Abstract
Endometriosis is a common gynecological inflammatory disorder characterized by immune system dysregulation, which is involved in lesion initiation and progression. Studies have demonstrated that several cytokines are associated with the evolution of endometriosis, including tumor necrosis factor-α (TNFα). TNFα is a non-glycosylated cytokine protein with potent inflammatory, cytotoxic, and angiogenic potential. In the current study, we examined the ability of TNFα to induce dysregulation of microRNAs (miRNAs) linked to NFkB signaling pathways, thus contributing to the pathogenesis of endometriosis. Using RT-qPCR, the expression of several miRNAs was quantified in primary cells derived from eutopic endometrium of endometriosis subjects (EESC) and normal endometrial stromal cells (NESC), and also TNFα-treated NESCs. The phosphorylation of the pro-inflammatory molecule NF-κB and the candidates of the survival pathways PI3K, AKT, and ERK was measured by western blot analysis. The elevated secretion of TNFα in EESCs downregulates the expression level of several miRNAs significantly in EESCs compared to NESCs. Also, treatment of NESCs with exogenous TNFα significantly reduced the expression of miRNAs in a dose-dependent manner to levels similar to EESCs. In addition, TNFα significantly increased the phosphorylation of the PI3K, AKT, ERK, and NF-κB signaling pathways. Notably, treatment with curcumin (CUR, diferuloylmethane), an anti-inflammatory polyphenol, significantly increased the expression of dysregulated miRNAs in EESC in a dose-dependent manner. Our findings demonstrate that TNFα is upregulated in EESCs, which subsequently dysregulates the expression of miRNAs, contributing to the pathophysiology of endometriotic cells. CUR effectively inhibits the expression of TNFα, subsequently altering miRNA levels and suppressing the phosphorylation of AKT, ERK, and NF-κB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
BACKGROUND
Endometriosis is a benign, estrogen-dependent inflammatory disease characterized by the presence of endometrial tissue (specifically glands and stroma) outside of the uterus [1,2,3]. The exact causes of endometriosis remain unknown. The theory of retrograde menstruation, an efflux of menstrual blood and cells via the fallopian tubes to extrauterine sites, is considered an important origin of endometriosis lesions [1, 3]. While 90% of reproductive-aged women experience retrograde menstruation, only 10% are diagnosed with endometriosis [4]. Therefore, in addition to retrograde menstruation, other factors are likely involved in the pathogenesis of endometriosis, including hormonal imbalance, metabolic environment, epithelial-mesenchymal transition, altered immunity, and abnormal regulation of inflammation in endometrial cells (ECs) of genetically susceptible women [3]. In the peritoneal cavity, resident or recruited immune cells secrete excessive levels of proinflammatory cytokines that trigger inflammatory reactions in endometrial cells and promote lesion development and disease progression [1, 5,6,7].
Cytokines are small, soluble, diverse pleiotropic immunoregulatory signaling proteins with a short half-life. Women with endometriosis have elevated levels of certain cytokines, including TNFα, that can stimulate EC proliferation, survival, migration, and adhesion to the peritoneal cavity, angiogenesis, and inflammation, which ultimately promote progression of the disease [8,9,10,11]. Cytokines, including TNFα, mediate their action through their receptors that activate a cascade of intracellular events, including nuclear factor-kappa B (NF-κB) signaling pathways [8,9,10,11,12]. NF-κB has been shown to orchestrate various physiological and pathophysiological responses of ECs and endometriosis [12,13,14,15,16,17]. Previous studies have demonstrated that women with endometriosis have increased NF-κB expression that regulates the expression of aberrant cytokines through autocrine self-amplifying cycles of cytokine release and NF-κB activation. These lead to amplification and maintenance of the proinflammatory local environment, promoting the survival and growth of ECs in endometriosis patients and reducing the clearance of retrogradely transported endometrial fragments [13,14,15,16,17,18,19].
Recent studies also demonstrated the aberrant dysregulation of microRNA (miR) expression in circulation as well as in ectopic and eutopic endometrium tissues of endometriotic patients [20,21,22,23,24,25,26,27,28,29,30,31,32]. miRNAs are a large family of short, non-coding, single-stranded RNAs that are involved in the post-transcriptional regulation of cellular processes by binding to complementary sequences in the coding, 5′- or 3′-untranslated region (UTR) of target mRNAs that are subsequently silenced or degraded [33,34,35,36]. Several pieces of evidence suggest that NF-κB signaling is overactive in endometriotic lesions and plays a vital role in the onset, progression, and recurrence of endometriosis [37]. As important transcriptional regulators, miRNAs can modify many target genes involved in cytokine expression and the NF-κB signaling pathway via negative or positive feedback loops, and these have been identified as potentially robust biomarkers for endometriosis both in circulation and tissues [20,21,22, 28, 32,33,34, 38].
The TNFα-dependent regulation of the expression of miRNAs associated with endometriosis in eutopic ECs is not well defined. Based on the proinflammatory nature of the disease, combined with the published data [11, 13, 14, 17, 33, 34, 39] and our comparative nanostring analysis of miRNAs (unpublished) between the stromal cells of women with (EESC) and without endometriosis (NESC), we aimed to analyze whether upregulation of TNFα expression in the eutopic stromal cells of endometriotic patients induces the dysregulation of miRNAs linked to NF-kB signaling pathways thus contributing to the pathogenesis of the disease. To evaluate this theory, the expression levels of proinflammatory and proangiogenic miRNAs were compared between the NESCs and EESCs. As TNFα is upregulated in the EESCs [11], therefore, to mimic the environment of the diseased cells, the NESCs were treated with exogenous TNFα. Followed by NESCs were evaluated for the expression of those selected miRNAs and whether their altered expressions have been linked to the phosphorylation of NF-κB, PI3K, AKT, and ERK1/2 pathways. Our previous studies established that curcumin (CUR), a natural medicinal Asian herb with strong anti-inflammatory and antioxidant properties, attenuates proangiogenic and proinflammatory factors in human eutopic EESCs through the NF‐κB signaling pathway. Thus, in a further study, we evaluated the effects of CUR in altering the expression of proinflammatory miRNAs that are linked to the NF-κB signaling pathway. Taken together, we established that TNFα is upregulated in EESCs which subsequently increases the expression of proangiogenic and proinflammatory miRNAs, potentially contributing to the pathophysiology of endometriotic cells. We have determined that CUR effectively reduces the expression of TNFα and dysregulation of miRNA levels and attenuates the phosphorylation status of PI3K, AKT, ERK, and NF-κB pathways.
MATERIALS AND METHODS
Human Subjects and Tissue Acquisition
The details about the source of primary endometrial stromal cells (ESCs) used in this study were described previously [11]. The current studies were approved by the institutional review boards of Emory University and Morehouse School of Medicine, Atlanta.
