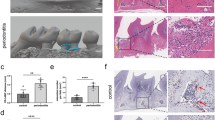
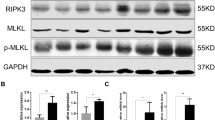
Abstract
Periodontitis, an infectious inflammatory disease influenced by various factors, disrupts the delicate balance between the host microbiota and immunity. The resulting excessive immune response exacerbates the progressive destruction of the supporting periodontal tissue. Macrophages are essential elements of the host innate immune system. They are pivotal components in the periodontal immune microenvironment and actively participate in both physiological and pathological processes of periodontal tissue. When confronted with periodontitis-related irritant factors, macrophages may differentiate to pro- or anti-inflammatory subtypes that affect tissue homeostasis. Additionally, macrophages may die in response to bacterial infections, potentially affecting the severity of periodontitis. This article reviews the typical mechanisms underlying macrophage death and its effects on periodontitis. We describe five forms of macrophage death in periodontitis: apoptosis, pyroptosis, necroptosis, ferroptosis, and ETosis. Our review of macrophage death in the pathophysiology of periodontitis enhances comprehension of the pathogenesis of periodontitis that will be useful for clinical practice. Although our review elucidates the complex mechanisms by which macrophage death and inflammatory pathways perpetuate periodontitis, unresolved issues remain, necessitating further research.



Similar content being viewed by others
Data Availability
All relevant data are within the paper. The data are available from the corresponding author on reasonable request.
References
Slots, Jørgen. 2017. Periodontitis: Facts, fallacies and the future. Periodontology 2000 (75): 7–23. https://doi.org/10.1111/prd.12221.
Kinane, Denis F., Panagiota G. Stathopoulou, and Panos N. Papapanou. 2017. Periodontal diseases. Nature Reviews. Disease Primers 3: 17038. https://doi.org/10.1038/nrdp.2017.38.
Bouchard, Philippe, Maria Clotilde Carra, Adrien Boillot, Francis Mora, and Hélène. Rangé. 2017. Risk factors in periodontology: A conceptual framework. Journal of Clinical Periodontology 44: 125–131. https://doi.org/10.1111/jcpe.12650.
Lourenço, Talita Gomes, Débora Heller. Baêta, Carina Maciel Silva-Boghossian, Sean L. Cotton, Bruce J. Paster, and Ana Paula Vieira. Colombo. 2014. Microbial signature profiles of periodontally healthy and diseased patients. Journal of Clinical Periodontology 41: 1027–1036. https://doi.org/10.1111/jcpe.12302.
Abdulkareem, Ali A., Firas B. Al-Taweel, Ali J. B. Al-Sharqi, Sarhang S. Gul, Aram Sha, and Iain L. C. Chapple. 2023. Current concepts in the pathogenesis of periodontitis: From symbiosis to dysbiosis. Journal of Oral Microbiology 15: 2197779. https://doi.org/10.1080/20002297.2023.2197779.
Gaibani, P., C. Vocale, S. Ambretti, F. Cavrini, J. Izard, L. Miragliotta, M.T. Pellegrino, and V. Sambri. 2010. Killing of Treponema denticola by mouse peritoneal macrophages. Journal of Dental Research 89: 521–526. https://doi.org/10.1177/0022034510363105.
Yang, Xue, Yaping Pan, Xu. Xiaoyu, Tong Tong, Yu. Shiwen, Yue Zhao, Li. Lin, Jingbo Liu, Dongmei Zhang, and Chen Li. 2018. Sialidase deficiency in Porphyromonas gingivalis increases IL-12 secretion in stimulated macrophages through regulation of CR3, IncRNA GAS5 and miR-21. Frontiers in Cellular and Infection Microbiology 8: 100. https://doi.org/10.3389/fcimb.2018.00100.
Makkawi, Hasnaa, Shifra Hoch, Elia Burns, Kavita Hosur, George Hajishengallis, Carsten J. Kirschning, and Gabriel Nussbaum. 2017. Porphyromonas gingivalis stimulates TLR2-PI3K signaling to escape immune clearance and induce bone resorption independently of MyD88. Frontiers in Cellular and Infection Microbiology 7: 359. https://doi.org/10.3389/fcimb.2017.00359.
