Abstract
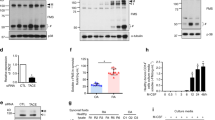
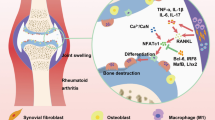
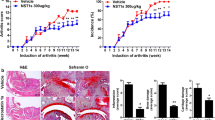
Rheumatoid arthritis (RA) is an autoimmune disease characterized by a notably high disability rate, primarily attributed to cartilage and bone degradation. The involvement of heat shock protein 90 (HSP90) as a molecular chaperone in the inflammatory response of RA has been established, but its role in bone destruction remains uncertain. In the present study, the expression of HSP90 was augmented in osteoclasts induced by the receptor activator of nuclear factor-κB ligand. Additionaly, it was observed that the outcomes revealed a noteworthy inhibition of osteoclast formation and differentation when triptolide was utilized to hinder the expression of HSP90. Furthermore, the positive influence of HSP90 in osteoclast differentiation was substantiated by overexpressing HSP90 in osteoclast precursor cells. Mechanically, HSP90 significantly activated the TNF receptor-associated factor 6 (TRAF6)/Nuclear factor of activated T cells 1 (NFATc1) signaling axis, accompanied by markedly promoting osteoclast differentiation. This effect was consistently observed in the destructive joint of rats with collagen-induced arthritis, where HSP90 effectively activated osteoclasts and contributed to arthritic bone destruction by activating the TRAF6/NFATc1 signaling. Overall, the findings of this study provide compelling evidence that HSP90 exacerbates bone destruction in RA by promoting osteoclast differentiation through the activation of TRAF6/NFATc1 signaling, and interference with HSP90 may be a promising strategy for the discovery of anti-arthritic bone destruction agents.







Similar content being viewed by others
DATA AVAILABILITY
Data are available from the corresponding author on reasonable request.
REFERENCES
Jarlborg, M. and C. Gabay. 2022. Systemic effects of IL-6 blockade in rheumatoid arthritis beyond the joints. Cytokine 149: 155742.
Choi, B.-Y., C.-H. Park, Y.H. Na, H.-W. Bai, J.-Y. Cho, and B.Y. Chung. 2016. Inhibition of RANKL-induced osteoclast differentiation through the downregulation of c-Fos and NFATc1 by Eremochloa ophiuroides (centipedegrass) extract. Molecular Medicine Reports 13: 4014–4022.
Greenblatt, M.B., J.N. Tsai, and M.N. Wein. 2017. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clinical Chemistry 63: 464–474.
Rui, X., Y. Yang, Q. Chen, J. Wu, J. Chen, Q. Zhang, R. Ren and D. Yin. 2020. Imperative and effective reversion of synovial hyperplasia and cartilage destruction in rheumatoid arthritis through multiple synergistic effects of O2 and Ca2+. Materials Science and Engineering C Materials for Biological Applications.114: 111058.
Li, Y., C. Yang, K. Jia, J. Wang, J. Wang, R. Ming, T. Xu, X. Su, Y. Jing, Y. Miao, C. Liu, and N.Lin. 2022. Fengshi Qutong capsule ameliorates bone destruction of experimental rheumatoid arthritis by inhibiting osteoclastogenesis. Journal of Ethnopharmacology 282: 114602.
Su, X., W. Guo, B. Yuan, D. Wang, L. Liu, X. Wu, Y. Zhang, X. Kong and N. Lin. 2021. Artesunate attenuates bone erosion in rheumatoid arthritis by suppressing reactive oxygen species via activating p62/Nrf2 signaling. Biomedicine & Pharmacotherapy 137: 111382.
De Vries, T.J., T. Schoenmaker, D. Aerts, L.C. Grevers, P.P.C. Souza, K. Nazmi, M.A. Van De Wiel, B. Ylstra, P.L. Van Lent, P.J.M. Leenen, and Everts, V. 2015. M-CSF priming of osteoclast precursors can cause osteoclastogenesis-insensitivity, which can be prevented and overcome on bone. Journal of Cellular Physiology 230: 210–225.
Gao, Y., B. Wang, C. Shen, and W. Xin. 2018. Overexpression of miR-146a blocks the effect of LPS on RANKL-induced osteoclast differentiation. Molecular Medicine Reports 18: 5481–5488.
Park, J.H., N.K. Lee, and S.Y. Lee. 2017. Current understanding of RANK signaling in osteoclast differentiation and maturation. Molecules and Cells. 40: 706–713.
Nakashima, T., and H. Takayanagi. 2011. New regulation mechanisms of osteoclast differentiation. Annals of the New York Academy of Sciences 1240: E13–E18.
Takayanagi, H. 2021. RANKL as the master regulator of osteoclast differentiation. Journal of Bone and Mineral Metabolism 39: 13–18.
Hoter, A., M. El-Sabban and H. Naim. 2018. The HSP90 family: structure, regulation, function, and implications in health and disease. International Journal of Molecular Sciences 19: 2560.
Zuehlke, A.D., M.A. Moses and L. Neckers. 2017. Heat shock protein 90: its inhibition and function. Philosophical Transactions of the Royal Society B: Biological Sciences 373: 20160527.
Schopf, F.H., M.M. Biebl, and J. Buchner. 2017. The HSP90 chaperone machinery. Nature Reviews Molecular Cell Biology 18: 345–360.
Khandia, R.K., A. Munjal, H. MN Iqbal, and K. Dhama. 2017. Heat shock proteins- therapeutic perspectives in inflammatory disorders. Recent Patents on Inflammation & Allergy Drug Discovery 10: 94–104.