Endometrial Stromal Cell (ESC) Cultures
Primary endometrial stromal cells (ESCs) from human eutopic endometrial biopsies from women with (EESC) and without evidence of endometriosis (NESC) were prepared according to the previously published method [40]. Cells (passages 3–5) were cultured and routinely maintained in Dulbecco’s Modified Eagle’s Medium/Ham’s Nutrient Mixture F-12 (DMEM/Ham’s F-12; Life Technologies, Inc.-BRL) supplemented with 12% fetal bovine serum (FBS; Thermo Fisher Scientific, Grand Island, NY, USA), 1% non-essential amino acids, 1% sodium pyruvate, and 1% penicillin–streptomycin (Penstrep, Sigma-Aldrich, St Louis, MO, USA), within a 5% CO2 atmosphere at 37 °C in a humidified incubator. Cells were grown to 80% confluency in 100-mm plates (Corning, NY, USA). The culture media was replaced with low serum-containing media overnight before any experiments. After 24 h, cells were treated or untreated in the DMEM/Ham’s F-12 medium supplemented with 0.4% FBS, 1% non-essential amino acids, 1% sodium pyruvate, and 1% Penstrep, and incubated at 37 °C in a humidified incubator with 5% CO2 for 24 h. Images of ESC cultures were taken at 24 and 48 h posttreated or untreated condition using an inverted phase contrast microscope. Unless specified differently, 20 random phase contrast images were acquired per well at 200 × magnification.
TNFα Treatment of Normal Endometrial Stromal Cells (NESCs)
NESCs were grown up to 80% confluency in 100-mm plates as described above. Cells were serum-starved for 24 h and then treated with TNFα (10 and 50 ng/mL, Sigma-Aldrich, USA) for 24 h. The dose and time of treatment for TNFα are based on our unpublished work and published literature [41]. Cells were harvested for the estimation of total RNA and protein.
Curcumin (CUR) Treatment of Normal and Eutopic Endometriotic Stromal Cells (NESCs, EESCs)
NESC and EESC cultures were grown to 80% confluency in 100-mm plates, as described above. Cells were treated with CUR (molecular weight 368.41, purity 99%, Sigma-Aldrich, USA) at a concentration of 5 and 10 μg/mL for 48 h [11]. CUR was dissolved in dimethyl sulfoxide (DMSO) and diluted to the desired concentrations in DMEM/Ham’s F-12 media with 0.4% serum-containing media followed by sterilization through 0.22-μm membrane filtration. Cells were treated with the equivalent concentrations of DMSO added to the medium for the parallel vehicle control experiments. The final concentration of DMSO was less than 0.1%.
Isolation of Total RNA
Total RNA from NESC and EESC and corresponding curcumin or TNFα-treated ESCs was extracted using Qiagen miRNeasy Mini kit (Germantown, MD, USA) according to the manufacturer’s instructions. The quality of the extracted RNA was verified via absorbance measurements at wavelengths of 230, 260, and 280 nm using a spectrophotometer (NanoDrop, 2000; Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNA 260/280 ratio of 1.9 or greater and 260/230 ratio of 1.8 or greater were used to obtain optimal results for the miR analysis.
microRNA (miR) Expression Analysis
The RNA samples were transcribed using the miRCURY LNA RT kit (Germantown, MD, USA) according to the manufacturer’s protocol. quantitative Real-time PCR (qRT-PCR) was performed using miRCURY LNA SYBR® Green PCR Kit (Germantown, MD, USA) and LNA-enhanced and Tm-normalized miRNA primers from Qiagen on CFX connected Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). All steps were performed according to the Qiagen MicroRNA assay protocol (Germantown, MD, USA). The relative expression of the gene was calculated using 2−ΔΔCT methods with 5S rRNA (hsa) and U6 snRNA (hsa), as the reference miRs.
Assessment of TNFα in Secretion Media
TNFα was measured in postculture media collected at 24 and 48 h using Bio-Plex ProTM Human Cytokine, Chemokine, and Growth Factor Magnetic Bead-Based Assays (BioRad, Hercules, CA, USA) coupled with the Luminex 200™ system (Austin, TX, USA) according to the manufacturer’s protocol. Samples were tested at a 1:2 dilution using optimal concentrations of standards and antibodies according to the manufacturer’s protocol.
Western Blot Analysis
Total protein was extracted from different treatment conditions from untreated and treated NESC and EESC and subjected to one-dimensional gel electrophoresis and western blot (WB) analysis. For one-dimensional gel electrophoresis, equal amounts of protein (25 μg) were applied to each lane. Primary antibodies were used as described in Table 1. Membranes were incubated with the appropriate secondary antibodies for 1 h at room temperature, and protein-antibody complexes were visualized using SuperSignal™ West Pico detection reagent (Thermo Fisher Scientific, Waltham, MA) on an iBright™ FL1500 Imaging System (Thermo Fisher Scientific, Waltham, MA). Results of representative chemiluminescence were scanned and densitometrically analyzed using a Power Macintosh Computer (G3; Apple Computer, Cupertino, CA) equipped with a Scan Jet 6100C Scanner (Hewlett-Packard, Greeley, CO). Quantification of the scanned images was performed using NIH Image version 1.61 software (NIH, Bethesda, MD) (34).
Statistical Analysis
Data are expressed as mean ± SEM of three independent experiments. Statistical analysis was performed by one-way ANOVA using SPSS version 11.0 software (SPSS, Chicago, IL) to test the significance of differences in dose, duration, and interaction between dose and duration. Post hoc corrections for multiple comparisons were done by Newman-Keuls’ test or unpaired Student’s t-test. Differences were considered significant at P ≤ 0.05. For miR expression analysis, fold change was calculated using a selected miR expression in a target sample relative to a control sample, normalized over a reference miR. The 2–∆∆Ct method was used and the ∆∆Ct was calculated using the average of the control values. That generates multiple values close to 1 for the control and gives a standard error of the mean.
RESULTS
EESC Secrete Higher Concentrations of TNFα
We compared the secretion of TNFα in the culture media of serum-starved NESC and EESC in vitro. Although under phase contrast microscopy, there was no significant morphological difference observed between NESC and EESC at 24 and 48 h (Fig. 1a), however, the concentration of TNFα was significantly higher in the culture media of EESCs compared to NESC at both 24 and 48 h (Fig. 1b). Moreover, a higher TNFα secretion was observed after 48 h in EESC media.
Analysis of morphological changes and pleiotropic cytokine tumor necrosis factor α (TNFα) expression in normal human endometrial stromal cells (NESCs) and cells derived from eutopic endometrium of endometriosis subjects (EESCs) in vitro. NESCs and EESCs were cultured as described in “MATERIALS AND METHODS.” a The representative photographs showed the morphological changes in live cells taken under a phase contrast microscope at 200 × magnification at 24 and 48 h. b Bar graph represents the concentrations of TNFα in the supernatants as mean ± SEM of results from three individual experiments (n = 3). Post hoc corrections for multiple comparisons were done by Newman-Keuls’ test. Star (*) represents significant differences (∗ ∗ P ≤ 0.01, ∗ ∗ ∗ P ≤ 0.001) between NESCs and EESCs groups.
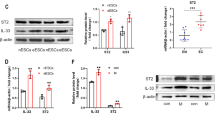
miRNAs Linked to Inflammation are Differentially Expressed Between NESC and EESC
To better understand the correlative changes in the abundantly expressed miRNAs linked to the inflammation in endometriosis, the selected miRNAs (miR-125b-5p, miR-126-5p, miR-132-3p, miR-146a-5p, miR-15b-5p, miR-152-3p, miR-155-5p, miR-181a-5p, miR-196b-5p, miR199a-5p, miR-21-5p, miR-214-3p, miR-222a-3p, miR-23a-5p, miR-29b-3p, and miR-98-5p) [42,43,44,45,46,47,48,49,50,51,52] were analyzed in NESCs and EESCs. The expression of the miRNA was measured at 48 h in ESC culture which conforms to the significant upregulation of TNFα secretion at 48 h compared to 24 h in EESCs. The expression level of miR-126-5p, miR-132-3p, miR-15b-5p, miR-152-3p, miR-155-5p, miR-181a-5p, miR-196b-5p, miR199a-5p, miR-21-5p, miR-214-3p, miR-222a-3p, miR-23a-5p, miR-29b-3p, and miR-98-5p was downregulated significantly (P < 0.05) in EESCs compared to NESCs, except for miRNA-125b-5p which showed a substantial upregulation in expression (Fig. 2). There were no significant changes in expression levels of miR-146a-5p.
