et al., 1997). Such cooperation can lead to gang attacks and the killing of females from other groups (Martínez-Íñigo et al., 2021). Females exhibit a cyclic swelling of their perianal skin that indicates ovulation timing reliably (Higham et al., 2012). Reproduction is year-round and male mating, and reproductive success are highly biased toward alpha males (Engelhardt et al., 2017; Higham et al., 2021). Males usually take over alpha-male positions from other groups (Marty et al., 2017). Neighbouring groups have overlapping home ranges (Fig. 1), where exclusive areas are rare. For example, between October 2015 and June 2016, only one of three study groups had an exclusive area in their home range, representing 27.72% of it (Rismayanti et al., this issue). Neighbouring groups encounter each other frequently (0.18–1.67 encounters/day (Kinnaird & O'Brien, 2000; Martínez-Íñigo et al., 2021)), mainly when defensible food resource patchiness is higher (Kinnaird & O'Brien, 2000).
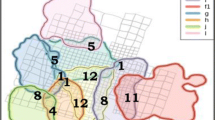
Location of the Macaca Nigra Project (MNP) field site (top left, green flag) in Tangkoko Reserve, North Sulawesi (Indonesia), and field site map (right) with the home ranges (95% BBMM) and core areas (50% BBMM) of the three crested macaques (Macaca nigra) study groups between October 2015 and July 2016. The display also shows the intergroup encounters (IGEs) recorded between November 2015 and July 2016 among the study groups (N = 158) and between a study group and a nonstudy group (N = 146).
In the present study, our first goal was to test the relative importance of reproductive strategies and group size and IGE location value in predicting IGE outcome and intensity (Table I) in free-ranging, crested macaques (Macaca nigra). Our second goal was to test what factors (Table II) best predict female and male participation in intergroup aggression in our model species.
The social tolerance of female, crested macaques, their involvement in severe intergroup aggression, and the increment in intergroup frequency when the patchiness of food resources increases suggest that female crested macaques may defend access to food resources against neighbouring groups (Duboscq et al., 2013; Martínez-Íñigo et al., 2021; Sterck et al., 1997). However, given the high between-male mating competition in the species and its sexual dimorphism, we hypothesized that males would defend mating opportunities through intergroup and intragroup aggression, limiting female participation in intergroup conflicts (Engelhardt et al., 2017; Henzi et al., 1998; Higham et al., 2021; Kitchen & Beehner, 2007; Marty et al., 2017). As such, we hypothesized that there would be a conflict of interests between males and females during intergroup encounters, leading to the low predictive power of models assuming homogeneous group interest. Thus, we predicted models representing male mate access defence would have more explanatory power than models focused on group size-location and female food defence. Moreover, we hypothesised predictors related to male direct mate-access defence would have more explanatory power than those associated with male indirect mate-access defence (Table I). We hypothesized that the probability of female and male intergroup aggression would depend on their reproductive strategies and potential costs (Table II). We hypothesized that the male direct mate access defence model would be more explanatory of male intergroup aggression than the male indirect mate access defence model.
Methods
Study Population
We collected data at Tangkoko-Batuangus Nature Reserve (1˚33'N, 125˚10'E, 0–100 m above sea level, North Sulawesi, Indonesia). The eastern part of the study area encompasses mostly primary forest, defined as undisturbed habitat abundant in trees of the families Anacardiaceae (e.g., Koordersiodedron pinnatum and Dracontomelon dao), Sapotaceae (e.g., Palaquium obovatum), Annonaceae (e.g., Cananga odorata), and Lamiaceae (e.g., Vitex quinata) (Nangoy et al., 2021). In the western part of the field site, secondary forests predominate, comprising regrown forest in disturbed habitat where trees of the families Moraceae (e.g., Ficus sp.) and Combretaceae (e.g., Terminalia catappa) dominate (Nangoy et al., 2021). The landscape is completed by regenerating gardens, and heavily disturbed areas (O'Brien & Kinnaird, 1997; Nailufar et al., 2015). Fruiting is continuous (Kinnaird and O’Brien, 2000), although the available fruit biomass varies between months and years (Kinnaird and O’Brien, 1999). The temperature ranged between 24 to 28 ºC (MNP, unpublished data). Rainfall is heavier between October and May, reaching an annual range between 1,410 and 2,352 mm (Kerhoas et al., 2014).
We collected data for a long-term project, the Macaca Nigra Project (MNP, https://www.macaca-nigra.org/). During the study, four researchers, including LMI and R, collected data on intergroup encounters. We observed three neighbouring groups of habituated crested macaques with overlapping home ranges (Fig. 1): PB1, R1, and R2. We monitored PB1 and R1 4–5 and 3–4 times per week between November 2015 and July 2016. We followed PB1 for 189 days and R1 for 153 days. We observed R2 2–3 days/week between April and July 2016 for 30 days. We followed the groups from when they came down from their sleeping trees in the morning until they settled in their sleeping trees at night (ca.12 h/day). We recorded the data using tablets (Odys Wintabs 10, AXDIA International GmbH and Acer One 10, Acer Inc.) and Ptab (3.0 for Windows, Z4Soft) software.
On each observation day, our intergroup-encounter team and the other MNP researchers recorded the number of adults in the group, the state of the sexual swelling of the adult females, and the number of infants. We considered females as adults once they gave birth for the first time. We regarded males as adults once their canines had fully erupted and their testes had descended. We classified macaques younger than one-year-old as infants. We judged females to be sexually receptive if their sexual skin was inflating or maximally tumescent (Higham et al., 2012). Throughout the manuscript, we refer specifically to adult individuals when we write about females and males without specifying age class. Sometimes, when two study groups encountered, there were observers from the intergroup-encounter team following one of the encountering groups but not the other group. However, in most cases, the group without intergroup-encounter team observers was being followed by other MNP researchers. In such cases, we used the data of those MNP researchers to complete the number of adults, infants, and sexually receptive females for the group which we (i.e., the intergroup-encounter team) were not following. We also completed our tracklog dataset with those collected by the rest of the MNP researchers.
Intergroup Encounters
We defined IGEs as events in which two fully habituated groups were in proximity (≤ 100 m) (Brown, 2011; Fashing, 2001). We recorded the start of an encounter as soon as individuals of different groups were within 100 m or as soon as we detected a closer proximity. IGEs ended once groups moved ≥ 100 m apart for at least one hour (Lawes & Henzit, 1995). At times, one to two subadult or adult males of one group continued following the other group after the rest of their original group had moved away ≥ 100 m. We also considered IGEs to have ended then, because we considered these as attempted migrations rather than intergroup encounters (Marty et al., 2017).