Xue, Ying, Han Xiao, Songhe Guo, Xu. Banglao, Yuehua Liao, Wu. Yixian, and Ge. Zhang. 2018. Indoleamine 2,3-dioxygenase expression regulates the survival and proliferation of Fusobacterium nucleatum in THP-1-derived macrophages. Cell Death & Disease 9: 355. https://doi.org/10.1038/s41419-018-0389-0.
Jorgensen, Ine, and Edward A. Miao. 2015. Pyroptotic cell death defends against intracellular pathogens. Immunological Reviews 265: 130–142. https://doi.org/10.1111/imr.12287.
Johansson, Anders. 2011. Aggregatibacter actinomycetemcomitans leukotoxin: A powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins 3: 242–259. https://doi.org/10.3390/toxins3030242.
Zhao, Pengfei, Ziqi Yue, Lulingxiao Nie, Zhihao Zhao, Qian Wang, Jiao Chen, and Qi. Wang. 2021. Hyperglycaemia-associated macrophage pyroptosis accelerates periodontal inflamm-aging. Journal of Clinical Periodontology 48: 1379–1392. https://doi.org/10.1111/jcpe.13517.
Darveau, Richard P. 2010. Periodontitis: A polymicrobial disruption of host homeostasis. Nature Reviews. Microbiology 8: 481–490. https://doi.org/10.1038/nrmicro2337.
Pérez-Chaparro, P.J., C. Gonçalves, L.C. Figueiredo, M. Faveri, E. Lobão, N. Tamashiro, P. Duarte, and M. Feres. 2014. Newly identified pathogens associated with periodontitis: A systematic review. Journal of Dental Research 93: 846–858. https://doi.org/10.1177/0022034514542468.
Feres, Magda, Flavia Teles, Ricardo Teles, Luciene Cristina Figueiredo, and Marcelo Faveri. 2016. The subgingival periodontal microbiota of the aging mouth. Periodontology 2000 (72): 30–53. https://doi.org/10.1111/prd.12136.
Zhu, Bin, Lorna C. Macleod, Todd Kitten, and Xu. Ping. 2018. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Future Microbiology 13: 915–932. https://doi.org/10.2217/fmb-2018-0043.
Kolenbrander, Paul E., Robert J. Palmer, Alexander H. Rickard, Nicholas S. Jakubovics, Natalia I. Chalmers, and Patricia I. Diaz. 2006. Bacterial interactions and successions during plaque development. Periodontology 2000 (42): 47–79. https://doi.org/10.1111/j.1600-0757.2006.00187.x.
Kaplan, Christopher W., Renate Lux, Susan Kinder Haake, and Wenyuan Shi. 2009. The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm. Molecular Microbiology 71: 35–47. https://doi.org/10.1111/j.1365-2958.2008.06503.x.
Socransky, S.S., A.D. Haffajee, M.A. Cugini, C. Smith, and R.L. Kent. 1998. Microbial complexes in subgingival plaque. Journal of Clinical Periodontology 25: 134–144. https://doi.org/10.1111/j.1600-051x.1998.tb02419.x.
Holt, Stanley C., and Jeffrey L. Ebersole. 2005. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 2000 (38): 72–122. https://doi.org/10.1111/j.1600-0757.2005.00113.x.
Hajishengallis, George, Richard P. Darveau, and Michael A. Curtis. 2012. The keystone-pathogen hypothesis. Nature Reviews. Microbiology 10: 717–725. https://doi.org/10.1038/nrmicro2873.
Kelk P, Abd H, Claesson R, Sandström G, Sjöstedt A, and Johansson A. 2011. Cellular and molecular response of human macrophages exposed to Aggregatibacter actinomycetemcomitans leukotoxin. Cell death & disease 2. Cell Death Dis. https://doi.org/10.1038/cddis.2011.6.
Hassell, T.M. 1993. Tissues and cells of the periodontium. Periodontology 2000 (3): 9–38. https://doi.org/10.1111/j.1600-0757.1993.tb00230.x.