Analysis of selected miRNAs in normal human endometrial stromal cells (NESCs) and cells derived from eutopic endometrium of endometriosis subjects (EESCs) in vitro. Cells were cultured for 48 h as described in “MATERIALS AND METHODS.” Total RNA was isolated, and selected miRNAs were analyzed by quantitative RT-PCR, normalized by 5S rRNA, and represented as fold changes between NESCs and EESCs. All bar graphs represent the mean ± SEM of results from three individual experiments (n = 3). Unpaired Student’s t-test represents significant differences (∗ P ≤ 0.05, ∗ ∗ P ≤ 0.01, ∗ ∗ ∗ P ≤ 0.001) between NESCs and EESCs groups. NS, no significant differences.
TNFα Treatment Alters the Expression of miRNAs and Phosphorylation of PI3K, AKT, ERK, and NF-κB in NESCs
To investigate the possible role of the increased level of the proinflammatory cytokine TNFα in ESCs with altered expression of miRNAs tied to the NF-κB and survival pathways, NESCs were treated with exogenous recombinant TNFα (10 and 50 ng/mL) for 24 h in vitro [19, 53]. The expression of miR-132-3p, miR-196b-5p, and miR-98-5p was downregulated whereas 146a-5p was significantly upregulated with TNFα treatment (10 and 50 ng/mL) after 24 h (Fig. 3). Whereas low dose of TNFα (10 ng/mL) had no significant effect on the expression of any of the miRNAs mentioned here (miR-125b-5p, miR-126-5p, miR-15b-5p, miR-152-3p, miR-155-5p, miR-181a-5p, miR199a-5p, miR-21-5p, miR-214-3p, miR-222a-3p, miR-23a-5p, and miR-29b-3p) (Fig. 3), a higher dose of TNFα (50 ng/mL for 24 h) induced a strong inhibitory effect on all miRNAs except miR146a-5p and miR199a-5p, which was significantly upregulated (Fig. 3).
The effects of tumor necrosis factor α (TNFα) treatment on miRNA gene expression in normal human endometrial stromal cells (NESCs) in vitro. Cells were cultured and treated with TNFα for 24 h as described in “MATERIALS AND METHODS.” Total RNA was isolated, and the expression of selected miRNAs was analyzed by quantitative RT-PCR, normalized for 5S rRNA concentrations, and represented as fold change of TNFα-treated cells compared to untreated NESCs. All bar graphs represent the mean ± SEM of results from three individual experiments (n = 3). One-way ANOVA analysis of TNFα effects on miRNA expression in NESCs in vitro [miR-125b-5p, F(5,12) = 30.51, P ≤ 0.001; miR-126-5p, F(5,12) = 19.64, P ≤ 0.002; miR-132-3p, F(5,12) = 9.26, P ≤ 0.015; miR-146a-5p, F(5,12) = 25.06, P ≤ 0.001; miR-15b-5p, F(5,12) = 24.14, P ≤ 0.001; miR-152-3p, F(5,12) = 56.94, P ≤ 0.0001; miR-155-5p, F(5,12) = 63.34, P ≤ 0.001; miR-181a-5p, F(5,12) = 10.19, P ≤ 0.012; miR-196b-5p, F(5,12) = 82.03, P ≤ 0.0001; miR199a-5p, F(5,12) = 5.22, P ≤ 0.05; miR-21-5p, F(5,12) = 27.47, P ≤ 0.001; miR-214-3p, F(5,12) = 9.88, P ≤ 0.013; miR-222a-3p, F(5,12) = 25.57, P ≤ 0.001; miR-23a-5p, F(5,12) = 27.90, P ≤ 0.001; miR-29b-3p, F(5,12) = 19.89, P ≤ 0.001; and miR-98-5p, F(5,12) = 11.67, P ≤ 0.01]. Post hoc corrections for multiple comparisons were done by Newman-Keuls’ test. Star (*) represents significant differences (∗ P ≤ 0.05, ∗ ∗ P ≤ 0.01, ∗ ∗ ∗ P ≤ 0.001) between NESCs and EESCs groups. NS, no significant differences.
TNFα is known to activate the PI3K/AKT pathway, which in turn activates the NF-κB signaling pathway [54] and are essential steps for proinflammatory gene expression. So we explored whether TNFα treatment affects phosphorylation of PI3K, AKT, ERK, and NF-κB in NESC. As shown in Fig. 4a and b, the treatment of NESCs with TNFα at 50 ng/mL for 24 h significantly increased the phosphorylation of PI3K, AKT, ERK, and NF-κB, whereas no significant effects on phosphorylation were noted at lower concentrations of TNFα except the phosphorylation of PI3K that is significantly higher in lower dose of TNFα.
The effects of tumor necrosis factor α (TNFα) treatment on kinases in normal human endometrial stromal cells (NESCs) in vitro. Cells were cultured and treated with TNFα for 24 h as described in “MATERIALS AND METHODS.” Total protein was isolated and the phosphorylation of AKT, PI3K, ERK1/2, and NF-κB was analyzed. A Representative western blot (WBs) analysis for phospho- and total AKT, PI3K, ERK1/2, and NF-κB protein levels in NESCs treated with or without TNFα. β-Actin was used as an internal constitutive control. B The bar graphs represent the ratios of phospho-Akt, phosphor-PI3K, phospho-Erk1/2, and phospho-NF-κB protein levels normalized to total AKT, PI3K, ERK1/2, and NF-κB, respectively. All bar graphs represent the mean ± SEM of results from three individual experiments (n = 3). One-way ANOVA analysis of TNFα effects on pAKT/AKT [F(5,12) = 170, P ≤ 0.0001], pPI3K/PI3K [F(5,12) = 168.18, P ≤ 0.0001], pERK/ERK [F(5,12) = 36.31, P ≤ 0.0001], and pNF-κB/NF-κB [F(5,12) = 188.37, P ≤ 0.0001] expression in NESCs in vitro. Post hoc corrections for multiple comparisons were done by Newman-Keuls’ test. Star (*) represents significant differences (∗ P ≤ 0.05, ∗ ∗ P ≤ 0.01, ∗ ∗ ∗ P ≤ 0.001) between TNFα treated and untreated groups. NS, no significant differences.
Curcumin Treatment Inhibits TNFα Secretion and Alters the Expression of miRNAs
To determine whether CUR treatment modulates the expression of miRNAs, EESCs and NESCs were treated with different doses of CUR for 48 h. To understand the mechanism better, TNFα secretion was analyzed post-CUR treatment. As shown in Fig. 5a, CUR treatment inhibited significantly the secretion (P ≤ 0.05) of TNFα in a dose-dependent manner in EESCs. In subsequent studies, the expression of selected miRNAs was analyzed under these experimental conditions. As shown in Fig. 5b, CUR treatment significantly promoted the expression of selected miRNAs, precisely at 5 µg/mL (miR-146a-5p) and at 10 µg/mL (miR-132-3p, miR-23a-5p) at 48 h in EESC compared to NESC. Moreover, there is a downregulation of miRNA expression after 48 h (miR-152-3p, miR-181a-5p, miR-199a-5p, miR-214-3p) at 5 µg/mL dose. However, there were no significant differences in the expression of most of the miRNAs in post-CUR-treated EESCs compared to NESCs at 48 h (Fig. 5b).
