Abstract
Sleep is an important and time-consuming activity, during which animals may be particularly vulnerable. Selecting a suitable sleeping site is therefore essential for an individual’s fitness. Here we test the importance of antipredator and thermoregulatory hypotheses for the sleeping site preference of a nocturnal primate, the Northern lesser galago (Galago senegalensis), in Northern Tanzania. During June to August of 2015 and 2016 we conducted daily surveys of sleeping sites to record the number of galagos and their location within the sleeping tree, and used focal follows to record when galagos reached and left sleeping sites. We collected vegetation data for sleeping sites (N = 47) and matched controls, and placed data loggers in sleeping (N = 14) and control locations to compare temperature and humidity. Sleeping group sizes were similar to that of G. senegalensis in The Gambia, and the mean proportion of visits in which galagos were present at each site was 27 ± SD 25%. Galagos slept on branches (N = 29), nests (N = 6), palm leaves (N = 6), and in tree cavities (N = 1). Palm leaves have not been previously recorded as regular sleeping sites for galagos and were overrepresented relative to their occurrence in the habitat. Random forest classification analysis revealed that galagos sleep in areas with greater canopy cover and connectivity, greater mid-level vegetation cover, higher tree density, and a greater number of Acacia trees. Sleeping locations had significantly lower mean temperatures but greater mean humidity than control locations. Our findings support predator avoidance and thermoregulation as drivers of nesting behavior. In particular, the characteristics of galago sleeping sites correspond well to those expected for protection from aerial predators, while those related to protection from terrestrial predators seem to play a minor role.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep is a widespread behavior, argued to provide essential restorative effects (Siegel 2005) and memory consolidation (Diekelmann and Born 2010). Many animals spend a large proportion of their time asleep, and can be especially vulnerable to predation and other environmental risks during this time (Lima et al.2005). Selecting a suitable sleeping site is therefore crucial for fitness, as it provides shelter and safety, and can facilitate social contact (Di Bitetti et al.2000; Hamilton 1982; Takahashi 1997). Studies of sleeping behavior in mammals generally find that either predation avoidance (e.g., degus, Octodon degus: Lagos et al.1995) or thermoregulation (e.g., koalas, Phascolarctos cinereus: Briscoe et al.2014), or both (e.g., pine martens, Martes martes: Birks et al.2005; Eastern spotted skunks, Spilogale putorius: Lesmeister et al.2008; North American porcupines, Erethizon dorsatum: Mabille and Berteaux 2014; and roe deer, Capreolus capreolus: Van Moorter et al.2009) are prominent factors affecting sleeping behavior, including site selection.
Predation risk appears to be the main factor driving sleeping site selection in primates. Olive baboons (Papio anubis) prefer to sleep in higher areas that are less accessible to leopards (Panthera pardus: Hamilton 1982); black-tufted marmosets (Callithrix penicillata) in urban areas choose tall trees to avoid predation from cats (Duarte and Young 2011); pileated gibbons (Hylobates pileatus) seek tall trees with few lower branches to avoid terrestrial predators (Phoonjampa et al.2010); and chimpanzees (Pan troglodytes ellioti) choose to build terrestrial nests only in areas where they are not under threat from humans (Last and Muh 2013). However, it is unlikely that predation risk exclusively influences where primates sleep, and some species such as pigtailed macaques (Macaca leonine) combine predation avoidance with other environmental factors (e.g., distance to food resources) when selecting sleeping sites (Albert et al.2011).
Microhabitat features known to influence primate sleeping site selection may offer antipredator benefits; e.g., tree height (Albert et al.2011; Di Bitetti et al.2000; Rode et al.2013) offers a vantage point from which to spot terrestrial predators, and inaccessibility to predators; greater tree diameter at breast height (DBH; Cheyne et al.2013; Di Bitetti et al.2000; Hankerson et al.2007; Rode et al.2013) indicates structural stability in case of need for evasive or defensive action; greater tree connectivity (Kenyon et al.2014) and density of undergrowth (Dagosto et al.2001) offer escape routes; and canopy cover (Hankerson et al.2007; Rode et al.2013) may offer concealment, especially from aerial predators. By sleeping in dense foliage, animals such as Northern giant mouse lemurs (Mirza zaza: Rode et al.2013) and green monkeys (Cercopithecus sabaeus: Harrison 1985) remain cryptic yet able to sense vibrations from approaching scansorial and aerial predators. Dense vegetation is also favored by Neotropical primates for parasite avoidance (Nunn and Heymann 2005). Alternatively, dense ground vegetation may provide cover for terrestrial predators, thus increasing predation risk (Bettridge and Dunbar 2012; Cowlishaw 1997a, b). The relative importance of each of the foregoing factors is subject to the ecological pressures on the population, and to gain further insight into the sleeping site ecology of primates, repeated use of sites should be monitored (Anderson 1984). Individuals may trade off conflicting pressures, meaning that to reduce detection by predators, even the most desirable sleeping sites may not be used consistently (Day and Elwood 1999).
Thermoregulatory hypotheses, where animals adapt their sleeping site behavior for thermoregulatory advantages, also explain elements of primate sleeping site selection (Anderson 1984; Stewart et al.2018). Western chimpanzees (Pan troglodytes verus) use a humidity avoidance strategy when building arboreal nests (Koops et al.2012). Thermoregulation is likely to be a greater consideration for smaller primate species; golden-brown mouse lemurs (Microcebus ravelobensis) use leaf nests more frequently in low temperatures (Thorén et al.2010) and Japanese macaques (Macaca fuscata) sleep on lower ground to facilitate larger groupings for huddling in cold winters (Takahashi 1997). Thermoregulatory pressures may also vary seasonally, or throughout an animal’s life. For example, the importance of thermoregulation in sleeping site choice of female gray mouse lemurs (Microcebus murinus) changes with seasonality and increases when they have offspring (Lutermann et al.2010). Some nocturnal primates may select particular microclimates to sleep in to reduce overheating from the sun. For example, mouse lemurs (Microcebus spp.) favor insulated sleeping sites with less extreme fluctuations in temperature when ambient daytime temperatures are high (Karanewsky and Wright 2015; Schmid 1998), and less insulated sites during periods of heavy rainfall (Lutermann et al.2010). Dense canopy cover can be an important factor in providing protection from the ambient temperature by shading animals from the sun (Duncan and Pillay 2013).
Much of the previous research into primate sleeping behavior has focused on diurnal primates. The earliest primates were thought to be small (Soligo and Martin 2006) and nocturnal (Ross et al.2007), similar to the nocturnal strepsirrhines of today (Crook and Gartlan 1966). Therefore, knowledge on the behavior and ecology of extant nocturnal primates aids our understanding of the selection pressures that acted on some of the earliest primate species before the appearance of diurnality. Some nocturnal primates (owl monkeys, Aotus spp.; and mouse lemurs, Microcebus spp.) are “marathon sleepers”, spending a much greater time asleep than diurnal primates do (Nunn et al.2010). The importance of a safe sleeping site is therefore paramount in nocturnal primates, but detailed information on the sleeping behavior of many species in Asia and mainland Africa is unavailable (Bearder et al.2003; Svensson et al.2018).