Kourtzelis, Ioannis, Xiaofei Li, Ioannis Mitroulis, Daniel Grosser, Tetsuhiro Kajikawa, Baomei Wang, Michal Grzybek, et al. 2019. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nature Immunology 20: 40–49. https://doi.org/10.1038/s41590-018-0249-1.
Geng, Fengxue, Junchao Liu, Chengcheng Yin, Shuwei Zhang, Yaping Pan, and Hongchen Sun. 2022. Porphyromonas gingivalis lipopolysaccharide induced RIPK3/MLKL-mediated necroptosis of oral epithelial cells and the further regulation in macrophage activation. Journal of Oral Microbiology 14: 2041790. https://doi.org/10.1080/20002297.2022.2041790.
Yang, Yuhui, Yiping Huang, and Weiran Li. 2021. Autophagy and its significance in periodontal disease. Journal of Periodontal Research 56: 18–26. https://doi.org/10.1111/jre.12810.
Murray, Peter J., Judith E. Allen, Subhra K. Biswas, Edward A. Fisher, Derek W. Gilroy, Sergij Goerdt, Siamon Gordon, et al. 2014. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 41: 14–20. https://doi.org/10.1016/j.immuni.2014.06.008.
Schlundt, Claudia, Heilwig Fischer, Christian H. Bucher, Carsten Rendenbach, Georg N. Duda, and Katharina Schmidt-Bleek. 2021. The multifaceted roles of macrophages in bone regeneration: A story of polarization, activation and time. Acta Biomaterialia 133: 46–57. https://doi.org/10.1016/j.actbio.2021.04.052.
Huang, Yanlan, Cheng Tian, Qimeng Li, and Xu. Qiong. 2019. TET1 knockdown inhibits Porphyromonas gingivalis LPS/IFN-γ-induced M1 macrophage polarization through the NF-κB pathway in THP-1 cells. International Journal of Molecular Sciences 20: 2023. https://doi.org/10.3390/ijms20082023.
Becerra-Ruiz, Julieta Saraí, Celia Guerrero-Velázquez, Fernando Martínez-Esquivias, Luz Andrea Martínez-Pérez, and Juan Manuel Guzmán-Flores. 2022. Innate and adaptive immunity of periodontal disease. From etiology to alveolar bone loss. Oral Diseases 28: 1441–1447. https://doi.org/10.1111/odi.13884.
Yu, Ting, Li. Zhao, Xin Huang, Chanjuan Ma, Yixiong Wang, Jincai Zhang, and Dongying Xuan. 2016. Enhanced activity of the macrophage M1/M2 phenotypes and phenotypic switch to M1 in periodontal infection. Journal of Periodontology 87: 1092–1102. https://doi.org/10.1902/jop.2016.160081.
Gottlieb, T.M., and M. Oren. 1998. p53 and apoptosis. Seminars in Cancer Biology 8: 359–368. https://doi.org/10.1006/scbi.1998.0098.
Yin, X.M. 2000. Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Research 10: 161–167. https://doi.org/10.1038/sj.cr.7290045.
Xu, Yu., Yue Zhang, Xu. Yao, Guangyao Zang, Bo. Li, Hao Xia, and Wei Yuan. 2021. Activation of CD137 signaling promotes macrophage apoptosis dependent on p38 MAPK pathway-mediated mitochondrial fission. The International Journal of Biochemistry & Cell Biology 136: 106003. https://doi.org/10.1016/j.biocel.2021.106003.
Kato, S., M. Muro, S. Akifusa, N. Hanada, I. Semba, T. Fujii, Y. Kowashi, and T. Nishihara. 1995. Evidence for apoptosis of murine macrophages by Actinobacillus actinomycetemcomitans infection. Infection and Immunity 63: 3914–3919. https://doi.org/10.1128/iai.63.10.3914-3919.1995.
Muro, M., T. Koseki, S. Akifusa, S. Kato, Y. Kowashi, Y. Ohsaki, K. Yamato, M. Nishijima, and T. Nishihara. 1997. Role of CD14 molecules in internalization of Actinobacillus actinomycetemcomitans by macrophages and subsequent induction of apoptosis. Infection and Immunity 65: 1147–1151. https://doi.org/10.1128/iai.65.4.1147-1151.1997.