Effects of curcumin (CUR) on tumor necrosis factor α (TNFα) secretion and miRNA expression in human normal endometrial stromal cells (NESCs) and cells derived from eutopic endometrium of endometriosis (EESCs) subjects. Cells were treated with or without curcumin (CUR, 5 µg/mL or 10 µg/mL) for 48 h as described in “MATERIALS AND METHODS.” a Bar graph represents the concentrations of TNFα in the supernatants. b Total RNA was isolated, and selected miRNAs were analyzed by quantitative RT-PCR, normalized over 5 s rRNA, and represented as fold changes of the treated group over the untreated ones in both NESCs and EESCs. All bar graphs represent the mean ± SEM of results from three individual experiments (n = 3). One-way ANOVA analysis of CUR effects on TNFα [F(5,12) = 99, P ≤ 0.0001] and miRNA expression [miR-125b-5p, F(5,12) = 28, P ≤ 0.0001; miR-126-5p, F(5,12) = 6.7, P ≤ 0.003; miR-132-3p, F(5,12) = 20.34, P ≤ 0.0001; miR-146a-5p, F(5,12) = 12.2, P ≤ 0.0001; miR-15b-5p, F(5,12) = 18.6, P ≤ 0.0001; miR-152-3p, F(5,12) = 20.3, P ≤ 0.0001; miR-155-5p, F(5,12) = 4.43, P ≤ 0.016; miR-181a-5p, F(5,12) = 4.46, P ≤ 0.016; miR-196b-5p, F(5,12) = 7.84, P ≤ 0.002; miR199a-5p, F(5,12) = 36.95, P ≤ 0.0001; miR-21-5p, F(5,12) = 23.05, P ≤ 0.0001; miR-214-3p, F(5,12) = 9.86, P ≤ 0.001; miR-222a-3p, F(5,12) = 42.03, P ≤ 0.0001; miR-23a-5p, F(5,12) = 50.13, P ≤ 0.0001; miR-29b-3p, F(5,12) = 26.7, P ≤ 0.0001; and miR-98-5p, F(5,12) = 6.94, P ≤ 0.003] in NESCs and EESCs in vitro. Post hoc corrections for multiple comparisons were done by Newman-Keuls’ test. Star (*) represents significant differences (∗ P ≤ 0.05, ∗ ∗ P ≤ 0.01, ∗ ∗ ∗ P ≤ 0.001) between NESCs and EESCs groups treated with CUR at 48 h. NS, no significant differences.
DISCUSSION
The current findings suggest a new basis for understanding the mechanism of TNFα in the pathogenesis of endometriosis. The acute inflammatory response to TNFα is mediated by local dysregulation of miRNAs linked to NFkB signaling pathways, thus contributing to the pathogenesis of the disease. It is well established that EESCs function differently in women with endometriosis compared with NESCs from disease-free women [55]. The current findings corroborate previously published data that EESCs have increased basal production of TNFα, which promotes a chronic inflammatory environment within the pelvis of these women [11, 56]. TNFα, along with other cytokines, is involved in the recruitment and activation of macrophages, neutrophils, eosinophils, basophils, monocytes, and NK cells to the sites of endometriosis implants, enhancing EC proliferation and angiogenesis through increased production of VEGF and the adhesion of endometrium cells to the peritoneal cavity [57, 58]. Moreover, elevated levels of TNFα in peritoneal fluid activate NFκB signaling along with other proinflammatory factors, which ultimately promote the proliferative and inflammatory characteristics of endometriosis [13, 14, 17,18,19, 37, 59,60,61].
The current study suggests that under basal conditions, increased production of TNFα in EESC is associated with dysregulation of the expression of selected miRNAs (miR-125-5p, miR-126-5p, miR-132-3p, miR-146a-5p, miR-15b-5p, miR-152-3p, miR-155-5p, miR-181a-5p, miR-196b-5p, miR199a-5p, miR-21-5p, miR-214-3p, miR-222a-3p, miR-23a-5p, miR-29b-3p, and miR-98-5p). It is well established that numerous miRNAs are altered in the eutopic and ectopic endometrium and lesions in women with endometriosis [22, 23, 25, 27, 29,30,31,32, 42, 48, 49]. Some downregulated miRNAs (miR-126-5p, miR-132-3p, miR-146a-5p, miR-15b-5p, miR-152-3p, miR-155-5p, miR-181a-5p, miR-196b-5p, miR199a-5p, miR-21-5p, miR-214-3p, miR-222a-3p, miR-23a-5p, miR-29b-3p, and miR-98-5p) are directly linked to the activation of inflammatory signaling molecule NF-κB which could be involved in the pathogenesis and progression of endometriosis [33, 50, 62, 63].
Studies have demonstrated in endometriotic and other cells and tissues that miR-125b is involved in cell proliferation and migration [64], miR-126 suppresses inflammation and reactive oxygen species (ROS) production [65,66,67], miR-15b-5p suppresses angiogenesis [68,69,70], and miR-152-5p acts as a tumor suppressor, inhibits cell proliferation, and is downregulated in endometrial cancer [71]. miR-155 is involved in the attenuation of inflammatory pathways [46, 72], miR-196b is involved in self-renewal and proliferation [42, 73], and miR-199a activates NFκB and inflammatory signaling pathways [74,75,76]. Similarly, miR-21 plays an essential role in the resolution of inflammation by negative feedback of inflammatory pathways [27, 77]; miR-214-3p inhibits the proliferation, migration, and invasion of EC cells [47]; miR-222-3p promotes proliferation, proangiogenesis, and invasion [51, 78, 79]; and miR23a is involved in local steroidogenesis-dependent inflammation and growth of ectopic ECs [80, 81]. MicroRNA-29b is involved in a wide range of functions, including apoptosis, cell proliferation, invasion, adhesion, metabolism, and progression in endometrial cancer cells by direct regulation of PTEN [82,83,84]. MiR-98 expression was found to be reduced in diseased EC tissues compared to normal tissues [85].
Our results further indicate that TNFα stimulation of NESCs dysregulates miRNA expression, phenocopying EESC and implying that these cells are TNFα responsive, with effects more pronounced at higher concentrations. These findings are consistent with previous studies indicating that a higher concentration of TNFα for a more extended exposure period promotes dysregulation of miRNA expression, which may partly govern NF-κB signaling molecules [19, 53]. Moreover, we found that exogenous TNFα significantly downregulated several miRNAs in NESCs except for 146a-5p which was upregulated with TNFα treatment at both doses (10 and 50 ng/mL) and miR-199a-5p, which was upregulated at the higher dosage (50 ng/mL) after 24 h. This apparent discrepancy could be a compensatory upregulation induced by a very high concentration of exogenous TNFα for an extended period or could be a part of a negative feedback loop reducing the impact of TNFα [76]. Furthermore, exogenous TNFα-dependent activation of PI3K/AKT/ERK1/2 signaling and NF-κB phosphorylation in NESCs suggest that TNFα may be an important cytokine contributing to the cascade of kinase signaling with dysregulation of miRNA expression in ECs. Previous studies also established that TNFα-mediated activation of the PI3K/Akt and the NF-κB signaling pathway are essential steps for proinflammatory gene expression [54]. In endometriotic cells, NK-κB signaling is activated by TNFα [8, 37, 86, 87] and the aberrant activation of NF-κB signaling leads to chronic inflammation, increased cell proliferation, and survival of ECs in endometriosis [13,14,15,16,17,18,19, 88]. Previous studies have also demonstrated that the phosphorylation states of NF-κB signaling molecules, including IKKα, IKKβ, NF-κB, JNK, and STAT3, are higher in EESCs, which are involved in the downstream participation of various kinases linked to cytokine- and chemokine-specific membrane receptor complexes and adaptor proteins, that converge on NF-κB signaling pathway [11, 63, 89]. Thus, TNFα-dependent dysregulation of miRNA expression in conjunction with altered phosphorylation of pPI3K/pAKT/pERK1/2/pNF-κB suggests a regulatory link that supports the idea of transformation of NESCs to a pathophysiological state similar to that of EESC (Fig. 6).