Nocturnal primates are likely to be vulnerable to a different predator guild than their diurnal counterparts because of high levels of inactivity during daytime hours. Small, arboreal, nocturnal primates are estimated to be predated on at a greater rate than other primate groups (Hart 2007) but reports of predation on nocturnal primates are scarce (Burnham et al.2012; Hart 2007). Known predators of nocturnal primates include snakes, felids, nonfelid carnivores, raptors, and other primates including humans (Burnham et al.2012; Svensson et al.2018). Nocturnal primates may be especially vulnerable to predation during the daytime (Butynski 1982; Pruetz and Bertolani 2007); therefore a level of crypsis is required when sleeping (Bearder et al.2002; Burnham et al.2012; Nekaris and Bearder 2011; Svensson et al.2018).
Galagos are nocturnal, arboreal strepsirrhine primates distributed across sub-Saharan Africa, comprising six genera: Euoticus, Galago, Galagoides, Otolemur, Paragalago, and Sciurocheirus. Taxa vary in sleeping behavior and ecology (Svensson et al.2018), but generally, galagos sleep in groups with variable membership during the day and forage alone at night (Bearder 1999; Bearder and Doyle 1974; Bearder et al.2003; Charles-Dominique 1977; Harcourt and Nash 1986), with particular sleeping sites used repeatedly by different individuals (Bearder et al.2003). Galago nests, built by a number of species within the Galagoides, Galago, and Otolemur genera, are usually leaf and twig, open platform constructions within thorny trees, presumably to provide protection from predators (Bearder et al.2003). In addition, Galago spp. and the thick-tailed galago (Otolemur crassicaudatus) use tree cavities, dense tangles of vegetation, and branches as sleeping sites (Bearder et al.2003; Svensson et al.2018). Some species sleep in areas of dense forest canopy and understory cover (e.g., Allen’s squirrel galago, Sciurocheirus alleni; and elegant needle-clawed galago, Euoticus elegantulus: Laurance et al.2008). Brief periods of torpor have been recorded in the Southern lesser galago (Galago moholi) but they largely favor behavioral and ecological adaptations, such as increased huddling behavior and choosing insulated sleeping sites (enclosed cavities and nests rather than open branches) over torpor use for survival in cold, dry winters (Nowack et al.2013).
Reptiles such as snakes (Svensson et al.2018), and raptors such as Verreaux’s eagle (Aquila verreauxii: Baker 2013, given in Svensson et al.2018) and hawks (Ambrose and Butynski, given in Svensson et al.2018), are either observed or suspected predators of galago species. Genets (Genetta spp.) are also predators of galagos (Burnham et al.2012; Mzilikazi et al.2006), but are more likely to hunt galagos at night or at dusk when they are active, rather than when they are sleeping (Bearder et al.2002). Western chimpanzees (Pruetz and Bertolani 2007) hunt Galago senegalensis from tree cavities but are not present at our study site, and researchers have observed vervet monkeys (Chlorocebus pygerythrus) eating (though not catching) a Northern lesser galago (G. senegalensis: Phyllis Lee, pers. comm. to C. Bettridge).
Galago senegalensis is the most widely distributed of the galagos, ranging across sub-Saharan Africa in a variety of habitats, such as woodland, bushland, savannah, and montane forest (Bearder et al.2008). Details of G. senegalensis sleeping site ecology and sociality featured in a review of galagos (Bearder et al.2003) and two short-term studies focused on the habitat ecology of populations in Kenya (Off et al.2008) and The Gambia (Svensson and Bearder 2013). However, no studies have addressed the sleeping site ecology of this species in great detail. In East Africa, populations of G. senegalensis are associated with Acacia spp. (Nash and Whitten 1989; Off et al.2008), which provide both sleeping sites (Haddow and Ellice 1964; Nash and Whitten 1989) and food sources (Acacia gum: Nash and Whitten 1989; Off et al.2008). Non-Acacia trees also provide suitable sleeping sites for the Kenyan sub-species G. s. braccatus (Nash and Whitten 1989). Known structure types to support sleep in G. senegalensis are nests (Bearder et al.2003), in tree cavities (Haddow and Ellice 1964; Svensson and Bearder 2013), on tree branches, or in dense tangles of vegetation (Nash and Whitten 1989; Svensson and Bearder 2013).
Here we describe the nesting behavior and sleeping site preferences of a population of Galago senegalensis in the Kwakuchinja wildlife corridor, Northern Tanzania, investigating the ecological importance of environmental variables on their sleeping site choice. If predation risk is a strong selective pressure influencing sleeping behavior galagos will sleep in trees with greater connectivity and canopy cover, and in areas with greater tree and mid-level vegetation density, but lower levels of ground cover. If thermoregulation is important to G. senegalensis we predict that cooler, more sheltered sites will be preferred. We aim to identify the levels of tree density, canopy cover, and vegetation cover that are preferred for sleeping sites with a view to establishing the habitat requirements for this and similar species.
Methods
Study Site
The study took place in a 9.2 km2 area within the Kwakuchinja wildlife corridor, located in the Babati District of Northern Tanzania, between Lake Manyara and Tarangire National Parks (3°46′19′′S, 35°51′29′′E; Fig. 1). The Tarangire–Manyara ecosystem has a semiarid climate, with a mean annual rainfall of around 650 mm (Foley and Faust 2010; TCP 1997, given in Msoffe et al.2007). The habitat in the study area is mainly woodland dominated by Acacia and Commiphora species, with scattered Baobab (Adansonia digitate) and palm (Hyphaene and Borassus) trees.
The site is home to two species of galago, Garnett’s greater galago (Otolemur garnettii) and the focus of this study, Galago senegalensis.
Location of Galagos and Sleeping Trees
We conducted a pilot study in 2014 to assess the feasibility of the site and methodology, but collected the majority of the data over two 6-week periods from June to August of 2015 and 2016. In 2015, we generated six 1-km2 blocks using QuantumGIS (QGIS Development Team 2017). For the first 4 weeks, we surveyed a block each morning before sunrise (04:30–07:00 h) for galagos and followed them back to their sleeping sites. Sleeping sites are the places where individuals sleep, and we define them in our study as the area around and including the sleeping tree within a 10 m radius. We define the sleeping tree as the tree that galagos sleep in. In the evening (19:00–23:00 h) we searched for galagos on foot to determine the best areas for locating sleeping sites in the morning. For the final two weeks, we concentrated our survey effort on one of the blocks, as we observed very few galagos in the other areas. In 2016 we increased the total survey area to 9.2 km2, including the previous area. We used a similar sampling strategy: we divided the area into five larger blocks (mean area 1.8 ± SD 0.4 km2, range = 1.2–2.3 km2), and randomized the order in which we sampled them. As in the previous year we initially sampled all blocks equally (this time for 3 weeks) to determine galago distribution, and then concentrated on the most heavily used areas (two blocks or 3.83 km2 in total in 2016) for the final 3 weeks.
We used torches fitted with red filters (LED Lenser, Clulite and Maglite 3-Cell D LED flashlight) to minimize disturbance to the animals (Bearder and Doyle 1974; Finley 1959; Svensson and Bearder 2013). We relied on visual detections from galagos’ eye shine reflecting in the torch light, and also used their vocalizations as an aid to locate them. We recorded the time each galago settled into its sleeping location, stopped moving, and, if in view, closed its eyes. When a galago is settling into sleep it is clear that its movements become very deliberate and slow and it assumes a recognizable position, often curling its tail over its head or around its body. We waited a minimum of 10 min or until light levels increased to make sure that the individual had settled. We recorded the location of sleeping trees with a handheld Garmin 62 GPS, and marked sleeping trees with biodegradable colored tape to assist in identification on return. We revisited occupied galago sleeping sites in the evening (17:30–19:30 h) to record time of awakening, the time the first galago left its sleeping location in the tree, and the time of departure from the sleeping tree.