Wan, Qiang, Jiabao Zhou, Wu. Yansheng, Liqiang Shi, Weiwei Liu, Ou. Jiaoying, and Jiandong Gao. 2022. TNF-α-mediated podocyte injury via the apoptotic death receptor pathway in a mouse model of IgA nephropathy. Renal Failure 44: 1216–1226. https://doi.org/10.1080/0886022X.2022.2079527.
Muro, M., K. Nakashima, J. Tomioka, S. Kato, K. Nonaka, T. Yoshida, M. Inoue, T. Nishihara, and Y. Kowashi. 1999. Inhibitory effect of lipopolysaccharide on apoptotic cell death in macrophages infected with Actinobacillus actinomycetemcomitans. FEMS microbiology letters 175: 211–216. https://doi.org/10.1111/j.1574-6968.1999.tb13622.x.
Lam, Roselind S., Neil M. O’Brien-Simpson, James A. Holden, Jason C. Lenzo, Shao B. Fong, and Eric C. Reynolds. 2016. Unprimed, M1 and M2 macrophages differentially interact with Porphyromonas gingivalis. PLoS ONE 11: e0158629. https://doi.org/10.1371/journal.pone.0158629.
Stowe, Irma, Bettina Lee, and Nobuhiko Kayagaki. 2015. Caspase-11: Arming the guards against bacterial infection. Immunological Reviews 265: 75–84. https://doi.org/10.1111/imr.12292.
Liu, X, Zhang Z, Ruan J, Pan Y, Magupalli Vg, Wu H, and Lieberman J. 2016. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535. Nature. https://doi.org/10.1038/nature18629.
Huang, Max Tze-Han., Debra TaxmanDebra, Elizabeth AElizabeth A. Holley-GuthrieTaxman, Chris B. Moore, Stephen B. Willingham, Victoria Madden, Rebecca Keyser Parsons, et al. 2009. Critical role of apoptotic speck protein containing a caspase recruitment domain (ASC) and NLRP3 in causing necrosis and ASC speck formation induced by Porphyromonas gingivalis in human cells. Journal of Immunology (Baltimore, Md.: 1950) 182: 2395–2404. https://doi.org/10.4049/jimmunol.0800909.
Fleetwood, Andrew J., Man K. S. Lee, William Singleton, Adrian Achuthan, Ming-Chin. Lee, Neil M. O’Brien-Simpson, Andrew D. Cook, et al. 2017. Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by Porphyromonas gingivalis and its outer membrane vesicles. Frontiers in Cellular and Infection Microbiology 7: 351. https://doi.org/10.3389/fcimb.2017.00351.
Coats, Stephen R., Jace W. Jones, Christopher T. Do, Pamela H. Braham, Brian W. Bainbridge, Thao T. To, David R. Goodlett, Robert K. Ernst, and Richard P. Darveau. 2009. Human toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1- and 4’-phosphatase activities. Cellular Microbiology 11: 1587–1599. https://doi.org/10.1111/j.1462-5822.2009.01349.x.
He, D., Li X, Zhang F, Wang C, Liu Y, Bhawal Uk, and Sun J. 2022. Dec2 inhibits macrophage pyroptosis to promote periodontal homeostasis. Journal of periodontal & implant science 52. J Periodontal Implant Sci. https://doi.org/10.5051/jpis.2101380069.
Jun, Hye-Kyoung., Young-Jung. Jung, and Bong-Kyu. Choi. 2017. Treponema denticola, Porphyromonas gingivalis, and Tannerella forsythia induce cell death and release of endogenous danger signals. Archives of Oral Biology 73: 72–78. https://doi.org/10.1016/j.archoralbio.2016.09.010.
De Andrade, Kq, Almeida-da-Silva Clc, Ojcius Dm, and Coutinho-Silva R. 2021. Differential involvement of the canonical and noncanonical inflammasomes in the immune response against infection by the periodontal bacteria Porphyromonas gingivalis and Fusobacterium nucleatum. Current research in microbial sciences 2. Curr Res Microb Sci. https://doi.org/10.1016/j.crmicr.2021.100023.
Jun, H.K., Lee S.h., Lee H.r., and Choi B.k., 2012. Integrin α5β1 activates the NLRP3 inflammasome by direct interaction with a bacterial surface protein. Immunity 36. Immunity. https://doi.org/10.1016/j.immuni.2012.05.002.