A schematic model showing the effects of tumor necrosis factor α (TNFα) induced activation of phosphatidylinositol-3-kinase (PI3K)/serine/threonine kinase (AKT)/Ras/extracellular signal-regulated kinase ½ (ERK1/2) signaling and nuclear factor κ-light-chain-enhancer of activated B (NF-κB) phosphorylation with dysregulation of miRNAs (miRs) expression in endometrial stromal cells that tilt the balance with the initiation and progression endometriosis. Interestingly, curcumin (CUR) attenuates this imbalance. ESCs, endometrial stromal cells; N, normal; E, endometriotic; the upward arrow represents an increase, and the blunt arrow represents inhibition.
Further studies revealed that CUR is a potent inhibitor of TNFα secretion from EESCs [11]. Moreover, our data showed that curcumin treatment could modulate TNFα mediated dysregulation of miRNAs in EESCs. The inhibitory effect of CUR is extended further to the attenuation of IKKα, IKKβ, and NF-κB [11, 33, 63, 89, 90]. IKKα and IKKβ are part of a multiprotein complex mediating the transcription of multiple chemokine and cytokine genes through Ikβ. Thus, our results are consistent with published reports showing that CUR has strong anti-inflammatory and antiangiogenic properties [11].
In conclusion, the current study provides new insights into how elevated levels of TNFα secretion are associated with aberrant expression of miRNAs in ECs, which subsequently alter phosphorylation of the proinflammatory molecule NF-κB and survival pathways. Moreover, CUR treatment modulates the dysregulation of miRNAs. Further studies are needed using genetic gain or loss-of-function models of individually selected miRNAs to pinpoint the pathophysiological effects of those miRNAs in inflammation during endometriosis. Based on the dynamic nature of miRNA expression combined with diverse actions and multiple targets of NF-κB signaling molecules, we believe that an NF-κB-miRNA feedback loop should be considered in inflammatory responses and initiation, progression, and development of endometriosis. Moreover, understanding the intersection of NF-κB signaling molecules and miRNA regulatory networks may offer opportunities for pharmacological exploitation and personalized treatment for endometriosis pain management.
Availability of Data and Materials
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. The authors confirm that the data supporting the findings of this study are available within the article. Therefore, any other declaration is “not applicable.”
References
Zondervan, K.T., C.M. Becker, and S.A. Missmer. 2020. Endometriosis. New England Journal of Medicine 382 (13): 1244–1256. https://doi.org/10.1056/NEJMra1810764.
Zondervan, K.T., C.M. Becker, K. Koga, S.A. Missmer, R.N. Taylor, and P. Vigano. 2018. Endometriosis. Nat Rev Dis Primers. 4 (1): 9. https://doi.org/10.1038/s41572-018-0008-5.
Chantalat, E., M.C. Valera, C. Vaysse, E. Noirrit, M. Rusidze, A. Weyl, et al. 2020. Estrogen receptors and endometriosis. International Journal of Molecular Sciences 21(8). https://doi.org/10.3390/ijms21082815.
Mehedintu, C., M.N. Plotogea, S. Ionescu, and M. Antonovici. 2014. Endometriosis still a challenge. Journal of Medicine and Life 7 (3): 349–357.
Lin, Y.H., Y.H. Chen, H.Y. Chang, H.K. Au, C.R. Tzeng, and Y.H. Huang. 2018. Chronic niche inflammation in endometriosis-associated infertility: current understanding and future therapeutic strategies. International Journal of Molecular Sciences 19(8). https://doi.org/10.3390/ijms19082385.
Malutan, A.M., T. Drugan, N. Costin, R. Ciortea, C. Bucuri, M.P. Rada, et al. 2015. Pro-inflammatory cytokines for evaluation of inflammatory status in endometriosis. Cent Eur J Immunol. 40 (1): 96–102. https://doi.org/10.5114/ceji.2015.50840.
Panir, K., J.E. Schjenken, S.A. Robertson, and M.L. Hull. 2018. Non-coding RNAs in endometriosis: A narrative review. Human Reproduction Update 24 (4): 497–515. https://doi.org/10.1093/humupd/dmy014.
Miyamoto, A., F. Taniguchi, Y. Tagashira, A. Watanabe, T. Harada, and N. Terakawa. 2009. TNFalpha gene silencing reduced lipopolysaccharide-promoted proliferation of endometriotic stromal cells. American Journal of Reproductive Immunology 61 (4): 277–285. https://doi.org/10.1111/j.1600-0897.2009.00691.x.
Yamauchi, N., T. Harada, F. Taniguchi, S. Yoshida, T. Iwabe, and N. Terakawa. 2004. Tumor necrosis factor-alpha induced the release of interleukin-6 from endometriotic stromal cells by the nuclear factor-kappaB and mitogen-activated protein kinase pathways. Fertility and Sterility 82 (Suppl 3): 1023–1028. https://doi.org/10.1016/j.fertnstert.2004.02.134.
Harada, T., T. Iwabe, and N. Terakawa. 2001. Role of cytokines in endometriosis. Fertility and Sterility 76 (1): 1–10. https://doi.org/10.1016/s0015-0282(01)01816-7.
Chowdhury, I., S. Banerjee, A. Driss, W. Xu, S. Mehrabi, C. Nezhat, et al. 2019. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF-kappaB signaling pathway. Journal of Cellular Physiology 234 (5): 6298–6312. https://doi.org/10.1002/jcp.27360.
Lawrence, T. 2009. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspectives Biology 1(6):a001651. https://doi.org/10.1101/cshperspect.a001651.
Kaponis, A., T. Iwabe, F. Taniguchi, M. Ito, I. Deura, G. Decavalas, et al. 2012. The role of NF-kappaB in endometriosis. Frontiers in Bioscience (Scholar Edition) 4 (4): 1213–1234. https://doi.org/10.2741/s327.
Cao, W.G., M. Morin, V. Sengers, C. Metz, T. Roger, R. Maheux, et al. 2006. Tumour necrosis factor-alpha up-regulates macrophage migration inhibitory factor expression in endometrial stromal cells via the nuclear transcription factor NF-kappaB. Human Reproduction 21 (2): 421–428. https://doi.org/10.1093/humrep/dei315.
Gonzalez-Ramos, R., A. Van Langendonckt, S. Defrere, J.C. Lousse, M. Mettlen, A. Guillet, et al. 2008. Agents blocking the nuclear factor-kappaB pathway are effective inhibitors of endometriosis in an in vivo experimental model. Gynecologic and Obstetric Investigation 65 (3): 174–186. https://doi.org/10.1159/000111148.