Sleeping Site Surveys
We surveyed all previously recorded sleeping trees each day to monitor their use and record group size of galagos. We recorded height of the sleeping location, number of individuals, and structure type. We use the term “structure type” to refer to the kind of structure used to support the individual when sleeping, and “sleeping location” to refer to the location of the individual in the tree. To calculate the height of sleeping locations, one researcher used a TruPulse range finder while another stood directly beneath the sleeping galago(s). The range finder required three points to calculate height: the horizontal distance to the researcher who stood beneath the animal(s); the angle up toward the animal(s); and the base angle, toward the feet of the researcher who stood beneath the galago(s). We defined four categories of structure types: 1) branch: no visible leaf structure; 2) nest: platform or cup constructed of plant material; 3) palm leaf: individuals sleeping in the folded sections of a palm tree; and 4) tree cavity: within a tree trunk or branch. In some sleeping trees (N = 5) galagos were seen in both nests and on branches, in such cases we report the most commonly used structure type for each sleeping tree. When we observed different numbers of galagos using a particular sleeping location on different days we report the first recorded group size for each tree instead of all observations in the results. For the same purpose, we report mean height of sleeping location and mean distance of sleeping location from the top of the tree canopy for each sleeping site. We calculated the mean occupancy of all sleeping sites, where occupancy for each sleeping site was the proportion of visits in which at least one sleeping galago was using the site.
Vegetation Data
We used sleeping trees as the centers of vegetation plots 10 m in radius. We marked them using 2 × 20 m bisecting measuring tape transects, each pointing in the direction of the four cardinal points. We selected control plots by using the minute hand on an analog watch and traveling 100 m in this direction from the sleeping tree; the closest tree to this point acted as the center of control plots. To assess the habitat, we recorded nine variables from all plots: 1) the number, 2) species, 3) height and 4) diameter at breast height (DBH) of all trees in the plot, 5) the number of trees with any part of their canopy touching any part of the sleeping or control tree, 6) canopy cover, 7) the number of shrubs, and 8) the number of shrub species within the plot. Lastly, where applicable, 9) we estimated the diameter of the sleeping branch where the galago(s) slept (0–5 cm, 5–10 cm, >10 cm). We defined trees as having a DBH ≥7.5 cm (Benavides et al.2016; Motta et al.2006) and shrubs as woody plants with a DBH of <7.5 cm. We measured DBH at 1.3 m from the ground using a diameter measuring tape; for multistemmed trees we calculated the mean for all stems ≥7.5 cm. We used either a clinometer or a TruPulse range finder to measure tree height and then calculated the mean height and DBH of all trees in the plot for use in analyses. We recorded unidentified tree or shrub species and included them in total number of trees or shrubs in the plot but not in any other analysis. We categorized nonsleeping tree species into Acacia or non-Acacia to reduce the number of levels in the tree species variable. We chose these categories because the Acacia species share broadly similar structural features such as thorns, and seasonally shed their leaves in the dry season; they also comprised a large proportion of the habitat. We grouped together non-Acacia trees, as each individual species were represented in relatively low numbers. Similarly, we categorized distance to nearest yellow fever tree (Acacia xanthophloea), a known food source for Galago senegalensis (Nash and Whitten 1989; Off et al.2008), into two levels (<30 m, ≥30 m), based on our usual range of sight.
We measured canopy cover every 5 m along the plot transects and at the center point, recording nine points for each plot. One observer looked through the reverse end of binoculars perpendicular to the ground and estimated the proportion of canopy cover as 0%, 1–20%, 21–40%, 41–60%, 61–80%, and 81–100%. To measure mid-level vegetation density one researcher held a pole with 1-cm red bands positioned every 10 cm along its length horizontally at ca. 1 m above the ground at each of the four cardinal points; another researcher stood at the center of the plot and counted the number of red bands it was possible to see using binoculars (8 × 42 or 10 × 42). We subtracted the total number of red bands in view from the total number of bands on the pole; a lower value represents lower mid-level density. There was a small variation in the number of red bands used in the different years (10 in 2015 and 15 in 2016), so we calculated proportions before any analyses. To record ground cover; in 2015, we used four quadrats of 1 m2, each 5 m from the central tree along the plot transects; in 2016 we used a circle with a 10 cm diameter every 1 m along the measuring tape (41 points in total). We then recorded the percentage of bare ground, and the height of vegetation in each quadrat or circle. The second method is more thorough, so we tested whether this difference in method affected the results using “year” as a factor in our model with two levels: the 2015 and 2016 field seasons. To measure human disturbance, we counted the number of cut tree and shrub stems in the vegetation plot and in 2016 also recorded counts of dung in the vegetation plot, categorized into “domestic herbivore”, “wild herbivore”, and “wild carnivore”.
Abiotic Measures
We compared abiotic measures between sleeping and control trees. Portable Reed ST-171 data loggers recorded temperature and humidity of the microhabitat around galago sleeping locations every 30 min. We used a 4 m long wooden stick to place data loggers as close as possible to the galago sleeping locations, and at the same height in control trees (defined as the closest tree of the same species and of similar height to the sleeping tree). In total we placed 28 data loggers (14 in sleeping trees and 14 in controls) for 7 days each in 2016 (10 in week 4, 10 in week 5, and 8 in week 6). We were unable to reach palm trees to place any data loggers, and we analyzed only data from currently unoccupied sleeping locations to ensure that body heat from the animals did not bias the results.
Statistical Analysis
Comparison between Sleeping Trees and the General Population of Trees
We compared data on height and DBH of sleeping trees to the general population of trees (the other trees in sleeping site plots and all trees in control plots from 2016). Data on both height and DBH violated assumptions of normality (Shapiro–Wilks test) in at least one of the levels (sleeping trees/general population of trees); all variables P < 0.05. Therefore, we used Wilcoxon matched-pairs signed-rank tests to compare the ranks of data on height and DBH between sleeping and control trees from the general population. We also compared tree species of sleeping trees with the composition of the wider habitat. We created four categories of tree species, the first two being the most used tree species, Acacia tortilis and Acacia kirkii, and all others were categorized into “palm” (Hyphaene and Borassus spp.) or “other.” We used a chi-squared test of goodness-of-fit to test whether the number of sleeping tree species were significantly different from the expected proportion of the general population of trees.
Predictors of Galago Sleeping Sites
We used a random forest classification analysis (Breiman 1996, 2001) to determine the best predictors of galago sleeping sites when compared to control sites. Random forests are built as a combination of generated classifier trees, which “vote” for the best predicted class and ultimately provide a measure of variable importance. The approach uses bootstrapping to create new samples, with replacement, from the known observations. For each of the bootstrap samples, the mean classification error is calculated using only the predictions from the trees not containing the training data in their bootstrap sample. This is called the “out of bag” error, generalized across the number of trees constructed, and a lower value indicates greater strength and lower correlation between classifiers. We included the following variables in the analysis: height of sleeping/control tree, DBH of sleeping/control tree, number of trees in the plot (including the sleeping/control tree), number of Acacia trees in the plot (including the sleeping/control tree), number of shrubs in the plot, mean height of surrounding trees in the plot, mean DBH of surrounding trees in the plot, species of sleeping/control tree (Acacia/non-Acacia), number of connected trees, distance to nearest yellow fever tree (<30 / ≥30 m), number of Acacia tortilis trees in the plot, percentage of canopy cover, percentage of mid-level vegetation density, number of trees and shrubs cut down, proportion of bare ground in ground cover, mean height of ground cover, counts of domestic herbivore dung, counts of wild herbivore dung, and year (field season). We excluded all other variables because of insufficient data.