Okinaga, T., Ariyoshi, W., and Nishihara, T., 2015. Aggregatibacter actinomycetemcomitans invasion induces interleukin-1β production through reactive oxygen species and cathepsin B. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 35. J Interferon Cytokine Res. https://doi.org/10.1089/jir.2014.0127.
Inoue, M., Okinaga, T., Usui, M., Kawano, A., Thongsiri, C., Nakashima, K., Ariyoshi, W., and Nishihara, T., 2019. β-glucan suppresses cell death of ASC deficient macrophages invaded by periodontopathic bacteria through the caspase-11 pathway. FEMS microbiology letters 366. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnz093.
Ziauddin, S.M., Mohammad Ibtehaz Alam, Megumi Mae, Masayuki Oohira, Kanako Higuchi, Yasunori Yamashita, Yukio Ozaki, and Atsutoshi Yoshimura. 2022. Cytotoxic effects of dental calculus particles and freeze-dried Aggregatibacter actinomycetemcomitans and Fusobacterium nucleatum on HSC-2 oral epithelial cells and THP-1 macrophages. Journal of Periodontology 93: e92–e103. https://doi.org/10.1002/JPER.21-0196.
Uchiyama, R., and Tsutsui, H., 2015. Caspases as the key effectors of inflammatory responses against bacterial infection. Archivum immunologiae et therapiae experimentalis 63. Arch Immunol Ther Exp (Warsz). https://doi.org/10.1007/s00005-014-0301-2.
Miao, Edward A., Irina A. Leaf, Piper M. Treuting, Dat P. Mao, Monica Dors, Anasuya Sarkar, Sarah E. Warren, Mark D. Wewers, and Alan Aderem. 2010. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nature Immunology 11: 1136–1142. https://doi.org/10.1038/ni.1960.
Li, C., Yin, W., Yu, N., Zhang, D., Zhao, H., Liu, J., Pan, Y., and Lin, L., 2019. miR-155 promotes macrophage pyroptosis induced by Porphyromonas gingivalis through regulating the NLRP3 inflammasome. Oral diseases 25. Oral Dis. https://doi.org/10.1111/odi.13198.
Pasparakis, M., and Vandenabeele, P., 2015. Necroptosis and its role in inflammation. Nature 517. Nature. https://doi.org/10.1038/nature14191.
Chen, X., Li, W., Ren, J., Huang, D., He, W., Song, Y., Yang, C., et al. 2014. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell research 24. Cell Res. https://doi.org/10.1038/cr.2013.171.
Ke, X., Lei, L., Li, H., and Yan, F., 2016. Manipulation of necroptosis by Porphyromonas gingivalis in periodontitis development. Molecular immunology 77. Mol Immunol. https://doi.org/10.1016/j.molimm.2016.07.010.
Yang, Yanan, Lingxia Wang, Haibing Zhang, and Lijun Luo. 2022. Mixed lineage kinase domain-like pseudokinase-mediated necroptosis aggravates periodontitis progression. Journal of Molecular Medicine (Berlin, Germany) 100: 77–86. https://doi.org/10.1007/s00109-021-02126-7.
Shi, J., Li, J., Su, W., Zhao, S., Li, H., and Lei, L., 2019. Loss of periodontal ligament fibroblasts by RIPK3-MLKL-mediated necroptosis in the progress of chronic periodontitis. Scientific reports 9. Sci Rep. https://doi.org/10.1038/s41598-019-39721-1.
Ryter, Sw., Bhatia, D., and Choi, Me., 2019. Autophagy: a lysosome-dependent process with implications in cellular redox homeostasis and human disease. Antioxidants & redox signaling 30. Antioxid Redox Signal. https://doi.org/10.1089/ars.2018.7518.
Deretic, V., and Levine, B., 2009. Autophagy, immunity, and microbial adaptations. Cell host & microbe 5. Cell Host Microbe. https://doi.org/10.1016/j.chom.2009.05.016.
Kroemer, G., Mariño, G., and Levine, B., 2010. Autophagy and the integrated stress response. Molecular cell 40. Mol Cell. https://doi.org/10.1016/j.molcel.2010.09.023.