For each encounter, we recorded start and end times, start location (using Garmin GPSMAP 64 and 62 Handheld Navigator, Garmin Ltd., US), the maximum level of aggression reached in the encounter (no aggression, noncontact aggression, or contact aggression), and whether there was male and female participation in intergroup aggression and within-group herding. We recorded these categorical data regardless of whether aggression was uni-or bi-directional (e.g., in an IGE categorised as involving “contact aggression,” such aggression could be displayed by one of the two opposing groups or both groups). We recorded “contact aggression” when we observed biting, slapping, pushing, hitting and/or pulling between two or more individuals of different groups. We recorded a “non-contact” aggression level when we observed charges and chases between any two or more individuals of different groups but no contact aggression.
We considered a group to be the winner of an encounter if it stayed in the encounter area and the other group moved away ≥ 100 m. If both groups travelled after the encounter, the group that maintained the travelling direction they had before the encounter was considered the winner (i.e., the angle formed between the direction of the travelling route 10 min before and 10 min after the encounter differed by less than 10º). If both groups retreated or followed the same direction they travelled to before encountering each other, we deemed the encounter to end in a draw (i.e., undecided).
Home Range Use
We recorded the travelling routes of the macaque groups between October 2015 and July 2016 as tracklogs using handheld GPS Garmin (GPSMAP 64 and 62 Handheld Navigator, Garmin Ltd., US), collecting, on average, 1 location every 70 s. We used tracklogs to calculate each group’s home ranges and core areas 30 days before each IGE. We chose 30 days because monthly home ranges are reliable predictors of IGE outcomes in other species (Koch et al., 2016a; Roth & Cords, 2016). In addition, crested macaques are highly frugivorous (O'Brien & Kinnaird, 1997), and monthly home ranges better reflect the turnover of fruit resources and their use than larger time scales. We calculated the home range employing Brownian Bridge Movement Models (BBMM, Bullard, 1998) (R1 = 222 tracklogs (299,182 points); R2 = 201 tracklogs (221,924 points); PB1 = 247 tracklogs (303,167 points), ~ 11.9 h/track). BBMMs calculate the most likely route between each consecutive pair of relocations, considering the time lag between them. By doing so, BBMMs estimate the relative time spent in an area during the time interval between relocations (Horne et al., 2007). BBMMs calculate the utilisation distribution, which is the probability of finding the study group in a particular area. Thus, the method provides a measure of the intensity of use of each location within the home range.
We calculated the BBMMs in R 4.2.2 (R Core Team, 2022) using the brownian.bridge function from the BBMM v.3.0 R package (Nielson et al., 2013) (Online Resource 1). We set the location error to 15 m, which gives a 95% accuracy (Garmin Ltd, 2017). We set the cell size to 190 m, the smallest size that was coarse enough to account for differences between observers in the recording of the starting location of an encounter. We allowed a maximum time lag of 15 min between consecutive locations. We used the rasterFromXYZ function from the raster v.3.6–14 R package (Hijmans, 2023) to write the calculated home ranges into a raster format. Then, we used the extract function to extract the intensity of using the IGE starting location. We extracted one value for each group involved in the IGE, using the home range calculated with the tracklogs collected for each group in the 30 days before the IGE. We also extracted the distance between the IGE location and the edge of the closest core area calculated with the tracklogs of the 30 days before the IGE. Core areas were areas where the probability of finding the focal group was 50% in the corresponding BBMM (Koch et al., 2016a; Scarry, 2012). We created the 50% isopleth of each corresponding BBMM with the bbmm function. We used the contour function of the BBMM package to extract the contour from the raster with the rasterToContour function of the same package. Then, we calculated the distance between the IGE starting location and the edge of the closest core area using the st_distance function of the sf v.1.0.9 R package (Pebesma, 2018) (Online Resource 1).
Statistical Analysis
We ran a series of binomial generalised linear mixed models (GLMM) in R 4.2.2 (R Core Team, 2022) to investigate the factors affecting IGE outcome (two models: decided vs. undecided; win vs. loss), IGE intensity (two models: no aggression vs. aggression; noncontact aggression vs. contact-aggression), female participation (presence vs. absence of female intergroup aggression) and male participation (presence vs. absence of male intergroup aggression). We used LMM to investigate the factors affecting IGE duration. IGE duration was log-transformed to correct skewness. We used the glmmTMB function of the package glmmTMB v.1.1.5. (Brooks et al., 2017). We set the family to binomial (link = "logit") for the binomial models and to gaussian for the linear model.
In the models for outcome and intensity of IGE, we aimed to express test variables (Table I) with two fixed effects each: as differences between groups (e.g., number of adults in group 1 – number of adults in group 2) and percentages of relative differences (e.g., [(number of adults in group 1 – number of adults in group 2)/[( number of adults in group 1 + number of adults in group 2)]/2]*100). We used absolute values of the differences between groups (i.e., the number without the sign) in all analyses except winning probability. We expected the winning probability to depend on the sign of the difference between groups and the magnitude of the difference (e.g., the group with the bigger size wins; Majolo et al., 2020). Accordingly, we used real values (i.e., indicating whether the difference was positive or negative) for winner-loser analyses. We randomly assigned an analytical focal group per dyad, which we used as such for all the encounters of that dyad, as in Roth and Cords (2016). By analytical focal, we mean the group we chose to take as a reference to subtract the values from the other group. For example, if group A had 15 adults and group B had 20 adults, taking group A as the analytical focal, we would obtain − 5 as the real value of the difference between groups. If, instead, we took B as the analytical focal, the real value of the difference would be 5. In both cases, the absolute value of the difference would be 5 and the percentage of the relative difference would be 28.57%.
Whenever there was a correlation greater than 0.85 between the two fixed factors for the same variable in a model (i.e., the difference between groups and the percentage of the relative difference between groups), we excluded the percentage of the relative difference to avoid multicollinearity.
Whenever we encountered convergence issues during the model fitting process, we re-fitted the model with all the independent variables centred (i.e. we subtracted the mean and divided it by the standard deviation of the variable) and included only that last version in the model selection process (i.e., the model with the centred variables). We included the number of observers (range, 1–4) as a control fixed effect for all models and log-transformed IGE duration as an offset in all binomial models. In addition, we included the total number of females in the encounter as a control fixed effect in the model exploring female participation of intergroup aggression (Table II). We included the total number of males in the encounter as a control fixed effect in the models exploring male participation of intergroup aggression (Table II).
We included dyad as a random factor with random intercepts to account for variation between dyads in all models. The random factor often caused a singular fit since it tended to have zero variance. As such, it could have been eliminated since it had no mathematical effect on the significance of fixed factors (Pasch et al., 2013). We kept it to maintain the repeated-measure structure of the models.