We used the cforest function in the party package (Hothorn et al.2015) in R version 3.3.1 (R Development Core Team 2016). Cforest takes into account potential collinearity between predictor variables and is argued to provide unbiased variable selection (Strobl et al.2007). The cforest function allows both continuous and categorical variables to be used in the analysis, with continuous variables varying in range (Strobl et al.2007). Variable importance is measured by mean decrease in classification accuracy, where greater values indicate that they are more important to the classification.
Abiotic Measures
We obtained the mean temperature and humidity from each full day (06:00–18:00 h) of data collection for each data logger and with these values calculated the mean of all full days for each data logger (N = 13). We calculated the standard deviation of the mean temperature (Tsd) and humidity (Hsd) for each day to measure fluctuation of the two variables (Bettridge et al.2010; Korstjens et al.2010). We did not use data from one data logger and its control in the analysis, as there was always a galago present at the sleeping location.
We tested all variables for normality using the Shapiro–Wilk test. Mean humidity and Hsd were normally distributed (mean humidity: sleeping: W = 0.91, P = 0.16, control: W = 0.92, P = 0.24; Hsd: sleeping: W = 0.89, P = 0.10, control: W = 0.92, P = 0.24), but mean temperature and Tsd did not have a normal distribution in at least one of the levels (mean temperature: sleeping: W = 0.85, P = 0.03, control: W = 0.95, P = 0.52; Tsd: sleeping: W = 0.95, P = 0.56, control: W = 0.85, P = 0.03). We used paired t-tests (for mean humidity and Hsd) and Wilcoxon matched-pairs signed-rank tests (for mean temperature and Tsd) for comparisons between sleeping and control locations.
Human Disturbance
We used Fisher’s exact tests to compare the counts of cut stems and domestic herbivore dung in sleeping and control sites. We also compared the number of wild herbivore dung, and data were insufficient for testing wild carnivore dung between sleeping sites and controls.
We conducted all statistical tests in R (version 3.3.1; R Development Core Team 2016).
Data Availability
The data sets generated and analyzed during the current study are not publicly available because the data form part of a PhD thesis but are available from the corresponding author on reasonable request.
Ethical Note
The project was approved by the Manchester Metropolitan University (MMU) Ethics Committee and the research complied with the International Primatological Society’s Code of best practice for field primatology. We adhered to the legal requirements of Tanzania at all times. MMU students registered at the College of African Wildlife Management collected the data under TAWIRI permission reference: AD.C.12 Vol.11.68.2014. The authors declare that they have no conflict of interest.
Results
Sleeping Sites
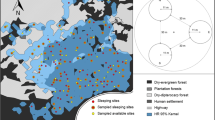
In 2015 we recorded 26 sleeping sites, including 3 that we had previously observed in our pilot study in 2014. In 2016 we located 31 sleeping sites, 8 of which were reused from previous years; giving a total of 49 independent sleeping sites (Fig. 2) located over 60 survey nights. Some sleeping trees from 2015 had been damaged in 2016; a small palm tree (Hyphaene petersiana) had mostly broken leaves and a large Acacia polycantha had fallen. We revisited sleeping sites 2–48 times, depending on when we first located them. Mean occupancy of all sleeping sites was 27 ± SD 25%.
Distribution of sleeping sites of Galago senegalensis found in July 2014, and from June to August of 2015 and 2016, within the Kwakuchinja wildlife corridor, Northern Tanzania (see Fig. 1 for the location of our study site).
Sleeping Tree Characteristics
We collected vegetation data for 47 of the 49 sleeping sites and 47 random controls. Mean height of sleeping trees was 9.78 ± SD 3.42 m and mean DBH was 18.95 ± SD 10.81 cm. Mean number of connected trees to the sleeping tree was 2.57 ± SD 1.96 and mean percentage of canopy cover at sleeping sites was 48 ± SD 21%.
The most commonly used tree species was Acacia tortilis (N = 21) followed by Acacia kirkii (N = 7); Hyphaene petersiana (N = 6); Acacia polycantha (N = 3); Borassus aethiopum (N = 2); Balanites glabra (N = 2); Acacia mellifera (N = 2); Lannea spp. (N = 1); Balanites aegyptiaca (N = 1); Albizia spp. (N = 1); and Acacia xanthophloea (N = 1). The species of sleeping trees differed from the composition of the wider habitat (χ23 = 8.68, P = 0.03). Galagos slept in palm trees more than expected (expected: 3; observed: 8), in A. tortilis and A. kirkii, as expected (A. tortilis: expected: 22; observed: 21; A. kirkii: expected: 6; observed: 7), and in “other species” less than expected (expected: 16; observed: 11).
Structure Type and Sleeping Location
The majority of the structure types used to support sleep were branches (65%; N = 32). Galagos used nests in 14% of sleeping trees (N = 7) and palm leaves in 18% (N = 9). We observed galagos using a tree cavity for the first time in 2016 (N = 1; Fig. 3), this was the only tree cavity recorded during the study. Of the 32 branches used to sleep on, the majority had a diameter of <5 cm (N = 24), four were 5–10 cm in diameter, and only one branch was >10 cm; we did not collect data for the remaining three branches owing to poor visibility. Nests were usually crude leaf constructions and we did not observe galagos building the nests. When using palm leaves, galagos slept toward the central spine of the leaf, usually covered by other towering palm leaves. The tree cavity was situated in the fork of an Acacia polycantha, at a height of ca. 9 m, with a closed canopy above (Fig. 3).
Examples of the four different structure types used by Galago senegalensis in the Kwakuchinja wildlife corridor, Northern Tanzania from June to August of 2015 and 2016. (a) Galago sleeping on a branch. (b) Galagos sleeping on a leaf nest. (c) Galago awake from sleeping in a palm leaf. (d) A tree cavity used by galagos.
Mean height of sleeping locations was 5.95 ± SD 2.38 m (range 2.50–12.00 m; N = 39) and mean distance of sleeping locations from the top of the tree was 4.30 ± SD 2.92 m (range 0.41–10.83 m; N = 38). We could not collect data from some trees with poor visibility.
Group Size
Of the first recorded group size for each sleeping site, 51% were solitary individuals (N = 22), 30% were a pair (N = 13), 14% were a group of three (N = 6), and 5% were a group of four (N = 2). We could not reliably determine group size for the remaining four trees.
Sleep Patterns
Galagos reached their sleeping trees at a mean of 29 ± SD 14 min before sunrise (N = 10, range = 12–53) and settled into their sleeping location, with no further movement, at a mean of 17 ± SD 17 min before sunrise (N = 7, range = 41 min before to 5 min after sunrise). In the evening, galagos first showed signs of awakening (eyes open or first movement) at a mean of 11 ± SD 10 min before sunset (N = 11, range = 34 min before to 1 min after sunrise). They left their sleeping location at a mean of 8 ± SD 8 min after sunset (N = 15, range = 5 min before to 23 min after sunset) and moved on from the sleeping tree at a mean of 15 ± SD 7 min after sunset (N = 16, range = 1 min before to 26 min after sunset). These sample sizes are variable because quite often the animals were not visible enough for us to detect small movements or whether their eyes were open or not.
