Abstract
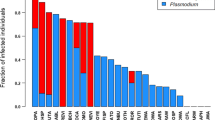
Parasites are ubiquitous in populations of free-ranging animals and impact host fitness, but virtually nothing is known about the factors that influence patterns of disease risk across species and the effectiveness of behavioral defenses to reduce this risk. We investigated the correlates of malaria infection (prevalence) in Neotropical primates using data from the literature, focusing on host traits involving group size, body mass, and sleeping behavior. Malaria is spread to these monkeys through anopheline mosquitoes that search for hosts at night using olfactory cues. In comparative tests that used two different phylogenetic trees, we confirmed that malaria prevalence increases with group size in Neotropical primates, as suggested by a previous non-phylogenetic analysis. These results are consistent with the hypothesis that larger groups experience increased risk of attack by mosquitoes, and counter to the hypothesis that primates benefit from the encounter-dilution effect of avoiding actively-seeking insects by living in larger groups. In contrast to non-phylogenetic tests, body mass was significant in fewer phylogeny-based analyses, and primarily when group size was included as a covariate. We also found statistical support for the hypothesis that sleeping in closed microhabitats, such as tree holes or tangles of vegetation, reduces the risk of malaria infection by containing the host cues used by mosquitoes to locate hosts. Due to the small number of evolutionary transitions in sleeping behavior in this group of primates, however, this result is considered preliminary until repeated with a larger sample size. In summary, risk of infection with malaria and other vector-borne diseases are likely to act as a cost of living in groups, rather than a benefit, and sleeping site selection may provide benefits by reducing rates of attack by malaria vectors.
Similar content being viewed by others
References
Abouheif E (1999) A method for testing the assumption of phylogenetic independence in comparative data. Evol Ecol Res 1:895–909
Alexander RD (1974) The evolution of social behavior. Ann Rev Ecol Syst 5:325–383
Anderson JR (1984) Ethology and ecology of sleep in monkeys and apes. Adv Study Behav 14:165–229
Anderson JR (1998) Sleep, sleeping sites, and sleep–related activities: awakening to their significance. Am J Primatol 46:63–75
Altizer S, Nunn CL, Thrall PH, Gittleman JL, Antonovics J, Cunningham AA, Dobson AP, Ezenwa V, Pedersen AB, Poss M, Pulliam JRC (2003) Social organization and parasite risk in mammals: integrating theory and empirical studies. Ann Rev Ecol Evol Syst 34:517–47
Blumstein DT, Evans CS, Daniel JC (1999) An experimental study of behavioural group size effects in tammar wallabies, Macropus eugenii. Anim Behav 58:351–360
Bock G, Cardew G (1996) Olfaction in mosquito–host interactions. In: Ciba Foundation symposium. Wiley, Chichester, New York.
Brown CR, Brown MB (1986) Ectoparasitism as a cost of coloniality in cliff swallows (Hirundo pyrrhonota). Ecology 67:1206–1218
Brown CR, Sethi RA (2002) Mosquito abundance is correlated with cliff swallow (Petrochelidon pyrrhonota) colony size. J Med Entomol 39:115–120
Brown CR, Komar N, Quick SB, Sethi RA, Panella NA, Brown MB, Pfeffer M (2001) Arbovirus infection increases with group size. Proc Roy Soc Lond B 268:1833–1840
Coatney GR, Collins WE, McWilson W (1971) The primate malarias. National Insitiute of Allergy and Infectious Diseases, Bethesda.
Cochrane AH, Matsumoto Y, Kamboj KK, Maracic M, Nussenzweig RS, Aikawa M (1988) Membrane–associated antigens of blood stages of Plasmodium brasilianum, a quartan malaria parasite. Infec Immunity 56:2080–2088
Coimbra-Filho AF (1977) Natural shelters of Leontopithecus rosalia and some ecological implications (Callitrichidae: Primates). In: Kleiman D (ed) The biology and conservation of the Callitrichidae. Smithsonian Institution Press, Washington, pp 79–89
Coimbra–Filho AF, Mittermeier R (1981) Ecology and behavior of neotropical primates 1. Academia Brasileira de Ciencias, Rio de Janeiro.