We assessed collinearity by calculating the generalised variance-inflation factors (vif) with the function vif of the R package car v.3.1.1. (Fox & Weisberg, 2019). We accepted values lower than 4 as indicating a non-problematic level of collinearity (Zuur et al., 2009). We used the functions in the DHARMa v.0.4.6 R package (Hartig, 2022) for post-model-fitting diagnostics, including the Moran's I test for distance-based autocorrelation and the Durbin-Watson test for time-based autocorrelation. The only models showing signs of spatial autocorrelation were the female and male-based models exploring the probability of winning an encounter. Consequently, we re-fitted these models, including the autoregressive order-1 (ar1) variance–covariance matrix. AR1 assumes that the correlation between two observations decreases exponentially as the distance between them increases. We used these last versions in the model comparison and discarded the female and male models without the ar1 variance–covariance matrix. We did not interpret the results of the control fixed effects.
We produced model selection tables with the aictab function from AICcmodavg v.2.3.1 package (Mazerolle, 2020), which included corrected Akaike Information Criteria (AICc) (Hurvich & Tsai, 1989). AICc is recommended to evaluate GLMMs run with small sample sizes (i.e., n/k < 40; n = sample size, k = number of fitted parameters in the most complex model) (Symonds & Moussalli, 2011). The smaller the AICc, the better the model fits the data. We also used evidence ratios between models to evaluate their suitability. Additionally, we calculated the marginal R2 GLMM, the variance explained by fixed factors (Nakagawa & Schielzeth, 2013), and the conditional R2 GLMM, the variance explained by both fixed and random factors (Nakagawa & Schielzeth, 2013), with the r.squaredGLMM function of MuMIn v.1.47.1 R package (Barton, 2022).
We created a control model for each response variable to test the effectiveness of the test fixed effects in explaining the response variables. The control models contained the control fixed factor(s), the random factor, the offset, and, when needed, the variance–covariance matrix specification to control for spatial autocorrelation.
The sample sizes of the binary GLMM are in Table III. For the LMM investigating the factors influencing IGE duration, we included 117 encounters (PB1-R1: 76 (168 ± 241 min); PB1-R2:1 (31 min), R1-R2: 40 (129 ± 150 min)). The descriptive statistics of the test fixed effects per dyad are available in Table S1 (Online Resource 4).
Ethical Note
The project underwent an ethical review at the University of Lincoln (UK) before the onset of the data collection. The research was conducted in accordance with the Animal Behaviour Society's guidelines for the treatment of animals in behavioural research and teaching and code of best practices for field primatology (IPS and ASP 2014). The project adhered to all relevant regulations of Indonesia and the United Kingdom. Permission to conduct the study in the Tangkoko Nature Reserve was granted by the Indonesian State Ministry of Research and Technology (RISTEK, permit 229/FRP/SM/VIII/2015), the Directorate General of Forest Protection and Nature Conservation (Direktorat Jenderal Perlindungan Hutan dan Konservasi Alam, PHKA) in Jakarta and the Department for the Conservation of Natural Resources (Balai Konservasi Sumber Daya Alam, BKSDA) in Manado. We collected the data for this manuscript as a part of a larger project to investigate intergroup interactions in crested macaques. Consequently, some data presented in this manuscript have been used elsewhere (Martínez-Íñigo, 2018; Martínez-Íñigo et al., 2021; Rismayanti et al., this issue).
Data Availability
The code for the BBMM calculation (Online Resource 1), the models (Online Resource 2), data (Online Resource 3), and supplementary tables (Online Resource 4) are available in Figshare.
Results
We recorded 158 dyadic encounters between the three study groups over 9 months. Most encounters were decided (80%) and aggressive (76% of IGEs with either non-contact or contact aggression). We observed contact aggression in 27% of encounters. We observed male intergroup aggression in 68% of encounters, and we recorded female intergroup aggression in 46%. We observed male intragroup aggression against females (i.e., herding) in 65% of encounters.
Draws
The best-fitting model was the group size-location model (Table S2 in Online Resource 4). The chances of decided encounter increased the more different the groups were in both the number of adults and relative intensity of use of IGE location (Fig. 2). The other test fixed effects were not statistically significant (Table S2 in Online Resource 4). The best-fitting model differed significantly from the control model (N = 117, degree of freedom [df] = 5, χ2 = 18.39, p = 0.01); it was separated from the next best-fitting model, the male strategies model, by a 4.68 evidence ratio and had a conditional ΔR2GLMM of 0.26 (Table S3 in Online Resource 4).
Predicted probability of decided encounter as a function of (a) the absolute between-group difference in the number of adults and (b) the relative difference in the intensity of use of the IGE starting location (Group size–location model in Tables S2 and S3 in Online Resource 4); N = 117. Data points represent encounters between three habituated groups of crested macaques (Macaca nigra) living at the Macaca Nigra Project field site in Tangkoko Reserve, North Sulawesi, Indonesia. The encounters took place between November 2015 and July 2016. We jittered the data points to prevent overplotting. We centred the data (i.e., subtracted the mean and divided it by the standard deviation) to attain model convergence.
Win-Loss
The best-fitting model was the control model accounting for spatial autocorrelation (Tables S4 and S5 in Online Resource 4). It was separated from the next best-fitting model by a 252.52 evidence ratio. We could not calculate the ΔR2GLMM due to the variance–covariance matrix in the model.
Presence of Aggression
The best-fitting model was the control model (Tables S6 and S7 in Online Resource 4). According to the evidence ratio, it was 13.39 times more likely than the next best-fitting model. The fixed and random effects of the best-fitting model explained almost no variance (the conditional ΔR2GLMM was 0.02).
Contact Aggression
The best-fitting model was the control model (Tables S8 and S9 in Online Resource 4). According to the evidence ratio, it was 20.26 times more likely than the next best-fitting model, and its fixed and random effects explained almost no variance (conditional ΔR2GLMM = 4*10–4).
IGE Duration
The best-fitting model was the control model (Tables S10 and S11 in Online Resource 4). It was 72.29 times more likely than the next best-fitting model, and its predictors contribute to explaining 15% of the variance (conditional ΔR2GLMM = 0.15).
Female and Male Aggression During IGEs
The best-fitting model for female aggression was the control model, which was 69.22 times more likely than the model with test fixed effects. Its effects explained 9% of the variance (conditional ΔR2GLMM = 0.09) (Tables S12 and S13 in Online Resource 4). The model with test fixed effects was not significantly different from the control model (N = 90, df = 9, χ2 = 13.84, P = 0.13). The addition of the test fixed effects explained 34% of the variance (conditional ΔR2GLMM = 0.34). The only statistically significant test fixed effect was male participation in intergroup aggression (N = 90, df = 1, χ2 = 8.11, P < 0.01). Females were more likely to participate in intergroup aggression when males did.