Habitat Predictors of Galago Sleeping Sites
Sleeping trees did not differ significantly from the general population of trees in height (Wilcoxon matched-pairs signed-rank test: Z = 2969, P = 0.72) or DBH (Wilcoxon matched-pairs signed-rank test: Z = 2999, P = 0.79). Random forest classification analysis (Table I and Fig. 4) showed that galagos select sleeping trees with greater connectivity than control trees. They also sleep in areas with greater mid-level vegetation and tree density, higher percentage of canopy cover, and more Acacia trees than control plots. Measures of ground cover (% bare ground and vegetation height) were not important predictors of sleeping sites (Electronic Supplementary Material [ESM] Table SI) and “year” was one of the least important predictors of all the variables we tested. The mean out of sample prediction error rate (“out of bag” error rate) for 500 repetitions was 9% (5% for sleeping trees and 12% for control trees; see the confusion matrix in Table II). Our model correctly predicted 42 sleeping sites as sleeping sites and 35 control sites as control sites.
The five best predictors of sleeping sites of Galago senegalensis within the Kwakuchinja wildlife corridor, Northern Tanzania from June to August of 2015 and 2016. They are ranked 19 to 15, with 19 being the best classifier. The importance rank of all other variables can be found in the Electronic Supplementary Material.
Abiotic Measures
Mean humidity was significantly higher, and mean temperature and Tsd were significantly lower in sleeping locations than in control locations. There was no significant difference in Hsd between sleeping and control locations (Table III).
Human Disturbance
There was no significant difference in the number of cut stems (Fisher’s exact test: P = 0.42; N = 47; mean: sleeping: 5.64 ± SD 6.20 control: 5.72 ± SD 7.14), domestic herbivore dung (Fisher’s exact test: P = 0.89; N = 21, mean: sleeping: 29.27 ± SD 18.58 control: 50.24 ± SD 36.96) or wild herbivore dung (Fisher’s exact test: P = 0.55; N = 21, mean: sleeping: 17.73 ± SD 11.29 control: 42.14 ± SD 33.03) between sleeping and control sites.
Discussion
We have shown that sleeping site selection by Galago senegalensis supports both antipredator and thermoregulatory hypotheses. The most important predictor of galago sleeping sites was connectivity between the sleeping tree and neighboring trees, a means of escape or safe departure from the sleeping tree. Greater mid-level vegetation and number of trees are also predictors of sleeping sites and provide escape routes from the sleeping tree. Dense canopy cover was also an important predictor, and can provide both antipredator benefits in concealment from aerial predators and thermoregulatory benefits in protection from direct sunlight.
Evidence for Antipredator Hypotheses
The most important predictors of sleeping sites in our study can all be attributed to the importance of predator avoidance by aiding cover and concealment (Albert et al.2011; Anderson 2000; Birks et al.2005; Duarte and Young 2011; Hamilton 1982; Last and Muh 2013; Phoonjampa et al.2010; Svensson et al.2018). Connectivity, and tree and mid-level density, relate to the galagos’ ability to easily move throughout their habitat and evade predators without the need for terrestrial locomotion. Mid-level vegetation density may be of particular importance for predator avoidance because galagos are light enough to move swiftly through shrubs, where their larger-bodied predators may not be. In our study galagos prioritized their defense from aerial predators over the threat from potential terrestrial predators. Dense canopy cover could increase crypsis from aerial predators and may provide more escape routes from other predators (Fan and Jiang 2008; Xiang et al.2010). In contrast to diurnal primates, which are argued to counter predation risk through increased gregariousness (Cheney and Wrangham 1987; Shultz et al.2004, 2011; van Schaik 1983), nocturnal primates probably counter predation risk through crypsis (Bearder et al.2002; Nekaris and Bearder 2011).
Reports of predation on nocturnal primates are far fewer than those related to their diurnal counterparts (Burnham et al.2012; Hart 2007), most likely because of the comparatively smaller number of studies addressing this. The main predators of galagos are thought to be genets and other viverrids, raptors, jackals (Canis mesomelas), snakes (Bearder et al.2002; Burnham et al.2012; Svensson et al.2018), and in some populations, chimpanzees (Pruetz and Bertolani 2007). There are also reports of blue monkeys (Cercopithecus mitis: Butynski 1982) and vervet monkeys (Phyllis Lee, pers. comm. to C. Bettridge) predating lesser galagos. Aside from reports of genets (Simon Bearder, pers. obs., Burnham et al.2012) all other reports of galago predation occur during the day, when galagos are asleep and at their most vulnerable (Lima et al.2005), reflecting the importance of predator avoidance in selecting a sleeping site. Galagos do not have the means for active defense against predators, so their only options for reducing predation risk are avoidance through crypsis or escape.
While the influence of predation risk is considered a major evolutionary pressure on sociality in diurnal primates (Hill and Lee 1998; van Schaik 1983), its effect on the behavioral ecology of nocturnal primates is less understood. The tendency for animals in this study to sleep in groups is similar to that observed in Galago senegalensis in The Gambia (Svensson and Bearder 2013), where animals slept alone only 30% of the time, and the closely related Galago moholi (Bearder and Doyle 1974), which slept alone around 40% of the time. We mostly found sleeping sites in small clusters within 150 m of each other. It is possible that the sharing of sleeping sites and/or being in close vicinity to other sites provides safety from potential predators through the use of alarm calls and the mobbing response of other galagos (Bearder et al.2002). The clustering of sleeping sites may also be due to the limited suitable habitat available, as most of the areas in our control plots exhibited lower connectivity and vegetation density than the sleeping sites.
The low mean occupancy rate of sleeping sites in this study could suggest that galagos experience a trade-off between choosing favorable sites and avoiding detection from predators, and move to reduce their detectability (Day and Elwood 1999). The fact that galagos move between sleeping trees suggests that the population density is low enough to allow this; if the habitat were saturated, or sleeping trees were a limited resource, we might expect to see more and larger groups at sleeping sites and more consistent use of each site. Our model incorrectly predicted a small number (11 of 49) of our control sites as sleeping sites, again suggesting that the population does not saturate its habitat, although with a longer study we may well have observed these control trees being used as sleeping sites. Alternatively, there may be factors unaccounted for in our model, such as presence of sympatric galago species; one tree used by Galago senegalensis in 2015 instead housed Otolemur garnettii in 2016.
Evidence for Thermoregulatory Hypotheses
Although the most important variables in determining sleeping sites in this study can relate to predator avoidance, one of the best predictors (canopy cover) can also offer thermoregulatory benefits. Data from data loggers also showed that galagos sleep in cooler, more humid locations, with less temperature variation than control sites. Thermoregulatory hypotheses are well supported by data from diurnal primate nesting behavior (Fruth and Hohmann 1996), and are thought to be of greater importance than predator avoidance for chimpanzees (Koops et al.2012). Much of the existing literature focuses on the need for diurnal primates to insulate themselves against low ambient temperatures overnight (McGrew 2004; Takahashi 1997) or humidity avoidance (Koops et al.2012). In our study, the thermoregulatory pressure likely comes from reducing overheating in the high daytime temperatures.