Collins WE (1994) The owl monkey as a model for malaria. In: Baer JF, Weller RE, Kakoma I (eds) Aotus: The owl monkey. Academic Press, San Diego, CA, pp 217–244
Côté IM, Poulin R (1995) Parasitism and group size in social animals: a meta-analysis. Behav Ecol 6:159–165
Davies CR, Ayres JM, Dye C, Deane LM (1991) Malaria infection rate of Amazonian primates increases with body weight and group size. Funct Ecol 5:655–662
Day JF, Edman JD (1984) Mosquito engorgement on normally defensive hosts depends on host activity patterns. J Med Entomol 21:732–740
de Arruda M, Nardin EH, Nussenzweig RS, Cochrane AH (1989) Sero-epidemiological studies of malaria in Indian tribes and monkeys of the Amazon Basin of Brazil. Am J Trop Med Hyg 41:379–385
Deane LM, Ferreira Neto JA, Okumura M, Ferreira MO (1969) Malaria parasites of Brazilian monkeys. Rev Inst Med Trop Sao Paulo 11:71–86
Deane LM (1992) Simian malaria in Brazil. Mem Inst Oswaldo Cruz 87:1–20
Di Bitetti MS, Vidal EML, Baldovino MC, Benesovsky V (2000) Sleeping site preferences in tufted capuchin monkeys (Cebus apella nigritus). Am J Primatol 50:257–274
Dudley R, Milton K (1990) Parasite deterrence and the energetic costs of slapping in howler monkeys, Alouatta palliata. J Mammal 71:463–465
Edman JD (1988) Disease control through manipulation of vector-host interaction: some historical and evolutionary perspectives. In: Scott TW, Grumstrup-Scott J (eds) Proceedings of a symposium: the role of vector-host interactions in disease transmission, vol 68 Entomological Society of America, Lanham, MD, pp 43–50
Edman JD, Day JF, Walker ED (1984) Field confirmation of laboratory observations on the differential antimosquito behavior of herons. Condor 86:91–92
Ewald PW (1983) Host-parasite relations, vectors, and the evolution of disease severity. Ann Rev Ecol Syst 14:465–485
Fandeur T, Volney B, Peneau C, De Thoisy B (2000) Monkeys of the rainforest in French Guiana are natural reservoirs for P. brasilianum/P. malariae malaria. Parasitology 120:11–21
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Freeland WJ (1977) Blood-sucking flies and primate polyspecific associations. Nature 269:801–802
Garcia J, Braza F (1987) Activity rhythms and use of space of a group of Aotus azarae in Bolivia during the rainy season. Primates 28:337–342
Garnham PCC (1966) Malaria parasites and other Haemosporidia. Blackwell Scientific, Oxford.
Gillies MT (1955) The Density of adult anopheles in the neighbourhood of an East African village. Am J Trop Med Hyg 4:1103–1113
Goodman M, Porter CA, Czelusniak J, Page SL, Schneider H, Shoshani J, Gunnell G, Groves CP (1998) Toward a phylogenetic classification of primates based on DNA evidence complemented by fossil evidence. Mol Phylog Evol 9:585–598
Haddow AJ (1942) The mosquito fauna and climate of native huts at Kisumu, Kenya. Bull Entomol Res 33:91–142
Hallem EA, Fox AN, Zwiebel LJ, Carlson JR (2004) Olfaction-mosquito receptor for human-sweat odorant. Nature 427:212–213
Harvey PH, Pagel MD (1991) The comparative method in evolutionary biology. Oxford University Press, Oxford
Hawkey CM (1977) The haematology of exotic mammals. In: Archer RK, Jeffcott LB, Lehmann H (eds) Comparative clinical haematology. Blackwell Scientific Publications, Oxford
Heymann EW (1995) Sleeping habits of tamarins, Saguinus mystax and Saguinus fuscicollis (Mammalia: Primates; Callitrichidae), in north-eastern Peru. J Zool Lond 237:211–226
Heymann EW (2001) Malaria infection rate of Amazonian primates: the role of sleeping habits. Folia Primatol 72:153
Hoare DJ, Couzin ID, Godin JGJ, Krause J (2004) Context-dependent group size choice in fish. Anim Behav 67:155–164
Izawa K (1979) Studies on peculiar distribution pattern of Callimico. Kyoto University Overseas Research Reports of New World Monkeys 1:1–19
Janson CH (1988) Food competition in brown capuchin monkeys (Cebus apella): quantitative effects of group size and tree productivity. Behaviour 105:53–76
Kappeler PM (1998) Nests, tree holes and the evolution of primate life histories. Am J Primatol 46:7–33
Kinzey WG (1981) The titi monkeys, genus Callicebus. In: Coimbra-Filho A, Mittermeier R (eds) Ecology and behavior of Neotropical primates. Academia Brasileira de Ciencias, Rio de Janeiro, pp 241–276
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, Oxford
Lourenço de Oliveira R, Luz SLB (1996) Simian malaria at two sites in the Brazilian Amazon. 2. vertical distribution and frequency of anopheline species inside and outside the forest. Mem Inst Oswaldo Cruz 91:687–694
Mittermeier RA, Rylands AB, Coimbra-Filho AF, Fonseca GAB (1988) Ecology and behavior of Neotropical primates 2. World Wildlife Fund, Washington
Møller AP, Dufva R, Allander K (1993) Parasites and the evolution of host social behavior. Adv Study Behav 22:65–102