Park, HM., Jeong, Sy., Na, Hs., and Chung, J., 2017. Porphyromonas gingivalis induces autophagy in THP-1-derived macrophages. Molecular oral microbiology 32. Mol Oral Microbiol. https://doi.org/10.1111/omi.12153.
Bai, Lan, Bo-Yan. Chen, Yan Liu, Wu-Chang. Zhang, and Sheng-Zhong. Duan. 2022. A mouse periodontitis model with humanized oral bacterial community. Frontiers in Cellular and Infection Microbiology 12: 842845. https://doi.org/10.3389/fcimb.2022.842845.
Hirschhorn, T., and Stockwell, BR, 2019. The development of the concept of ferroptosis. Free radical biology & medicine 133. Free Radic Biol Med. https://doi.org/10.1016/j.freeradbiomed.2018.09.043.
Xu, T., Ding, W., Ji, X., Ao, X., Liu, Y., Yu, W., and Wang, J., 2019. Molecular mechanisms of ferroptosis and its role in cancer therapy. Journal of cellular and molecular medicine 23. J Cell Mol Med. https://doi.org/10.1111/jcmm.14511.
Xie, Y., Hou, W., Song, X., Yu, Y., Huang, J., Sun, X., Kang, R., and Tang, D., 2016. Ferroptosis: process and function. Cell death and differentiation 23. Cell Death Differ. https://doi.org/10.1038/cdd.2015.158.
杜雪纯,李保胜,乔树伟,欧燕珍,李珍 & 孟维艳.(2022).牙龈卟啉单胞菌脂多糖对巨噬细胞中铁死亡相关因子表达水平的影响. 吉林大学学报(医学版)(05),1148–1155. https://doi.org/10.13481/j.1671-587X.20220507.
Xu, Z., Tan, R., Li, X., Pan, L., Ji, P., and Tang, H., 2023. Development of a classification model and an immune-related network based on ferroptosis in periodontitis. Journal of periodontal research 58. J Periodontal Res. https://doi.org/10.1111/jre.13100.
Brinkmann, V., Reichard, U., Goosmann, C., Fauler, B., Uhlemann, Y., Weiss, Ds., Weinrauch, Y., and Zychlinsky, A., 2004. Neutrophil extracellular traps kill bacteria. Science (New York, N.Y.) 303. Science. https://doi.org/10.1126/science.1092385.
Chow, Oa., von Köckritz-Blickwede, M., Bright, At., Hensler, Me., Zinkernagel, As., Cogen, Al., Gallo, Rl., et al. 2010. Statins enhance formation of phagocyte extracellular traps. Cell host & microbe 8. Cell Host Microbe. https://doi.org/10.1016/j.chom.2010.10.005.
Weng, W., Hu, Z., and Pan, Y., 2022. Macrophage extracellular traps: current opinions and the state of research regarding various diseases. Journal of immunology research 2022. J Immunol Res. https://doi.org/10.1155/2022/7050807.
Liu, Yj., Chen, Jl., Fu, Zb., Wang, Y., Cao, Xz., and Sun, Y., 2022. Enhanced responsive formation of extracellular traps in macrophages previously exposed to Porphyromonas gingivalis. Inflammation 45. Inflammation. https://doi.org/10.1007/s10753-021-01611-y.
Marquardt, C., Fritsch-Decker, S., Al-Rawi, M., Diabaté, S., and Weiss, C., 2017. Autophagy induced by silica nanoparticles protects RAW264.7 macrophages from cell death. Toxicology 379. Toxicology. https://doi.org/10.1016/j.tox.2017.01.019.
Zhao, J., Geng, W., Wan, K., Guo, K., Xi, F., Xu, X., Xiong, X., Huang, X., Liu, J., and Kuang, X., 2021. Lipoxin A4 promotes autophagy and inhibits overactivation of macrophage inflammasome activity induced by Pg LPS. The Journal of international medical research 49. J Int Med Res. https://doi.org/10.1177/0300060520981259.