Tree holes provide good insulation properties and so may be preferred sleeping locations for small primates, especially those that undergo torpor in order to save energy, such as the Microcebus spp. (Schmid 1998). Previous studies on the closely related Galago moholi, which are subject to lower temperatures than our study population, show that although they are capable of torpor, they only rarely employ this tactic and instead rely on behavioral adjustments to thermoregulation (Nowack et al.2013). The most commonly used structure type in this study was open branches, where galagos hugged the branch with their four limbs (Fig. 3). Although this posture may allow the cool microenvironment of the branches to prevent the animals from overheating (Briscoe et al.2014), clinging to thinner lateral branches is likely to have been more important for avoiding a fall. We recorded only one tree cavity in this study, contrasting with other populations of G. senegalensis (Svensson and Bearder 2013) and closely related species (e.g., Galago moholi and Galago matschiei: Bearder et al.2003). It is unclear from our study why galagos did not use more tree cavities. It could be that the temperature conditions are not extreme enough for cavities to be important; female gray mouse lemurs show a seasonal shift from tree holes, which provide superior thermoregulatory advantages, to nests during the wet season (Lutermann et al.2010). Alternatively, owing to the level of human activity in the area, it is possible that there are not many trees large or old enough to house suitable cavities.
Human activity is prevalent in the area and we observed tree cutting almost daily. Deforestation of other tree species may be one reason that this study is the first to record the use of palm trees as sleeping sites for any galago species (Svensson et al.2018). Galagos used palms more than would be expected based on their representation in the habitat, which may be due to a lack of tree cavities (Haddow and Ellice 1964; Svensson and Bearder 2013) or other suitable resources. Palm leaves may provide shelter from direct sunlight, but based on personal observations they may not offer good protection from predators: on several occasions we saw vervet monkeys, baboons and raptors in the palm trees, and the raptors appeared to be actively searching amongst the leaves.
Habitat Requirements of Galago senegalensis
Our study reveals that unsurprisingly for an arboreal primate, galagos need areas with high tree connectivity and high tree and mid-level vegetation density. However, the height and DBH of trees (indicators of age, and structural stability) seem less important, and there was no difference in these variables between sleeping sites and control areas. Galagos preferred a higher density of Acacia trees in their sleeping sites. It is likely that galagos favor the protection from predators offered by the trees’ spines and thorns over their use as food resources, as we did not observe any animals feeding at sleeping sites. De Jong and Butynski (2004) made a similar argument for the use of Acacia trees by the closely related Galago gallarum. The prevalence of cut stems and domestic herbivore dung across both sleeping and control sites suggests that G. senegalensis are able to persist in areas with high human activity. Just how much they are affected by anthropogenic activity cannot be determined from this study, as we do not have any areas where humans are not having an impact. Now that we have provided a greater understanding of galago sleeping site ecology, the effect of anthropogenic pressure should be a focus of future research.
Conclusion
Our study reveals the nesting behavior of a poorly understood nocturnal primate and highlights the importance of antipredation and thermoregulation on sleeping site choice; it is possible that both influence sleeping site selection in other small nocturnal mammals. Future research should focus on the effects of human impact on galagos, aided by the knowledge of the sleeping site ecology provided here. Nocturnal primates are underrepresented in the scientific literature and further research into their behavioral ecology, including the effect of anthropogenic threats, is paramount for understanding the selective pressures on their evolutionary past and aiding their conservation.
References
Albert, A., Savini, T., & Huynen, M. C. (2011). Sleeping site selection and presleep behaviour in wild pigtailed macaques. American Journal of Primatology, 73(12), 1222–1230.
Anderson, J. R. (1984). Ethology and ecology of sleep in monkeys and apes. Advances in the Study of Behaviour, 14, 165–229.
Anderson, J. R. (2000). Sleep-related behavioural adaptations in free-ranging anthropoid primates. Sleep Medicine Reviews, 4(4), 355–373.
Bearder, S. K. (1999). Physical and social diversity among nocturnal primates: A new view based on long term research. Primates, 40(1), 267–282.
Bearder, S. K., & Doyle, G. A. (1974). Field and laboratory studies of social organization in bushbabies (Galago senegalensis). Journal of Human Evolution, 3, 37–50.
Bearder, S. K., Nekaris, K. A. I., & Buzzell, C. A. (2002). Dangers of the night: Are some primates afraid of the dark? In L. E. Miller (Ed.), Eat or be eaten: Predator sensitive foraging in primates (pp. 21–43). Cambridge: Cambridge University Press.
Bearder, S. K., Ambrose, L., Harcourt, C., Honess, P., Perkin, A., et al (2003). Species-typical patterns of infant contact, sleeping site use and social cohesion among nocturnal primates in Africa. Folia Primatologica, 74(5–6), 337–354.
Bearder, S., Butynski, T. M., & De Jong, Y. (2008). Galago senegalensis. The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2008.RLTS.T8789A12932627.en. Accessed 12 Dec 2017.
Benavides, R., Escudero, A., Coll, L., Ferrandis, P., Ogaya, R., et al (2016). Recruitment patterns of four tree species along elevation gradients in Mediterranean mountains: Not only climate matters. Forest Ecology and Management, 360, 287–296.
Bettridge, C. M., & Dunbar, R. I. M. (2012). Predation as a determinant of minimum group size in baboons. Folia Primatologica, 83(3–6), 332–352.
Bettridge, C., Lehmann, J., & Dunbar, R. I. M. (2010). Trade-offs between time, predation risk and life history, and their implications for biogeography: A systems modelling approach with a primate case study. Ecological Modelling, 221(5), 777–790.
Birks, J. D. S., Messenger, J. E., & Halliwell, E. C. (2005). Diversity of den sites used by pine martens Martes martes: A response to the scarcity of arboreal cavities? Mammal Review, 35(3–4), 313–320.
Breiman, L. (1996). Bagging predictors. Machine Learning, 24(2), 123–140.
Breiman, L. (2001). Random forests. Machine Learning, 45(1), 5–32.
Briscoe, N. J., Handasyde, K. A., Griffiths, S. R., Porter, W. P., Krockenberger, A., & Kearney, M. R. (2014). Tree-hugging koalas demonstrate a novel thermoregulatory mechanism for arboreal mammals. Biology Letters, 10(6), 140235. https://doi.org/10.1098/rsbl.2014.0235.
Burnham, D., Bearder, S. K., Cheyne, S. M., Dunbar, R. I. M., & MacDonald, D. W. (2012). Predation by mammalian carnivores on nocturnal primates: Is the lack of evidence support for the effectiveness of nocturnality as an antipredator strategy? Folia Primatologica, 83(3–6), 236–251.
Butynski, T. M. (1982). Blue monkey (Cercopithecus mitis stuhlmanni) predation on galagos. Primates, 23(4), 563–566.
Charles-Dominique, P. (1977). Ecology and behaviour of nocturnal primates: Prosimians of equatorial West Africa. New York: Columbia University Press.
Cheney, D. L., & Wrangham, R. W. (1987). Predation. In B. B. Smuts, D. L. Cheney, R. M. Seyfarth, R. W. Wrangham, & T. T. Struhsaker (Eds.), Primate societies (pp. 227–239). Chicago: University of Chicago Press.