Gonzalez-Ramos, R., A. Van Langendonckt, S. Defrere, J.C. Lousse, S. Colette, L. Devoto, et al. 2010. Involvement of the nuclear factor-kappaB pathway in the pathogenesis of endometriosis. Fertility and Sterility 94 (6): 1985–1994. https://doi.org/10.1016/j.fertnstert.2010.01.013.
Ponce, C., M. Torres, C. Galleguillos, H. Sovino, M.A. Boric, A. Fuentes, et al. 2009. Nuclear factor kappaB pathway and interleukin-6 are affected in eutopic endometrium of women with endometriosis. Reproduction 137 (4): 727–737. https://doi.org/10.1530/REP-08-0407.
Nowak, N.M., O.M. Fischer, T.C. Gust, U. Fuhrmann, U.F. Habenicht, and A. Schmidt. 2008. Intraperitoneal inflammation decreases endometriosis in a mouse model. Human Reproduction 23 (11): 2466–2474. https://doi.org/10.1093/humrep/den189.
Webster, J.D., and D. Vucic. 2020. The balance of TNF mediated pathways regulates inflammatory cell death signaling in healthy and diseased tissues. Front Cell Dev Biol. 8: 365. https://doi.org/10.3389/fcell.2020.00365.
Cosar, E., R. Mamillapalli, G.S. Ersoy, S. Cho, B. Seifer, and H.S. Taylor. 2016. Serum microRNAs as diagnostic markers of endometriosis: A comprehensive array-based analysis. Fertility and Sterility 106 (2): 402–409. https://doi.org/10.1016/j.fertnstert.2016.04.013.
Vanhie, A.O.D., D. Peterse, A. Beckers, A. Cuellar, A. Fassbender, et al. 2019. Plasma miRNAs as biomarkers for endometriosis. Human Reproduction 34(9):1650–60. https://doi.org/10.1093/humrep/dez116.
Bjorkman, S., and H.S. Taylor. 2019. MicroRNAs in endometriosis: Biological function and emerging biomarker candidates. Biology of Reproduction 100 (5): 1135–1146. https://doi.org/10.1093/biolre/ioz014.
Burney, R.O., A.E. Hamilton, L. Aghajanova, K.C. Vo, C.N. Nezhat, B.A. Lessey, et al. 2009. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Molecular Human Reproduction 15 (10): 625–631. https://doi.org/10.1093/molehr/gap068.
Filigheddu, N., I. Gregnanin, P.E. Porporato, D. Surico, B. Perego, L. Galli, et al. 2010 Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. Journal of Biomedicine and Biotechnology 2010:369549. https://doi.org/10.1155/2010/369549.
Hull, M.L., and V. Nisenblat. 2013. Tissue and circulating microRNA influence reproductive function in endometrial disease. Reproductive Biomedicine Online 27 (5): 515–529. https://doi.org/10.1016/j.rbmo.2013.07.012.
Jia, S.Z., Y. Yang, J. Lang, P. Sun, and J. Leng. 2013. Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Human Reproduction 28 (2): 322–330. https://doi.org/10.1093/humrep/des413.
Teague, E.M., C.G. Print, and M.L. Hull. 2010. The role of microRNAs in endometriosis and associated reproductive conditions. Human Reproduction Update 16 (2): 142–165. https://doi.org/10.1093/humupd/dmp034.
O’Connell, R.M., D.S. Rao, and D. Baltimore. 2012. microRNA regulation of inflammatory responses. Annual Review of Immunology 30: 295–312. https://doi.org/10.1146/annurev-immunol-020711-075013.
Yang, L., and H.Y. Liu. 2014. Small RNA molecules in endometriosis: Pathogenesis and therapeutic aspects. European Journal of Obstetrics, Gynecology, and Reproductive Biology 183: 83–88. https://doi.org/10.1016/j.ejogrb.2014.10.043.
Mari-Alexandre, J., D. Sanchez-Izquierdo, J. Gilabert-Estelles, M. Barcelo-Molina, A. Braza-Boils, and Sandoval J. 2016 miRNAs regulation and its role as biomarkers in endometriosis. International Journal of Molecular Sciences 17(1). https://doi.org/10.3390/ijms17010093.
Klemmt, P.A.B., and A. Starzinski-Powitz. 2018. Molecular and cellular pathogenesis of endometriosis. Curr Womens Health Rev. 14 (2): 106–116. https://doi.org/10.2174/1573404813666170306163448.
Moga, M.A., A. Balan, O.G. Dimienescu, V. Burtea, R.M. Dragomir, and Anastasiu CV. 2019. Circulating miRNAs as biomarkers for endometriosis and endometriosis-related ovarian cancer-an overview. Journal of Clinical Medicine 8(5). https://doi.org/10.3390/jcm8050735.
Banerjee, S., W.E. Thompson, and I. Chowdhury. 2021. Emerging roles of microRNAs in the regulation of Toll-like receptor (TLR)-signaling. Front Biosci (Landmark Ed). 26 (4): 771–796. https://doi.org/10.2741/4917.
Ying, S.Y., D.C. Chang, and S.L. Lin. 2008. The microRNA (miRNA): Overview of the RNA genes that modulate gene function. Molecular Biotechnology 38 (3): 257–268. https://doi.org/10.1007/s12033-007-9013-8.
O’Brien, J., H. Hayder, Y. Zayed, and C. Peng. 2018. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 9: 402. https://doi.org/10.3389/fendo.2018.00402.
Wang, J., J. Chen, and S. Sen. 2016. MicroRNA as biomarkers and diagnostics. Journal of Cellular Physiology 231 (1): 25–30. https://doi.org/10.1002/jcp.25056.
Liu, Y., J. Wang, and X. Zhang. 2022. An update on the multifaceted role of NF-kappaB in endometriosis. International Journal of Biological Sciences 18 (11): 4400–4413. https://doi.org/10.7150/ijbs.72707.
Alam, M.M., and L.A. O’Neill. 2011. MicroRNAs and the resolution phase of inflammation in macrophages. European Journal of Immunology 41 (9): 2482–2485. https://doi.org/10.1002/eji.201141740.
Nothnick, W., and Z. Alali. 2016. Recent advances in the understanding of endometriosis: the role of inflammatory mediators in disease pathogenesis and treatment. F1000Research 5. https://doi.org/10.12688/f1000research.7504.1.
Ryan, I.P., E.D. Schriock, and R.N. Taylor. 1994. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. Journal of Clinical Endocrinology and Metabolism 78 (3): 642–649. https://doi.org/10.1210/jcem.78.3.8126136.
Culpan, D., J. Palmer, J.S. Miners, S. Love, and P.G. Kehoe. 2011. The influence of tumour necrosis factor- alpha (TNF-alpha) on amyloid-beta (Abeta)-degrading enzymes in vitro. Int J Mol Epidemiol Genet. 2 (4): 409–415.
Ohlsson Teague, E.M., K.H. Van der Hoek, M.B. Van der Hoek, N. Perry, P. Wagaarachchi, S.A. Robertson, et al. 2009. MicroRNA-regulated pathways associated with endometriosis. Molecular Endocrinology 23 (2): 265–275. https://doi.org/10.1210/me.2008-0387.
Wang, H., L. Sha, L. Huang, S. Yang, Q. Zhou, X. Luo, et al. 2019. LINC00261 functions as a competing endogenous RNA to regulate BCL2L11 expression by sponging miR-132-3p in endometriosis. Am J Transl Res. 11 (4): 2269–2279.