Chung, E.J., Y.-I. Jeong, M.-R. Lee, Y.J. Kim, S.-E. Lee, S.-H. Cho, W.-J. Lee, M.-Y. Park and J.-W. Ju. 2017. Heat shock proteins 70 and 90 from Clonorchis sinensis induce Th1 response and stimulate antibody production. Parasites & Vectors 10: 118.
Shimp, S.K., C.B. Chafin, N.L. Regna, S.E. Hammond, M.A. Read, D.L. Caudell, and C.M. Reilly. 2012. Heat shock protein 90 inhibition by 17-DMAG lessens disease in the MRL/lpr mouse model of systemic lupus erythematosus. Cellular & Molecular Immunology 9: 255–266.
Tukaj, S., D. Zillikens, and M. Kasperkiewicz. 2015. Heat shock protein 90: A pathophysiological factor and novel treatment target in autoimmune bullous skin diseases. Experimental Dermatology 24: 567–571.
Hayem G., De Bandt M., Palazzo E., Roux S., Combe B., Eliaou J.F., Sany J., Kahn M.F. and Meyer O. 1999. Anti-heat shock protein 70 kDa and 90 kDa antibodies in serum of patients with rheumatoid arthritis. Annals of the Rheumatic Diseases 58: 291–296.
Mantej, J., K. Polasik, E. Piotrowska, and S. Tukaj. 2018. Autoantibodies to heat shock proteins 60, 70, and 90 in patients with rheumatoid arthritis. Cell Stress and Chaperones 24: 283–287.
Miki Murata, Y.M., A. Hashiramoto, H. Kitamura, H. Kawasaki, K. Shiozawa, S. Yoshiya, H. Baba, K. Chihara, and S. Shiozawa. 2005. Heat shock protein 90 is required for increased DNA binding activity of activator protein-1, a heterodimer of Fos-JunD, in rheumatoid synovial cells under inflammatory stimuli. International Journal of Molecular Medicine 15: 649–653.
Hashiramoto, A., M. Murata, T. Kawazoe, K. Yoshida, C. Akiyama, K. Shiozawa, and S. Shiozawa. 2010. Heat shock protein 90 maintains the tumour-like character of rheumatoid synovial cells by stabilizing integrin-linked kinase, extracellular signal-regulated kinase and protein kinase B. Rheumatology 50: 852–861.
Braun, T., and J. Zwerina. 2011. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Research & Therapy 13: 235.
Chen, X., Z. Wang, N. Duan, G. Zhu, E.M. Schwarz, and C. Xie. 2017. Osteoblast–osteoclast interactions. Connective Tissue Research 59: 99–107.
Schett, G., and E. Gravallese. 2012. Bone erosion in rheumatoid arthritis: Mechanisms, diagnosis and treatment. Nature Reviews Rheumatology 8: 656–664.
Zhang, F.Z., D.H.-H. Ho, and R.H.-F. Wong. 2018. Triptolide, a HSP90 middle domain inhibitor, induces apoptosis in triple manner. Oncotarget 9: 22301–22315.
Ganguly, S., T. Home, A. Yacoub, S. Kambhampati, H. Shi, P. Dandawate, S. Padhye, A.K. Saluja, J. Mcguirk, and R. Rao. 2015. Targeting HSF1 disrupts HSP90 chaperone function in chronic lymphocytic leukemia. Oncotarget 6: 31767–31779.
Kajiya, H., F. Okamoto, T. Nemoto, K. Kimachi, K. Toh-Goto, S. Nakayana, and K. Okabe. 2010. RANKL-induced TRPV2 expression regulates osteoclastogenesis via calcium oscillations. Cell Calcium 48: 260–269.
Takayanagi H., Kim S., Koga T., Nishina H., Isshiki M., Yoshida H., Saiura A., Isobe M., Yokochi T., Inoue J., Wagner E.F., Mak T.W., Kodama T. and Taniguchi T. 2002. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Developmental Cell 3: 889–901.
Zeng, X.-Z., Y.-Y. Zhang, Q. Yang, S. Wang, B.-H. Zou, Y.-H. Tan, M. Zou, S.-W. Liu, and X.-J. Li. 2019. Artesunate attenuates LPS-induced osteoclastogenesis by suppressing TLR4/TRAF6 and PLCγ1-Ca2+-NFATc1 signaling pathway. Acta Pharmacologica Sinica 41: 229–236.
Matsumoto, M., M. Kogawa, S. Wada, H. Takayanagi, M. Tsujimoto, S. Katayama, K. Hisatake, and Y. Nogi. 2004. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. Journal of Biological Chemistry 279: 45969–45979.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant number: 82174042), Scientific and technological innovation project of China Academy of Chinese Medical Sciences (Grant number: CI2021A03806), Special Funds for Basic Research Operations of Public Welfare Research Institutes (Grant number: ZZ13-YQ-043), Beijing Natural Science Foundation of China (Grant number: 7202142), and Special Funds for Basic Research Operations of Public Welfare Research Institutes (Grant number: ZXKT21016 and ZXKT20014).
Author information
Authors and Affiliations
Contributions
Xiaohui Su and Na Lin contributed to the study conception and design. Qian Wang, Wanyi Guo, Liling Liu, and Xueying Tao performed the experiments and analyzed the data. Qian Wang drafted the manuscript. Xiangying Kong and Yage Tian contributed to the discussion of this study and the revision of this manuscript. Xiaohui Su and Na Lin wrote and revised the manuscript, conducted the project administration, and Xiaohui Su acquired the funding grants. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This study was approved by approved by the Institutional Animal Ethics Committee of China Academy of Chinese Medical Sciences.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Q., Kong, X., Guo, W. et al. HSP90 Exacerbates Bone Destruction in Rheumatoid Arthritis by Activating TRAF6/NFATc1 Signaling. Inflammation 47, 363–375 (2024). https://doi.org/10.1007/s10753-023-01914-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01914-2