Cheyne, S. M., Rowland, D., Höing, A., & Husson, S. J. (2013). How orangutans choose where to sleep: Comparison of nest-site variables. Asian Primates Journal, 3(1), 13–17.
Cowlishaw, G. (1997a). Trade-offs between foraging and predation risk determine habitat use in a desert baboon population. Animal Behaviour, 53, 667–686.
Cowlishaw, G. (1997b). Refuge use and predation risk in a desert baboon population. Animal Behaviour, 54, 241–253.
Crook, J. H., & Gartlan, J. S. (1966). Evolution of primate societies. Nature, 210(5042), 1200–1203.
Dagosto, M., Gebo, D. L., & Dolino, C. (2001). Positional behaviour and social organization of the Philippine tarsier (Tarsius syrichta). Primates, 42(3), 233–243.
Day, R. T., & Elwood, R. W. (1999). Sleeping site selection by the golden-handed tamarin Saguinus midas midas: The role of predation risk, proximity to feeding sites, and territorial defence. Ethology, 105(12), 1035–1051.
De Jong, Y. A., & Butynski, T. M. (2004). Life in the thornbush—The Somali bushbaby. Swara, 27, 22.
Di Bitetti, M. S., Vidal, E. M. L., Baldovino, M. C., & Benesovsky, V. (2000). Sleeping site preference in tufted capuchin monkeys (Cebus apella nigritus). American Journal of Primatology, 50(4), 257–274.
Diekelmann, S., & Born, J. (2010). The memory function of sleep. Nature Reviews Neuroscience, 11(2), 114–126.
Duarte, M. H. L., & Young, R. J. (2011). Sleeping site selection by urban marmosets (Callithrix penicillata) under conditions of exceptionally high predator density. International Journal of Primatology, 32(2), 329–334.
Duncan, L. M., & Pillay, N. (2013). Shade as a thermoregulatory resource for captive chimpanzees. Journal of Thermal Biology, 38(4), 169–177.
Fan, P. F., & Jiang, X. L. (2008). Sleeping sites, sleeping trees, and sleep-related behaviours of black crested gibbons (Nomascus concolor jingdongensis) at Mt. Wuliang, Central Yunnan, China. American Journal of Primatology, 70(2), 153–160.
Finley, R. B. (1959). Observation of nocturnal animals by red light. Journal of Mammalogy, 40(4), 591–594.
Foley, C. A. H., & Faust, L. J. (2010). Rapid population growth in an elephant Loxodonta africana population recovering from poaching in Tarangire National Park, Tanzania. Oryx, 44(2), 205–212.
Fruth, B., & Hohmann, G. (1996). Nest building behaviour in the great apes: The great leap forward? In W. C. McGrew, L. F. Marchant, & T. Nishida (Eds.), Great ape societies (pp. 225–240). Cambridge: Cambridge University Press.
Haddow, A. J., & Ellice, J. M. (1964). Studies on bush-babies (Galago spp.) with special reference to the epidemiology of yellow fever. Transactions of the Royal Society of Tropical Medicine and Hygiene, 58(6), 521–538.
Hamilton, W. J. (1982). Baboon sleeping site preferences and relationships to primate grouping patterns. American Journal of Primatology, 3, 41–53.
Hankerson, S. J., Franklin, S. P., & Dietz, J. M. (2007). Tree and forest characteristics influence sleeping site choice by golden lion tamarins. American Journal of Primatology, 69(9), 976–988.
Harcourt, C. S., & Nash, L. T. (1986). Species differences in substrate use and diet between sympatric galagos in two Kenyan coastal forests. Primates, 27(1), 41–52.
Harrison, M. J. S. (1985). Time budget of the green monkey, Cercopithecus sabaeus: Some optimal strategies. International Journal of Primatology, 6(4), 351–376.
Hart, D. (2007). Predation on primates: A biogeographical analysis. In S. L. Gursky-Doyen & K. A. I. Nekaris (Eds.), Primate anti-predator strategies (pp. 27–59). Developments in primatology: Progress and prospects. New York: Springer Science+Business Media.
Hill, R. A., & Lee, P. C. (1998). Predation risk as an influence on group size in cercopithecoid primates: Implications for social structure. Journal of Zoology, 245(4), 447–456.
Hothorn, T., Hornik, K., & Strobl, C. (2015). Party: A laboratory for recursive partytioning. https://cran.r-project.org/web/packages/party/vignettes/party.pdf
Karanewsky, C. J., & Wright, P. C. (2015). A preliminary investigation of sleeping site selection and sharing by the brown mouse lemur Microcebus rufus during the dry season. Journal of Mammalogy, 96(6), 1344–1351.
Kenyon, M., Streicher, U., Loung, H., Tran, T., Tran, M., et al (2014). Survival of reintroduced pygmy slow Loris Nycticebus pygmaeus in South Vietnam. Endangered Species Research, 25(2), 185–195.
Koops, K., McGrew, W. C., de Vries, H., & Matsuzawa, T. (2012). Nest-building by chimpanzees (Pan troglodytes verus) at Seringbara, Nimba Mountains: Antipredation, thermoregulation, and antivector hypotheses. International Journal of Primatology, 33(2), 356–380.
Korstjens, A. H., Lehmann, J., & Dunbar, R. I. M. (2010). Resting time as an ecological constraint on primate biogeography. Animal Behaviour, 79(2), 361–374.
Lagos, V. O., Contreras, L. C., Meserve, P. L., Gutiérrez, J. R., & Jaksic, F. (1995). Effects of predation risk on space use by small mammals: A field experiment with a Neotropical rodent. Oikos, 74(2), 259–264.
Last, C., & Muh, B. (2013). Effects of human presence on chimpanzee nest location in the Lebialem-Mone Forest landscape, southwest region, Cameroon. Folia Primatologica, 84(1), 51–63.
Laurance, W. F., Croes, B. M., Guissouegou, N., Buij, R., Dethier, M., & Alonso, A. (2008). Impacts of roads, hunting, and habitat alteration on nocturnal mammals in African rainforests. Conservation Biology, 22(3), 721–732.
Lesmeister, D. B., Gompper, M. E., & Millspaugh, J. J. (2008). Summer resting and den site selection by eastern spotted skunks (Spilogale putorius) in Arkansas. Journal of Mammalogy, 89(6), 1512–1520.
Lima, S. L., Rattenborg, N. C., Lesku, J. A., & Amlaner, C. J. (2005). Sleeping under the risk of predation. Animal Behaviour, 40(4), 723–736.
Lutermann, H., Verburgt, L., & Rendigs, A. (2010). Resting and nesting in a small mammal: Sleeping sites as a limiting resource for female grey mouse lemurs. Animal Behaviour, 79(6), 1211–1219.
Mabille, G., & Berteaux, D. (2014). Hide or die: Use of cover decreases predation risk in juvenile north American porcupines. Journal of Mammalogy, 95(5), 992–1003.
McGrew, W. C. (2004). The cultured chimpanzee: Reflections on cultural primatology. Cambridge: Cambridge University Press.
Motta, R., Morales, M., & Nola, P. (2006). Human land-use, forest dynamics and tree growth at the treeline in the Western Italian Alps. Annals of Forest Science, 63(7), 739–747.
Msoffe, F., Mturi, F. A., Galanti, V., Tosi, W., Wauters, L. A., & Tosi, G. (2007). Comparing data of different survey methods for sustainable wildlife management in hunting areas: The case of Tarangire-Manyara ecosystem, northern Tanzania. European Journal of Wildlife Research, 53(2), 112–124.