Abe, W., K. Nasu, C. Nakada, Y. Kawano, M. Moriyama, and H. Narahara. 2013. miR-196b targets c-myc and Bcl-2 expression, inhibits proliferation and induces apoptosis in endometriotic stromal cells. Human Reproduction 28 (3): 750–761. https://doi.org/10.1093/humrep/des446.
Kolanska, K., S. Bendifallah, G. Canlorbe, A. Mekinian, C. Touboul, S. Aractingi, et al. 2021. Role of miRNAs in normal endometrium and in endometrial disorders: comprehensive review. Journal of Clinical Medicine 10(16). https://doi.org/10.3390/jcm10163457.
Tili, E., J.J. Michaille, A. Cimino, S. Costinean, C.D. Dumitru, B. Adair, et al. 2007. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. The Journal of Immunology 179 (8): 5082–5089. https://doi.org/10.4049/jimmunol.179.8.5082.
Fang, Y.Y., M.R. Tan, J. Zhou, L. Liang, X.Y. Liu, K. Zhao, et al. 2019. miR-214-3p inhibits epithelial-to-mesenchymal transition and metastasis of endometrial cancer cells by targeting TWIST1. Oncotargets and Therapy 12: 9449–9458. https://doi.org/10.2147/OTT.S181037.
Hawkins, S.M., C.J. Creighton, D.Y. Han, A. Zariff, M.L. Anderson, P.H. Gunaratne, et al. 2011. Functional microRNA involved in endometriosis. Molecular Endocrinology 25 (5): 821–832. https://doi.org/10.1210/me.2010-0371.
Saare, M., K. Rekker, T. Laisk-Podar, N. Rahmioglu, K. Zondervan, A. Salumets, et al. 2017. Challenges in endometriosis miRNA studies - from tissue heterogeneity to disease specific miRNAs. Biochimica et Biophysica Acta, Molecular Basis of Disease 1863 (9): 2282–2292. https://doi.org/10.1016/j.bbadis.2017.06.018.
Agrawal, S., T. Tapmeier, N. Rahmioglu, S. Kirtley, K. Zondervan, and C. Becker. 2018. The miRNA mirage: how close are we to finding a non-invasive diagnostic biomarker in endometriosis? A systematic review. International Journal of Molecular Science 19(2). https://doi.org/10.3390/ijms19020599.
Nothnick, W.B. 2017. MicroRNAs and endometriosis: Distinguishing drivers from passengers in disease pathogenesis. Seminars in Reproductive Medicine. 35 (2): 173–180. https://doi.org/10.1055/s-0037-1599089.
Ferlita, A., R. Battaglia, F. Andronico, S. Caruso, A. Cianci, M. Purrello, et al. 2018. Non-coding RNAs in endometrial physiopathology. International Journal of Molecular Sciences 19(7). https://doi.org/10.3390/ijms19072120.
Sedger, L.M., and M.F. McDermott. 2014. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine & Growth Factor Reviews 25 (4): 453–472. https://doi.org/10.1016/j.cytogfr.2014.07.016.
Bai, C., X. Yang, K. Zou, H. He, J. Wang, H. Qin, et al. 2016. Anti-proliferative effect of RCE-4 from Reineckia carnea on human cervical cancer HeLa cells by inhibiting the PI3K/Akt/mTOR signaling pathway and NF-kappaB activation. Naunyn-Schmiedeberg’s Archives of Pharmacology 389 (6): 573–584. https://doi.org/10.1007/s00210-016-1217-7.
Burney, R.O., S. Talbi, A.E. Hamilton, K.C. Vo, M. Nyegaard, C.R. Nezhat, et al. 2007. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148 (8): 3814–3826. https://doi.org/10.1210/en.2006-1692.
Vercellini, P., P. Vigano, E. Somigliana, and L. Fedele. 2014. Endometriosis: Pathogenesis and treatment. Nature Reviews. Endocrinology 10 (5): 261–275. https://doi.org/10.1038/nrendo.2013.255.
Reis, F.M., F. Petraglia, and R.N. Taylor. 2013. Endometriosis: Hormone regulation and clinical consequences of chemotaxis and apoptosis. Human Reproduction Update 19 (4): 406–418. https://doi.org/10.1093/humupd/dmt010.
Bedaiwy, M.A., T. Falcone, R.K. Sharma, J.M. Goldberg, M. Attaran, D.R. Nelson, et al. 2002. Prediction of endometriosis with serum and peritoneal fluid markers: A prospective controlled trial. Human Reproduction 17 (2): 426–431. https://doi.org/10.1093/humrep/17.2.426.
Keenan, J.A., T.T. Chen, N.L. Chadwell, D.S. Torry, and M.R. Caudle. 1995. IL-1 beta, TNF-alpha, and IL-2 in peritoneal fluid and macrophage-conditioned media of women with endometriosis. American Journal of Reproductive Immunology 34 (6): 381–385. https://doi.org/10.1111/j.1600-0897.1995.tb00968.x.
Paik, J., J.Y. Lee, and D. Hwang. 2002. Signaling pathways for TNFa-induced COX-2 expression: Mediation through MAP kinases and NFkB, and inhibition by certain nonsteroidal anti-inflammatory drugs. Advances in Experimental Medicine and Biology 507: 503–508. https://doi.org/10.1007/978-1-4615-0193-0_77.
Gonzalez-Ramos, R., J. Rocco, C. Rojas, H. Sovino, A. Poch, P. Kohen, et al. 2012. Physiologic activation of nuclear factor kappa-B in the endometrium during the menstrual cycle is altered in endometriosis patients. Fertility and Sterility 97 (3): 645–651. https://doi.org/10.1016/j.fertnstert.2011.12.006.
Nejad, C., H.J. Stunden, and M.P. Gantier. 2018. A guide to miRNAs in inflammation and innate immune responses. FEBS Journal 285 (20): 3695–3716. https://doi.org/10.1111/febs.14482.
Hoesel, B., and J.A. Schmid. 2013. The complexity of NF-kappaB signaling in inflammation and cancer. Molecular Cancer 12: 86. https://doi.org/10.1186/1476-4598-12-86.
Hajimaqsoudi, E., F. Darbeheshti, SM. Kalantar, A. Javaheri, SH. Mirabutalebi, and MH Sheikhha. 2020. Investigating the expressions of miRNA-125b and TP53 in endometriosis. Does it underlie cancer-like features of endometriosis? A case-control study. International Journal of Reproductive Biomedicine 18(10):825–36. https://doi.org/10.18502/ijrm.v13i10.7767.
Tang, S.T., F. Wang, M. Shao, Y. Wang, and H.Q. Zhu. 2017. MicroRNA-126 suppresses inflammation in endothelial cells under hyperglycemic condition by targeting HMGB1. Vascular Pharmacology 88: 48–55. https://doi.org/10.1016/j.vph.2016.12.002.
Wu, Y., L.T. Song, J.S. Li, D.W. Zhu, S.Y. Jiang, and J.Y. Deng. 2017. MicroRNA-126 regulates inflammatory cytokine secretion in human gingival fibroblasts under high glucose via targeting tumor necrosis factor receptor associated factor 6. Journal of Periodontology 88 (11): e179–e187. https://doi.org/10.1902/jop.2017.170091.
Meng, X., J. Liu, H. Wang, P. Chen and D. Wang. 2019. MicroRNA-126–5p downregulates BCAR3 expression to promote cell migration and invasion in endometriosis. Molecular and Cellular Endocrinology 494:110486. https://doi.org/10.1016/j.mce.2019.110486.