The best-fitting model for male aggression was the control model, but the evidence ratio that separated it from the direct mate-access defence model was 1.69 and ΔAICc of 1.05 (Tables S14 and S15 in Online Resource 4). Consequently, both fitted the data similarly well (N = 90, df = 4, χ2 = 8.26, P = 0.08) (Symonds & Moussalli, 2011). In contrast, the indirect mate-access defence model was 21.17 less likely than the control model. The predictors within the control model explained almost no variance (conditional ΔR2GLMM = 4*10–3), whereas the direct mate-access defence model explained a 13% (conditional ΔR2GLMM = 0.13). Such a model only had one statistically significant test fixed effect, female participation in intergroup aggression (N = 90, df = 1, χ2 = 5.54, P = 0.02; Fig. 3). Males were more likely to participate in intergroup aggression when females did.
Predicted probability of male participation in intergroup aggression as a function of female participation in intergroup aggression. Data points represent encounters between three habituated groups of crested macaques (Macaca nigra) living at the Macaca Nigra Project field site in Tangkoko Reserve, North Sulawesi, Indonesia. The encounters took place between November 2015 and July 2016. We jittered the data points to avoid overplotting.
Discussion
Intergroup encounters in crested macaques typically ended with a clear winner and were aggressive, although contact aggression was not frequent. Both sexes engaged in intergroup aggression, but males were more likely to do so. We observed herding in most encounters. Draws were more likely when encountering groups were more similar in size. When groups encountered in an area infrequently used by both of them, a draw was less likely, although still highly probable. The likelihood of winning an intergroup encounter was dependent on the encounter location. No test model predicted encounter intensity better than the control models, and all explained minimal variance. Female and male intergroup aggression were more likely when the other sex was aggressive.
We anticipated that models depicting male reproductive strategies would be superior to group size-location models and female food defence models in predicting the outcome and intensity of intergroup encounters. Specifically, we hypothesised that within the male reproductive strategies models, factors related to direct mate defence, such as the number of females per male and the number of sexually receptive females per male, would have greater explanatory power than those related to indirect mate-access defence. However, our results showed that the control models, which contained only the number of observers as a fixed effect and the IGE duration as an offset, were the best among each set of models, except for the set examining the probability of a decided encounter.
Despite lacking support for our hypotheses, we identified several intriguing patterns in crested macaque intergroup encounters. For instance, we found that the chances of a draw increased as the number of adults became more balanced, but draws were still possible when numerical disparities were sizeable. Furthermore, similarity in RHP was more important than RV in determining the chances of a decided encounter. This is consistent with findings in blue monkeys, where similar numerical odds lead to greater chances of an intergroup draw, but location-based payoffs have little effect (Roth & Cords, 2016). Similarly, in savannah baboons (Papio cynocephalus ursinus), draws are more likely when the number of males is similar across groups (Kitchen et al., 2004).
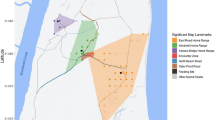
Maximum RHP was a poor predictor of who won an encounter, regardless of whether the proxy accounted for the number of adults, females, or males. R1 consistently had maximum RHP advantage in all three measurements and still frequently lost encounters against its smaller neighbours. In fact, R2 won against R1 more often than the other way around. Such lack of power of the imbalance in group size to predict encounter winner contrasts with findings in most primates (Majolo et al., 2020). Smaller groups may win over larger groups when encounters occur in areas highly valued by the smaller group (Crofoot et al., 2008; Koch et al., 2016a; Strong et al., 2018). In our study, location played a role in explaining the pattern of winners and losers. However, outcome spatial association was poorly related to the intensity of use of the IGE starting location and its distance to the closest core area, contrasting with our expectations and other studies (Gareta-García et al., 2022; Koch et al., 2016a). Instead, the spatial distribution of intergroup encounter outcomes (Fig. 4) suggests that the home range centre, a potential measure for residence effect, might be a better predictor of the winner. The group closer to its home range center might have higher chances of winning.
Macaca Nigra Project field site (Tangkoko Reserve, North Sulawesi, Indonesia) map displaying the home ranges (October 2015-July 2016) and encounters (November 2015-July 2016) among the three study groups of crested macaques (Macaca nigra). Each intergroup encounter point specifies the two groups encountering and the winner of the encounter.
Contrary to what other studies found (Cooper et al., 2004; Harris, 2005; Kitchen et al., 2004; Roth & Cords, 2016; Scarry, 2012), we observed that escalation in crested macaque intergroup encounters occurred even when maximum RHP symmetry was absent and regardless of differences in RV. All encounters that lasted more than 87 min were aggressive. All encounters over 306 min involved male IGE aggression, and females engaged in intergroup aggression in all IGEs over 381 min. Probability of contact aggression, however, seemed to be little affected by IGE duration. Conflict theory predicts that larger differences in RHP should lead to shorter conflicts (Riechert, 1998). For example, in blue monkeys and green woodhoopoes (Phoeniculus purpureus), encounters tend to be longer when groups are more similar in size (Radford & Du Plessis, 2004; Roth & Cords, 2016). While we observed such a tendency in our models, the effect of group size had little explanatory power.
Our data revealed that noncontact aggression was a common feature of most intergroup encounters, perhaps reflecting a general strategy of indiscriminate aggression designed to achieve intergroup dominance and its associated fitness advantages (Cowlishaw, 1995; Crofoot, 2007; Crofoot & Wrangham, 2010). Nevertheless, aggression also could be present in most encounters because the resource of interest is omnipresent. In crested macaques, reproductive success is highly skewed towards the alpha male (Higham et al., 2021). Migrant males, rather than ingroup mates, usually displace alpha males (Marty et al., 2017). The fact that intergroup aggression is common, independently of resource distribution, the proportion of females, and their reproductive status, is consistent with the use of intergroup encounters by males to assess their chances of taking over an alpha position.
While our analysis did not provide conclusive evidence that male direct mate-access defence influences the outcome and intensity of intergroup encounters in crested macaques, our results suggest that males engage in intergroup aggression more frequently when females also participate. This finding is consistent with the hypothesis that male crested macaques use direct mate-access defence as a strategy to prevent matings between ingroup females and outgroup males during intergroup encounters. Our observations of herding behaviour during these encounters further support this idea.
We believe that the lack of support for our hypotheses may be partially attributable to the particularities of our sample. First, our sample did not contain situations where maximum RHP was balanced. We predicted that a balanced maximum RHP would lead to draws and escalation. Secondly, almost all encounters in our sample involved one group (R1) against one of its two habituated neighbours (PB1 and R2). Such a limited number of dyads means that potential behavioural variability due to ecological conditions may be underrepresented in our data.
The lack of support for our hypotheses may also stem from using response variables that do not account accurately for the phenomena we aimed to measure. For instance, our response variable for intergroup aggression was binary, indicating the absence or presence of aggression. As a result, we may have faced a ceiling effect that prevented us from gaining a deeper understanding of factors that increase intergroup encounter intensity. For example, in a study on chacma baboons, Kitchen and colleagues (2004) found that the presence of oestrous females increased the number of males who joined aggressive displays and the duration of such displays. However, aggression was common in virtually all encounters. If their analysis had been conducted accounting for the presence-absence of aggression and contact aggression, the difference in intensity would have been disregarded.