Zhao, Z., Ming, Y., Li, X., Tan, H., He, X., Yang, L., Song, J., and Zheng, L., 2023. Hyperglycemia aggravates periodontitis via autophagy impairment and ROS-inflammasome-mediated macrophage pyroptosis. International journal of molecular sciences 24. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms24076309.
Zhou, Y., Shen, Yong., Cong, Chen., Xinbing, Sui., Jingjing, Yang., Linbo, Wang., and Jichun, Zhou., 2019. The crosstalk between autophagy and ferroptosis: what can we learn to target drug resistance in cancer? Cancer Biology & Medicine 16: 630–646. https://doi.org/10.20892/j.issn.2095-3941.2019.0158.
Taabazuing, Cy., Okondo, Mc., and Bachovchin, Da., 2017. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell chemical biology 24. Cell Chem Biol. https://doi.org/10.1016/j.chembiol.2017.03.009.
Conos, Sa., Chen, Kw., De Nardo, D., Hara, H., Whitehead, L., Núñez, G., Masters, Sl., et al. 2017. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proceedings of the National Academy of Sciences of the United States of America 114. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1613305114.
Kawahara, Y., T. Kaneko, Y. Yoshinaga, Y. Arita, K. Nakamura, C. Koga, A. Yoshimura, and R. Sakagami. 2020. Effects of sulfonylureas on periodontopathic bacteria-induced inflammation. Journal of Dental Research 99: 830–838. https://doi.org/10.1177/0022034520913250.
Zhou, X., Wang, Q., Nie, L., Zhang, P., Zhao, P., Yuan, Q., Ji, N., Ding, Y., and Wang, Q., 2020. Metformin ameliorates the NLPP3 inflammasome mediated pyroptosis by inhibiting the expression of NEK7 in diabetic periodontitis. Archives of oral biology 116. Arch Oral Biol. https://doi.org/10.1016/j.archoralbio.2020.104763.
Sordi, MB., Panahipour, L., Kargarpour, Z., and Gruber, R., 2022. Platelet-rich fibrin reduces IL-1β release from macrophages undergoing pyroptosis. International journal of molecular sciences 23. Int J Mol Sci. https://doi.org/10.3390/ijms23158306.
Sordi, Mariane Beatriz, Ariadne Cristiane Cabral, Layla da Cruz, and Panahipour, and Reinhard Gruber. 2022. Enamel matrix derivative decreases pyroptosis-related genes in macrophages. International Journal of Molecular Sciences 23: 5078. https://doi.org/10.3390/ijms23095078.
Lagha, Ben, Amy Howell Amel, and Daniel Grenier. 2019. Cranberry proanthocyanidins neutralize the effects of Aggregatibacter actinomycetemcomitans Leukotoxin. Toxins 11: 662. https://doi.org/10.3390/toxins11110662.
Niu, Li., Shuangshuang Chen, Xue Yang, Chunliang Ma, Chunling Pan, Hongyan Wang, Qian Li, Fengxue Geng, and Xiaolin Tang. 2021. Vitamin D decreases Porphyromonas gingivalis internalized into macrophages by promoting autophagy. Oral Diseases 27: 1775–1788. https://doi.org/10.1111/odi.13696.
Chung, Jin, Sumi Kim, Hyun Ah Lee, Mi Hee Park, Yu. Seyeon Kim, Ri. Song, and Hee Sam Na. 2018. Trans-cinnamic aldehyde inhibits Aggregatibacter actinomycetemcomitans-induced inflammation in THP-1-derived macrophages via autophagy activation. Journal of Periodontology 89: 1262–1271. https://doi.org/10.1002/JPER.17-0727.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81800974, No. 81970942) and the Natural Science Foundation of Liaoning Province (No. 2023-MS-173, No. 2023JH2/101300042).
Author information
Authors and Affiliations
Contributions
Li Lin and Hongyan Wang were responsible for the whole work design, funding support, and paper submission; Wen Luo and Chengying Du were responsible for the literature collection and manuscript writing; Hsiuwei Huang, Jie Kong, and Ziming Ge were responsible for the manuscript review and figures. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, W., Du, C., Huang, H. et al. The Role of Macrophage Death in Periodontitis: A Review. Inflammation (2024). https://doi.org/10.1007/s10753-024-02015-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-024-02015-4