Mooring MS, Hart BL (1992) Animal grouping for protection from parasites: Selfish herd and encounter-dilution effects. Behaviour 123:173–193
Nunn CL (2002) A comparative study of leukocyte counts and disease risk in primates. Evolution 56:177–190
Nunn CL, Gittleman JL, Antonovics J (2000) Promiscuity and the primate immune system. Science 290:1168–1170
Pålsson K, Jaenson TGT, Dias F, Laugen T, Bjorkman A (2004) Endophilic Anopheles mosquitoes in Guinea Bissau, West Africa, in relation to human housing conditions. J Med Entomol 41:746–752
Patz JA, Graczyk TK, Geller N, Vittor AY (2000) Effects of environmental change on emerging parasitic diseases. Int J Parasitol 30:1395–1405
Peres CA (1991) Ecology of mixed-species groups of tamarins in Amazonian terra firme forests. Ph.D. thesis, University of Cambridge
Petraitis PS, Dunham AE, Niewlarowski PH (1996) Inferring multiple causality: the limitations of path analysis. Funct Ecol 10:421–431
Port GR, Boreham PFL, Bryan JH (1980) The relationship of host size to feeding by mosquitos of the Anopheles gambiae Giles complex (Diptera, Culicidae). Bull Entomol Res 70:133–144
Porter CA, Page SL, Czelusniak J, Schneider H, Schneider MPC, SAmpaio I, Goodman M (1997) Phylogeny and evolution of selected primates as determined by sequences of the epsilon-globin locus and 5' flanking regions. Int J Primatol 18:261–295
Purvis A (1995) A composite estimate of primate phylogeny. Phil Tran Roy Soc Lond B 348:405–421
Purvis A, Rambaut A (1995) Comparative analysis by independent contrasts (CAIC): an Apple Macintosh application for analysing comparative data. Comp Applic Biosci 11:247–251
Reeve J, Abouheif E (2003) Phylogenetic Independence. 2.0 edn. McGill University, Montreal
Ribbands CR (1949) Studies on the attractiveness of human populations to anophelines. Bull Entomol Res 40:227–238
Rice WR, Gaines SD (1994) Heads I win, tails you lose - testing directional alternative hypotheses in ecological and evolutionary research. Trends Ecol Evol 9:235–237
Rubiopalis Y, Curtis CF (1992) Biting and resting behavior of anophelines in western Venezuela and implications for control of malaria transmission. Med Vet Entomol 6:325–334
Rylands AB (1981) Preliminary field observations on the marmoset, Callithrix humeralifer intermedius (Hershkovitz, 1977), at Dardanelos, Rio Aripuana, Mato Grosso. Primates 22:46–59
Schneider H (2000) The current status of the New World monkey phylogeny. Anais Da Academia Brasileira De Ciencias 72:165–172
Smith AC (1997) Comparative ecology of saddleback (Saguinus fuscicollis) and moustached (Saguinus mystax) tamarins. Thesis, University of Reading
Smith RJ, Jungers WL (1997) Body mass in comparative primatology. J Hum Evol 32:523–559
Soini P (1988) The pygmy marmoset, Cebuella. In: Mittermeier RA, Rylands AB, Coimbra-Filho AF, Fonseca GAB (eds), vol 2. World Wildlife Fund, Washington, pp 79–129
Stevenson MF, Rylands AB (1988) The marmosets, genus Callithrix. In: Mittermeier RA, Coimbra-Filho AF, da Fonseca GAB (eds) Ecology and behavior of Neotropical primates, vol 2. World Wildlife Fund, Washington, DC, pp 131–222
Taliaferro WH, Taliaferro LG (1934) Morphology, periodicity and course of infection in Panamanian monkeys. Am J Hyg 20:2–49
Tella JL (2002) The evolutionary transition to coloniality promotes higher blood parasitism in birds. J Evol Biol 15:32–41
Valderrama X, Robinson JG, Attygalle AB, Eisner T (2000) Seasonal anointment with millipedes in a wild primate: A chemical defense against insects? J Chem Ecol 26:2781–2790
Voorham J (2002) Intra–population plasticity of Anopheles darlingi's (Diptera, Culicidae) biting activity patterns in the state of Amapa, Brazil. Revista De Saude Publica 36:75–80
Webber LA, Edman JD (1972) Anti-mosquito behavior of ciconiiform birds. Anim Behav 20:228–232
Acknowledgments
We thank V. Ezenwa, P. M. Kappeler, C. Brown and three anonymous reviewers for helpful suggestions. This project was supported through funding from the NSF (Grant #DEB-0212096 to CN).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Brown
Electronic supplementary material
Tree 2
Phylogenetic tree of New World primates based on Porter et al. (1997), Schneider (2000) and Goodman et al. (1998). This tree is referred to as PSG in Table 2. Numbers indicate branch length in Ma. In addition to branch length differences, which were based on information in the references, the major differences to the Purvis tree involve the position of Aotus, the internal arrangement of the marmosets (Callithrix, Cebuella, Mico), and arrangement of species in the clade containing Ateles.
Rights and permissions
About this article
Cite this article
Nunn, C.L., Heymann, E.W. Malaria infection and host behavior: a comparative study of Neotropical primates. Behav Ecol Sociobiol 59, 30–37 (2005). https://doi.org/10.1007/s00265-005-0005-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0005-z