Finally, missing or imprecise predictors might be involved in the lack of support for our hypotheses. For example, whereas the proportion of infants and sexually receptive females may have an effect on male participation in intergroup aggression, this is likely to be mediated by their rank, which in crested macaques is a strong predictor of mating success and paternity (Higham et al., 2021). Thus, more infants and sexually receptive females may increase the participation of males likely to benefit from protecting them, but not in others. Also, whereas infant carrying often decreases female participation in intergroup aggression (Arseneau-Robar et al., 2017; Cheney & Seyfarth, 1987; Koch et al., 2016b), during the data collection, we observed females going to the front line of intergroup encounters regularly the days after giving birth, even if they were not often seen there previously, resembling patterns seen in Verreaux's sifaka (Propithecus verreauxi) (Lewis et al., 2020). Moreover, males often concentrate their herding efforts on cycling females (Henzi et al., 1998; Kitchen & Beehner, 2007). However, we observed males herding females with infants and even forming coalitions to attack them and drive them away from the front lines. Such indiscriminate aggression against females could be part of a sexual intimidation strategy by males (Baniel et al., 2017; Smit et al., 2022). Moreover, some infant deaths might be a byproduct of males herding females carrying infants, which could contribute to the high infant mortality in crested macaques (Kerhoas et al., 2014; Thierry, 2005).
Conclusions
Contrary to our predictions, factors related to male direct mate access defence did not explain better IGE outcome and intensity in crested macaques compared to models focused on group size-location and female food defence. Instead, draws seemed to reflect symmetries in group sizes and relative intensity of the use of intergroup encounter location. Groups tended to win in particular areas of their home range independently of how often they used them or how far they were from their core areas. Male and female participation was more likely when the other sex was involved in intergroup aggression. Male direct mate access defence was evident through herding in most encounters. Our factors of interest did not explain IGE intensity, which could be due to the binomial structure of the tests (e.g., presence vs. absence of aggression) and the permanent presence of incentives for intergroup aggression, such as the conflict between alpha males and aspirant immigrant males. Most of our models explained a low percentage of variance, which indicates that factors we did not consider might play a prominent role in determining IGE outcome and intensity in crested macaques. Such a situation might have arisen if individuals differ in their interests and thus threshold RV triggering participation, leading to group and sex-wise maximum RHP to be weakly correlated to realised RHP. Future studies should consider the number of active participants and explore alternative measurements of RV. Research on individual participation could serve as an orientation for which factors might be more relevant.
References
Arseneau-Robar, T. J. M., Taucher, A. L., Schnider, A. B., van Schaik, C. P., & Willems, E. P. (2017). Intra- and interindividual differences in the costs and benefits of intergroup aggression in female vervet monkeys. Animal Behaviour, 123, 129–137. https://doi.org/10.1016/j.anbehav.2016.10.034
Baniel, A., Cowlishaw, G., & Huchard, E. (2017). Male violence and sexual intimidation in a wild primate society. Current Biology, 27(14), 2163–2168.
Barton, K. (2022). MuMIn: Multi-model inference. https://cran.r-project.org/package=MuMIn. Accessed 1 Apr 2023
Brooks, M. E., Kristensen, K., Van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., & Bolker, B. M. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R journal, 9(2), 378–400.
Brown, M. (2011). Intergroup encounters in grey-cheeked mangabeys (Lophocebus albigena) and redtail monkeys (Cercopithecus ascanius): form and function. PhD Thesis. Columbia University. https://doi.org/10.7916/D8T159NZ
Brown, M. (2013). Food and range defence in group-living primates. Animal Behaviour, 85(4), 807–816. https://doi.org/10.1016/j.anbehav.2013.01.027
Brown, M., & Crofoot, M. C. (2013). Social and spatial relationships between primate groups. In E. J. Sterling, N. Bynum, & M. E. Blair (Eds.), Primate ecology and conservation: A handbook of techniques (pp. 151–176). Oxford University Press.
Bullard, F. (1998). Estimating the home range of an animal: A brownian bridge approach. University of North Carolina at Chapel Hill.
Cassidy, K. A., MacNulty, D. R., Stahler, D. R., Smith, D. W., & Mech, L. D. (2015). Group composition effects on aggressive interpack interactions of gray wolves in Yellowstone National Park. Behavioral Ecology, 26(5), 1352–1360. https://doi.org/10.1093/beheco/arv081
Cheney, D. L., & Seyfarth, R. M. (1987). Behavioral ecology and sociobiology the influence of intergroup competition on the survival and reproduction of female vervet monkeys. Behavioral Ecology and Sociobiology, 21, 375–386.
Cooper, M. A., Aureli, F., & Singh, M. (2004). Between-group encounters among bonnet macaques (Macaca radiata). Behavioral Ecology and Sociobiology, 56(3), 217–227. https://doi.org/10.1007/s00265-004-0779-4
Cords, M. (2007). Variable participation in the defence of communal feeding territories by blue monkeys in the Kakamega Forest, Kenya. Behaviour, 144, 1537–1550.
Cowlishaw, G. (1995). Behavioural patterns in baboon group encounters: The role of resource competition and male reproductive strategies. Behaviour, 132(1–2), 75–86.
Crofoot, M. C. (2007). Mating and feeding competition in white-faced capuchins (Cebus capucinus): the importance of short-and long-term strategies. Behaviour, 144, 1473–1495.
Crofoot, M. C., Gilby, I. C., Wikelski, M. C., & Kays, R. W. (2008). Interaction location outweighs the competitive advantage of numerical superiority in Cebus capucinus intergroup contests. Proceedings of the National Academy of Sciences, 105(2), 577–581. https://doi.org/10.1073/pnas.0707749105
Crofoot, M. C., & Wrangham, R. W. (2010). Intergroup aggression in primates and humans: the case for a unified theory. In P. M. Kappeler & J. B. Silk (Eds.), Mind the gap : tracing the origins of human universals (pp. 171–195). Springer. https://doi.org/10.1007/9783642027253
Di Bitetti, M. S. (2001). Home-range use by the tufted capuchin monkey (Cebus apella nigritus) in a subtropical rainforest of Argentina. Journal of Zoology, 253(1), 33–45. https://doi.org/10.1017/S0952836901000048
Duboscq, J., Micheletta, J., Agil, M., Hodges, K., Thierry, B., & Engelhardt, A. (2013). Social Tolerance in Wild Female Crested Macaques (Macaca nigra) in Tangkoko-Batuangus Nature Reserve, Sulawesi, Indonesia. American Journal of Primatology, 75(4), 361–375. https://doi.org/10.1002/ajp.22114
Engelhardt, A., Muniz, L., Perwitasari-Farajallah, D., & Widdig, A. (2017). Highly polymorphic microsatellite markers for the assessment of male reproductive skew and genetic variation in critically endangered crested macaques (Macaca nigra). International Journal of Primatology, 38(4), 672–691. https://doi.org/10.1007/s10764-017-9973-x
Fashing, P. J. (2001). Male and female strategies during intergroup encounters in guerezas (Colobus guereza): Evidence for resource defence mediated through males and a comparison with other primates. Behavioral Ecology and Sociobiology, 50(3), 219–230. https://doi.org/10.1007/s002650100358
Fox, J., & Weisberg, S. (2019). An {R} Companion to applied Regression. SAGE Publications Inc. https://socialsciences.mcmaster.ca/jfox/Books/Companion/. Accessed 1 Apr 2023
Furrer, R. D., Kyabulima, S., Willems, E. P., Cant, M. A., & Manser, M. B. (2011). Location and group size influence decisions in simulated intergroup encounters in banded mongooses. Behavioral Ecology, 22(3), 493–500. https://doi.org/10.1093/beheco/arr010
Gareta-García, M. G., de Guinea, M., Bshary, R., & van de Waal, E. (2022). Drivers and outcomes of between-group conflict in vervet monkeys. Philosophical Transactions of the Royal Society B, 377(1851), 20210145.