Mzilikazi, N., Masters, J. C., & Lovegrove, B. G. (2006). Lack of torpor in free-ranging southern lesser galagos, Galago moholi: Ecological and physiological considerations. Folia Primatologica, 77(6), 465–476.
Nash, L. T., & Whitten, P. L. (1989). Preliminary observations on the role of Acacia gum chemistry in Acacia utilization by Galago senegalensis in Kenya. American Journal of Primatology, 17(1), 27–39.
Nekaris, K. A. I., & Bearder, S. K. (2011). The Lorisiform primates of Asia and mainland Africa: Diversity shrouded in darkness. In C. Campbell, A. Fuentes, K. MacKinnon, S. K. Bearder, & R. Stumpf (Eds.), Primates in perspective (pp. 24–45). Oxford: Oxford University Press.
Nowack, J., Wippich, M., Mzilikazi, N., & Dausmann, K. H. (2013). Surviving the cold, dry period in Africa: Behavioural adjustments as an alternative to heterothermy in the African lesser bushbaby (Galago moholi). International Journal of Primatology, 34(1), 49–64.
Nunn, C. L., & Heymann, E. W. (2005). Malaria infection and host behaviour: A comparative study of Neotropical primates. Behavioural Ecology and Sociobiology, 59(1), 30–37.
Nunn, C. L., McNamara, P., Capellini, I., Preston, P., & Barton, R. A. (2010). Primate sleep in phylogenetic perspective. In Evolution of sleep: Phylogenetic and functional perspectives (pp. 123–144). Cambridge: Cambridge University Press.
Off, E. C., Isbell, L. A., & Young, T. P. (2008). Population density and habitat preferences of the Kenya lesser galago (Galago senegalensis braccatus) along the Ewaso Nyiro River, Laikipia, Kenya. Journal of East African Natural History, 97(1), 109–116.
Phoonjampa, R., Koenig, A., Borries, C., Gale, G. A., & Savini, T. (2010). Selection of sleeping trees in pileated gibbons (Hylobates pileatus). American Journal of Primatology, 72(7), 617–625.
Pruetz, J. D., & Bertolani, P. (2007). Savanna chimpanzees, Pan troglodytes verus, hunt with tools. Current Biology, 17(5), 412–417.
Quantum GIS Development Team. (2017). Quantum GIS geographic information system. Open source Geospatial Foundation project. http://qgis.osgeo.org
R Development Core Team. (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rode, J. E., Nekaris, K. A. I., Markolf, M., Schliehe-Diecks, S., Seiler, M., et al (2013). Social organisation of the northern giant mouse lemur Mirza zaza in Sahamalaza, North Western Madagascar, inferred from nest group composition and genetic relatedness. Contributions to Zoology, 82(2), 71–83.
Ross, C. F., Hall, M. I., & Heesy, C. P. (2007). Were basal primates nocturnal? Evidence from eye and orbit shape. In Primate origins: Adaptations and evolution (pp. 233–256). Developments in primatology: Progress and prospects. New York: Springer Science+Business Media.
Schmid, J. (1998). Tree holes used for resting by gray mouse lemurs (Microcebus murinus) in Madagascar: Insulation capacities and energetic consequences. International Journal of Primatology, 19(5), 797–809.
Shultz, S., Noe, R., McGraw, W. S., & Dunbar, R. I. M. (2004). A community-level evaluation of the impact of prey behavioural and ecological characteristics on predator diet composition. Proceedings of the Royal Society B: Biological Sciences, 271(1540), 725–732.
Shultz, S., Opie, C., & Atkinson, Q. D. (2011). Stepwise evolution of stable sociality in primates. Nature, 479(7372), 219–222.
Siegel, J. M. (2005). Clues to the functions of mammalian sleep. Nature, 437(7063), 1264–1271.
Soligo, C., & Martin, R. D. (2006). Adaptive origins of primates revisited. Journal of Human Evolution, 50(4), 414–430.
Stewart, F. A., Piel, A. K., Azkarate, J. C., & Pruetz, J. D. (2018). Savanna chimpanzees adjust sleeping nest architecture in response to local weather conditions. American Journal of Physical Anthropology, 166(3), 549–562.
Strobl, C., Boulesteix, A., Zeileis, A., & Hothorn, T. (2007). Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinformatics, 8(1), 25.
Svensson, M. S., & Bearder, S. K. (2013). Sightings and habitat use of the northern lesser galago (Galago senegalensis senegalensis) in Niumi National Park, the Gambia. African Primates, 8, 51–58.
Svensson, M. S., Nekaris, K. A. I., Bearder, S. K., Bettridge, C. M., Butynski, T. M., et al (2018). Sleep patterns, daytime predation, and the evolution of diurnal sleep site selection in lorisiforms. American Journal of Physical Anthropology, 166(3), 563–577.
Takahashi, H. (1997). Huddling relationships in night sleeping groups among wild Japanese macaques in Kinkazan Island during winter. Primates, 38(1), 57–68.
Thorén, S., Quietzsch, F., & Radespiel, U. (2010). Leaf nest use and construction in the golden-brown mouse lemur (Microcebus ravelobensis) in the Ankarafantsika National Park. American Journal of Primatology, 72(1), 48–55.
Van Moorter, B., Gaillard, J. M., McLoughlin, P. D., Delorme, D., Klein, F., & Boyce, M. S. (2009). Maternal and individual effects in selection of bed sites and their consequences for fawn survival at different spatial scales. Oecologia, 159(3), 669–678.
van Schaik, C. P. (1983). Why are diurnal primates living in groups? Behaviour, 87(1), 120–144.
Xiang, Z. F., Nie, S. G., Chang, Z. F., Wei, F. W., & Li, M. (2010). Sleeping sites of Rhinopithecus brelichi at Yangaoping, Guizhou. International Journal of Primatology, 31(1), 59–71.
Acknowledgments
We thank Augustino Mwageni, Nassoro Kapinga, and Michael Chuala for their hard work and assistance in the field; the many BSc and MSc students who assisted in data collection; and Edwin Harris for advice on the analysis. Thanks also to Victor Kakengi from TAWIRI for his support, to two anonymous reviewers for their helpful comments on a previous version of this manuscript, and two more anonymous reviewers and Professor Jo Setchell for her editorial assistance. C. M. Bettridge formulated the original idea and designed the study; C. M. Bettridge, M. Jones, G. Ellison, A. Wolfenden, L. Kahana, and A. Kisingo developed the methodology; G. Ellison, C. M. Bettridge, A. Wolfenden, and J. Jamieson conducted fieldwork; L. Kahana and A. Kisingo provided logistical advice; G. Ellison and M. Jones analyzed the data. G. Ellison and C. M. Bettridge wrote the manuscript; the other authors provided editorial advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Joanna M. Setchell

Badge earned for open practices: Open Materials. Experiment materials and data are available at: https://osf.io/pjudq/.
Electronic supplementary material
ESM 1
(DOC 53 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ellison, G., Wolfenden, A., Kahana, L. et al. Sleeping Site Selection in the Nocturnal Northern Lesser Galago (Galago senegalensis) Supports Antipredator and Thermoregulatory Hypotheses. Int J Primatol 40, 276–296 (2019). https://doi.org/10.1007/s10764-019-00085-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-019-00085-y