Garmin Ltd. (2017). Garmin BaseCamp. https://www.garmin.com/en-US/software/basecamp/. Accessed 15 May 2017
Giraldeau, L. A., & Ydenberg, R. (1987). The center-edge effect: The result of a war of attrition between territorial contestants? The Auk, 104(3), 535–538.
Green, P. A., Briffa, M., & Cant, M. A. (2021). Assessment during intergroup contests. Trends in Ecology and Evolution, 36(2), 139–150. https://doi.org/10.1016/j.tree.2020.09.007
Gros-Louis, J., Perry, S., & Manson, J. H. (2003). Violent coalitionary attacks and intraspecific killing in wild white-faced capuchin monkeys (Cebus capucinus). Primates, 44, 341–346.
Harris, T. R. (2005). Roaring, intergroup aggression, and feeding competition in black and white colobus monkeys (Colobus guereza) at Kanyawara, Kibale National Park, Uganda. PhD Thesis. Yale University.
Harris, T. R. (2006). Between-group contest competition for food in a highly folivorous population of black and white colobus monkeys (Colobus guereza). Behavioral Ecology and Sociobiology, 61(2), 317–329. https://doi.org/10.1007/s00265-006-0261-6
Hartig, F. (2022). DHARMa: Residual diagnostics for Hierarchical (Multi-Level/Mixed) regression models. R package version 0.4.6. https://CRAN.R-project.org/package=DHARMa. Accessed 1 Apr 23
Henkel, S., Heistermann, M., & Fischer, J. (2010). Infants as costly social tools in male Barbary macaque networks. Animal Behaviour, 79(6), 1199–1204. https://doi.org/10.1016/j.anbehav.2010.02.005
Henzi, S. P., Lycett, J. E., & Weingrill, T. (1998). Mate guarding and risk assessment by male mountain baboons during inter-troop encounters. Animal Behaviour, 55(6), 1421–1428.
Higham, J. P., Heistermann, M., Agil, M., Perwitasari-Farajallah, D., Widdig, A., & Engelhardt, A. (2021). Female fertile phase synchrony, and male mating and reproductive skew, in the crested macaque. Scientific Reports, 11(1), 4251. https://doi.org/10.1038/s41598-021-81163-1
Higham, J. P., Heistermann, M., Saggau, C., Agil, M., Perwitasari-Farajallah, D., & Engelhardt, A. (2012). Sexual signalling in female crested macaques and the evolution of primate fertility signals. BMC Evolutionary Biology, 12(1). https://doi.org/10.1186/1471-2148-12-89
Hijmans, R.J. (2023). raster: Geographic Data Analysis and Modeling. R Package version 3.6–14. https://CRAN.R-project.org/package=raster. Accessed 1 Apr 2023.
Horne, J. S., Garton, E. O., Krone, S. M., & Lewis, J. S. (2007). Analysing animal movements using Brownian bridges. Ecology, 88(9), 2354–2363. https://doi.org/10.1890/06-0957.1
Hurvich, C. M., & Tsai, C.-L. (1989). Regression and time series model selection in small samples. Biometrika, 76(2), 297–307.
IPS, & ASP. (2014). Code of best practices for field primatology. https://www.asp.org/resources/docs/Code%20of_Best_Practices%20Oct%202014.pdf [Accessed on the 1st April 2022]
Johnstone, R. A., Cant, M. A., Cram, D., & Thompson, F. J. (2020). Exploitative leaders incite intergroup warfare in a social mamm al., Proceedings of the National Academy of Sciences, 202003745. https://doi.org/10.1073/pnas.2003745117
Kerhoas, D., Perwitasari-farajallah, D., Agil, M., Widdig, A., & Engelhardt, A. (2014). Social and ecological factors influencing offspring survival in wild macaques. Behavioural Ecology, 25(5), 1164–1172. https://doi.org/10.1093/beheco/aru099
Kerhoas, D., Kulik, L., Perwitasari-farajallah, D., Agil, M., Engelhardt, A., & Widdig, A. (2016). Mother-male bond, but not paternity, influences male-infant affiliation in wild crested macaques. Behavioral Ecology and Sociobiology, 70, 1117–1130. https://doi.org/10.1007/s00265-016-2116-0
Kinnaird, M. F., & O’Brien, T. G. (2000). Comparative movement patterns of two semi-terrestrial Cercopithecine primates: The Tana river crested mangabey and the Sulawesi crested black macaque. In S. Boinski & P. A. Garber (Eds.), On the move: How and why animals travel in groups (pp. 327–350). University of Chicago Press.
Kinnaird, M. F., & O’Brien, T. G. (1999). Breeding ecology of the Sulawesi red-knobbed hornbill, Aceros cassidix. Ibis, 141(1), 60–69. https://doi.org/10.1111/j.1474-919x.1999.tb04263.x
Kitchen, D. M., & Beehner, J. C. (2007). Factors affecting individual participation in group-level aggression among non-human primates. Behaviour, 144(12), 1551–1581. https://doi.org/10.1163/156853907782512074
Kitchen, D. M., Cheney, D. L., & Seyfarth, R. M. (2004). Factors mediating inter-group encounters in savannah baboons (Papio cynocephalus ursinus). Behaviour, 141(2), 197–218.
Koch, F., Signer, J., Kappeler, P. M., & Fichtel, C. (2016a). The role of the residence-effect on the outcome of intergroup encounters in Verreaux’ s sifakas. Scientific Reports, 6, 28457. https://doi.org/10.1038/srep28457
Koch, F., Signer, J., Kappeler, P. M., & Fichtel, C. (2016b). Intergroup encounters in Verreaux’s sifakas (Propithecus verreauxi): Who fights and why? Behavioral Ecology and Sociobiology, 70(5), 797–808. https://doi.org/10.1007/s00265-016-2105-3
Kokko, H. (2013). Dyadic contests: Modelling fights between two individuals. In I. C. W. Hardy & M. Briffa (Eds.), Animal contest (pp. 5–32). Cambridge University Press.
Lawes, M. J., & Henzit, S. P. (1995). Inter-group encounters in blue monkeys: how territorial must a territorial species be? Anim al., Behaviour, 49(1), 240–243. https://doi.org/10.1016/0003-3472(95)80173-1
Lewis, R. J., Sandel, A. A., Hilty, S., & Barnett, S. E. (2020). The collective action problem but not numerical superiority explains success in intergroup encounters in Verreaux’s sifaka (Propithecus verreauxi): Implications for individual participation and free-riding. International Journal of Primatology, 41(2), 305–324. https://doi.org/10.1007/s10764-020-00155-6
Majolo, B., deBortoli Vizioli, A., Martínez-Íñigo, L., & Lehmann, J. (2020). Effect of group size and individual characteristics on intergroup encounters in primates. International Journal of Primatology, 41(2), 325–341. https://doi.org/10.1007/s10764-019-00119-5
Markham, A. C., Alberts, S. C., & Altmann, J. (2012). Intergroup conflict: Ecological predictors of winning and consequences of defeat in a wild primate population. Animal Behaviour, 84(2), 399–403. https://doi.org/10.1016/j.anbehav.2012.05.009
Markham, A. C., Alberts, S. C., & Altmann, J. (2016). Haven for the night : Sleeping site selection in a wild primate. Behavioral Ecology, 27(1), 29–35. https://doi.org/10.1093/beheco/arv118
Martínez-Íñigo, L. (2018). Intergroup interactions in crested macaques (Macaca nigra): Factors affecting intergroup encounter outcome and intensity. PhD thesis. University of Lincoln. Retrieved from: http://eprints.lincoln.ac.uk/id/eprint/42729/
Martínez-Íñigo, L., Engelhardt, A., Agil, M., Pilot, M., & Majolo, B. (2021). Intergroup lethal gang attacks in wild crested macaques, Macaca nigra. Animal Behaviour, 180, 81–91. https://doi.org/10.1016/j.anbehav.2021.08.002
Marty, P. R., Hodges, K., Agil, M., & Engelhardt, A. (2017). Alpha male replacements and delayed dispersal in crested macaques (Macaca nigra). American Journal of Primatology, 79(7), 1–8. https://doi.org/10.1002/ajp.22448
Mazerolle, M. J. (2020). AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package version 2.3–1. https://cran.r-project.org/package=AICcmodavg. Accessed 1 Apr 2023
Nailufar, B., Syartinilia, & Perwitasari, D. (2015). Landscape modeling for human – Sulawesi Crested black macaques conflict in North Sulawesi. Procedia Environmental Sciences, 24, 104–110https://doi.org/10.1016/j.proenv.2015.03.014
Nakagawa, S., & Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalised linear mixed-effects models. Methods in Ecology and Evolution, 4(2), 133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Nangoy, M., Ransaleleh, T., Lengkong, H., Koneri, R., Latinne, A., & Kyes, R.C. (2021). Diversity of fruit bats (Pteropodidae) and their ectoparasites in Batuputih Nature Tourism Park, Sulawesi, Indonesia. Biodiversitas, 22(6), 3075–3082. https://doi.org/10.13057/biodiv/d220609
Nielson, R. M., Sawyer, H., & McDonald, T. L. (2013). Package 'BBMM: Brownian bridge movement model. R package version 3.0.' https://cran.r-project.org/package=BBMM. Accessed 1 Apr 2023
Nunn, C. L., & Deaner, R. O. (2004). Patterns of participation and free riding in territorial conflicts among ringtailed lemurs (Lemur catta). Behavioral Ecology and Sociobiology, 57(1), 50–61. https://doi.org/10.1007/s00265-004-0830-5
O’Brien, T. G., & Kinnaird, M. F. (1997). Behavior, diet, and movements of the Sulawesi crested black macaque (Macaca nigra). International Journal of Primatology, 18(3), 321.
Palombit, R. A. (2014). Sexual conflict in nonhuman primates. In M. Naguib, J. C. Mitani, L. Simmons, H. J. Brockmann, T. Roper, & L. Barrett (Eds.), Advances in the Study of Behavior (1st ed., Vol. 46, pp. 191–280). Academic Press. https://doi.org/10.1016/B978-0-12-800286-5.00005-5
Parker, G. A. (1974). Assessment strategy and the evolution of fighting behaviour. Journal of Theoretical Biology, 47(1), 223–243. https://doi.org/10.1016/0022-5193(74)90111-8
Pasch, B., Bolker, B. M., & Phelps, S. M. (2013). Interspecific dominance via vocal interactions mediates altitudinal zonation in neotropical singing mice. American Naturalist, 182(5). https://doi.org/10.1086/673263
Pebesma, E. (2018). Simple Features for R: Standardised Support for Spatial Vector Data. The R Journal, 10 (1), 439–446. https://doi.org/10.32614/RJ-2018-009
R Core Team. (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.r-project.org/. Accessed 15 Mar 2022
Radford, A. N., & Du Plessis, M. A. (2004). Territorial vocal rallying in the green woodhoopoe: Factors affecting contest length and outcome. Animal Behaviour, 68(4), 803–810. https://doi.org/10.1016/j.anbehav.2004.01.010
Riechert, S. E. (1998). Game theory and animal contests. In L. A. Dugatkin & H. K. Reeve (Eds.), Game theory and animal behaviour (pp. 64–93). Oxford University Press.
Roth, A. M., & Cords, M. (2016). Effects of group size and contest location on the outcome and intensity of intergroup contests in wild blue monkeys. Animal Behaviour, 113, 49–58. https://doi.org/10.1016/j.anbehav.2015.11.011
Rosenbaum, S., Vecellio, V., & Stoinski, T. (2016). Observations of severe and lethal coalitionary attacks in wild mountain gorillas. Scientific Reports, 6(1), 1–8.
Rubenstein, D. I. (1986). Ecology and sociality in horses and zebras. In D. I. Rubenstein & R. W. Wrangham (Eds.), Ecological aspects of social evolution: Birds and mammals (pp. 282–302). Princeton University Press.
Scarry, C. J. (2012). The functions and consequences of intergroup aggression among Argentine Tufted Capuchine Monkeys (Cebus apella [Sapajus] nigritus). PhD Thesis. Stony Brook University. Retrived from: https://dspace.sunyconnect.suny.edu/handle/1951/60210. Accessed 1 Feb 2017
Scarry, C. J. (2017). Male resource defence during intergroup aggression among tufted capuchin monkeys. Animal Behaviour, 123, 169–178. https://doi.org/10.1016/j.anbehav.2016.10.015
Seiler, N., Boesch, C., Mundry, R., Stephens, C., & Robbins, M. M. (2017). Space partitioning in wild, non-territorial mountain gorillas: the impact of food and neighbours. Royal Society open science, 4(11), 170720. https://doi.org/10.1098/rsos.170720
Smit, N., Baniel, A., Roura-Torres, B., Amblard-Rambert, P., Charpentier, M. J., & Huchard, E. (2022). Sexual coercion in a natural mandrill population. Peer Community Journal, 2 (article e36), 1–17. https://doi.org/10.24072/pcjournal.134
Smith, J. M., & Parker, G. A. (1976). The logic of asymmetric contests. Animal Behaviour, 24(1), 159–175. https://doi.org/10.1016/S0003-3472(76)80110-8
Sterck, E. H. M., Watts, D. P., Van Schaik, C. P., & Van Schaik, C. P. (1997). The evolution of female social relationships in nonhuman primates. Behavioral Ecology and Sociobiology, 41, 291–309.
Strong, M. J., Sherman, B. L., & Riehl, C. (2018). Home field advantage, not group size, predicts outcomes of intergroup conflicts in a social bird. Animal Behaviour, 143, 205–213. https://doi.org/10.1016/j.anbehav.2017.07.006
Supriatna, J., & Andayani, N. (2008). Macaca nigra. The IUCN Red List of Threatened Species. https://dx.doi.org/https://doi.org/10.2305/IUCN.UK.2008.RLTS.T12556A3357272.en. Accessed 10 October 2020.
Symonds, M. R. E., & Moussalli, A. (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology, 65, 13–21. https://doi.org/10.1007/s00265-010-1037-6
Thierry, B. (2005). Order and noise in primate societies. In C. Hemelrijk (Ed.), Self-Organisation and Evolution of Biological and Social Systems (pp. 108–122). Cambridge University Press. https://doi.org/10.1017/CBO9780511542275.008
Thorén, S., Lindenfors, P., & Kappeler, P. M. (2006). Phylogenetic analyses of dimorphism in primates: Evidence for stronger selection on canine size than on body size. American Journal of Physical Anthropology, 130(1), 50–59. https://doi.org/10.1002/ajpa.20321
Trivers, R. L. (1972). Parental investment and sexual selection. In B. Campbell (Ed.), Sexual selection and the descent of men:1871–1971 (pp. 136–179). Aldine Publishing Company.
Van Belle, S., Garber, P. A., Estrada, A., & Di Fiore, A. (2014). Social and genetic factors mediating male participation in collective group defence in black howler monkeys. Animal Behaviour, 98, 7–17.
Waterman, J. O. (2021). Within-group consequences of the risk and occurrence of between-group conflict in crested macaques (Macaca nigra). PhD Thesis. Liverpool John Moores University. Retrived from: https://researchonline.ljmu.ac.uk/id/eprint/14619/. Accessed 2 Mar 2022
Williams, J. M., Oehlert, G. W., Carlis, J. V., & Pusey, A. E. (2004). Why do male chimpanzees defend a group range? Animal Behaviour, 68(3), 523–532. https://doi.org/10.1016/j.anbehav.2003.09.015
Wilson, M. L., Boesch, C., Fruth, B., Furuichi, T., Gilby, I. C., Hashimoto, C., ... & Wrangham, R. W. (2014). Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature, 513(7518), 414–417.
Wilson, M. L., Kahlenberg, S. M., Wells, M., & Wrangham, R. W. (2012). Ecological and social factors affect the occurrence and outcomes of intergroup encounters in chimpanzees. Animal Behaviour, 83(1), 277–291. https://doi.org/10.1016/j.anbehav.2011.11.004
Wrangham, R. W. (1999). Evolution of coalitionary killing. American Journal of Physical Anthropology, 110(S29), 1–30. https://doi.org/10.1002/(SICI)1096-8644(1999)110:29+%3c1::AID-AJPA2%3e3.0.CO;2-E
Zhao, Q. K. (1997). Intergroup interactions in Tibetan macaques at Mt. Emei, China. American Journal of Physical Anthropology, 104(4), 459–470.
Zuur, A. F., Ieno, E. N., Walker, N. J., & A., S. A. (2009). Mixed effect models and extensions in ecology with R. Springer.
Acknowledgements
We gratefully acknowledge the permission of the Indonesian State Ministry of Research and Technology (229/SIP/FRP/SM/VIII/2015), the Directorate General of Forest Protection and Nature Conservation in Jakarta and the Department for the Conservation of Natural Resources in Manado, to conduct this research in the Tangkoko-Batuangus Nature Reserve. We are grateful to all the researchers and field assistants of the Macaca Nigra Project. We are particularly in debt to E. Cahyaningrum, J.M. Berthier for their crucial contribution to the data collection. We are extremely grateful to the crowdfunding contributors who helped to fund this project and to the University of Lincoln for a PhD studentship awarded to LMI. We would like to thank Julie Duboscq and Jerôme Micheletta for inviting us to contribute to this special issue of the International Journal of Primatology on Macaca nigra. We thank two anonymous reviewers and the guest editor for their insightful comments and suggestions that helped to improve earlier versions of this manuscript. We are grateful to RedPony Analytics (http://www.redponyanalytics.com/) for the coding to calculate the home ranges from the 30 days before each intergroup encounter and for statistical advice. The authors declare that they have no conflict of interest.
Funding
This work was supported by a Research Investment Fund scholarship scheme (RIF2014S-32) from the University of Lincoln, awarded to LMI and crowdfunding. MP was supported by the Polish National Agency for Academic Exchange (NAWA; Polish Returns Fellowship PPN/PPO/2018/1/00037).
Author information
Authors and Affiliations
Contributions
LMI, MP, and BM obtained the funding, formulated the idea, and designed the methodology. LMI and R conducted fieldwork. LMI analysed the data, created the figures, and wrote the manuscript; all other authors revised the manuscript critically. AE and MA stewarded the field project of which this study was a part.
Corresponding author
Ethics declarations
Inclusion and Diversity Statement
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. One or more of the authors of this paper self-identifies as having lived with a disability. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list. The author list includes contributors from the location where the research was conducted, who participated in study conception, study design, data collection, analysis, and/or interpretation of the findings.
Additional information
Handling Editor: Jérôme Micheletta
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martínez-Íñigo, L., Rismayanti, Engelhardt, A. et al. Factors Affecting the Outcome and Intensity of Intergroup Encounters in Crested Macaques (Macaca nigra). Int J Primatol 44, 696–721 (2023). https://doi.org/10.1007/s10764-023-00382-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-023-00382-7