Liu, Z., D. Yang, P. Xie, G. Ren, G. Sun, X. Zeng, et al. 2012. MiR-106b and MiR-15b modulate apoptosis and angiogenesis in myocardial infarction. Cellular Physiology and Biochemistry 29 (5–6): 851–862. https://doi.org/10.1159/000258197.
Yang, Y., Y. Liu, Y. Li, Z. Chen, Y. Xiong, T. Zhou, et al. 2020. MicroRNA-15b targets VEGF and inhibits angiogenesis in proliferative diabetic retinopathy. Journal of Clinical Endocrinology and Metabolism 105 (11): 3404–3415. https://doi.org/10.1210/clinem/dgaa538.
Hua, Z., Q. Lv, W. Ye, CK. Wong, G. Cai, D. Gu, et al. 2006. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One 1(1):e116. https://doi.org/10.1371/journal.pone.0000116.
Liu, X., J. Li, F. Qin, and S. Dai. 2016. miR-152 as a tumor suppressor microRNA: Target recognition and regulation in cancer. Oncology Letters 11 (6): 3911–3916. https://doi.org/10.3892/ol.2016.4509.
Duan, Q., X. Mao, Y. Xiao, Z. Liu, Y. Wang, H. Zhou, et al. 2016. Super enhancers at the miR-146a and miR-155 genes contribute to self-regulation of inflammation. Biochimica et Biophysica Acta 1859 (4): 564–571. https://doi.org/10.1016/j.bbagrm.2016.02.004.
Rawat, V.P.S., M. Gotze, A. Rasalkar, N.M. Vegi, S. Ihme, S. Thoene, et al. 2020. The microRNA miR-196b acts as a tumor suppressor in Cdx2-driven acute myeloid leukemia. Haematologica 105 (6): e285–e289. https://doi.org/10.3324/haematol.2019.223297.
Chakrabarty, A., S. Tranguch, T. Daikoku, K. Jensen, H. Furneaux, and S.K. Dey. 2007. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci U S A. 104 (38): 15144–15149. https://doi.org/10.1073/pnas.0705917104.
Chen, R., A.B. Alvero, D.A. Silasi, M.G. Kelly, S. Fest, I. Visintin, et al. 2008. Regulation of IKKbeta by miR-199a affects NF-kappaB activity in ovarian cancer cells. Oncogene 27 (34): 4712–4723. https://doi.org/10.1038/onc.2008.112.
Koeck, I., A. Hashemi Gheinani, U. Baumgartner, E. Vassella, R. Bruggmann, F.C. Burkhard, et al. 2018. Tumor necrosis factor-alpha initiates miRNA-mRNA signaling cascades in obstruction-induced bladder dysfunction. American Journal of Pathology 188 (8): 1847–1864. https://doi.org/10.1016/j.ajpath.2018.05.008.
Lu, Y., J. Xiao, H. Lin, Y. Bai, X. Luo, Z. Wang, et al. 2009. A single anti-microRNA antisense oligodeoxyribonucleotide (AMO) targeting multiple microRNAs offers an improved approach for microRNA interference. Nucleic Acids Research 37(3):e24. https://doi.org/10.1093/nar/gkn1053.
Wang, D., Y. Sang, T. Sun, P. Kong, L. Zhang, Y. Dai, et al. 2021. Emerging roles and mechanisms of microRNA‑222‑3p in human cancer (review). International Journal of Oncology 58(5). https://doi.org/10.3892/ijo.2021.5200.
Liu, B., Q. Che, H. Qiu, W. Bao, X. Chen, W. Lu, et al. 2014. Elevated MiR-222–3p promotes proliferation and invasion of endometrial carcinoma via targeting ERalpha. PLoS One 9(1):e87563. https://doi.org/10.1371/journal.pone.0087563.
Vasquez, Y.M., S.P. Wu, M.L. Anderson, S.M. Hawkins, C.J. Creighton, M. Ray, et al. 2016. Endometrial expression of steroidogenic factor 1 promotes cystic glandular morphogenesis. Molecular Endocrinology 30 (5): 518–532. https://doi.org/10.1210/me.2015-1215.
Shen, L., S. Yang, W. Huang, W. Xu, Q. Wang, Y. Song, et al. 2013. MicroRNA23a and microRNA23b deregulation derepresses SF-1 and upregulates estrogen signaling in ovarian endometriosis. Journal of Clinical Endocrinology and Metabolism 98 (4): 1575–1582. https://doi.org/10.1210/jc.2012-3010.
Li, J., B. Cen, S. Chen, and Y. He. 2016. MicroRNA-29b inhibits TGF-beta1-induced fibrosis via regulation of the TGF-beta1/Smad pathway in primary human endometrial stromal cells. Molecular Medicine Reports 13 (5): 4229–4237. https://doi.org/10.3892/mmr.2016.5062.
Xie, Y., S. Naizabekov, Z. Chen, and T. Tokay. 2016. Power of PTEN/AKT: Molecular switch between tumor suppressors and oncogenes. Oncology Letters 12 (1): 375–378. https://doi.org/10.3892/ol.2016.4636.
Kong, J., X. He, Y. Wang, and J. Li. 2019. Effect of microRNA-29b on proliferation, migration, and invasion of endometrial cancer cells. Journal of International Medical Research 47 (8): 3803–3817. https://doi.org/10.1177/0300060519844403.
Panda, H., T.D. Chuang, X. Luo, and N. Chegini. 2012. Endometrial miR-181a and miR-98 expression is altered during transition from normal into cancerous state and target PGR, PGRMC1, CYP19A1, DDX3X, and TIMP3. Journal of Clinical Endocrinology and Metabolism 97 (7): E1316–E1326. https://doi.org/10.1210/jc.2012-1018.
Kim, K.H., J.K. Park, Y.W. Choi, Y.H. Kim, E.N. Lee, J.R. Lee, et al. 2013. Hexane extract of aged black garlic reduces cell proliferation and attenuates the expression of ICAM-1 and VCAM-1 in TNF-alpha-activated human endometrial stromal cells. International Journal of Molecular Medicine 32 (1): 67–78. https://doi.org/10.3892/ijmm.2013.1362.
Ohama, Y., T. Harada, T. Iwabe, F. Taniguchi, Y. Takenaka, and N. Terakawa. 2008. Peroxisome proliferator-activated receptor-gamma ligand reduced tumor necrosis factor-alpha-induced interleukin-8 production and growth in endometriotic stromal cells. Fertility and Sterility 89 (2): 311–317. https://doi.org/10.1016/j.fertnstert.2007.03.061.
Hayden, M.S., and S. Ghosh. 2012. NF-kappaB, the first quarter-century: Remarkable progress and outstanding questions. Genes & Development 26 (3): 203–234. https://doi.org/10.1101/gad.183434.111.
Israel, A. 2010. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harbor Perspectives Biology 2(3):a000158. https://doi.org/10.1101/cshperspect.a000158.
Huminiecki, L., J. Horbanczuk, and A.G. Atanasov. 2017. The functional genomic studies of curcumin. Seminars in Cancer Biology 46: 107–118. https://doi.org/10.1016/j.semcancer.2017.04.002.
Funding
This study was supported in part by National Institutes of Health Grants 1SC3 GM113751, 1SC1 GM130544, U01 HD66439, 1R01HD057235, U54 CA118948, HD41749, S21MD000101, and G12-RR03034. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Grant #C06 RR18386 from NIH/NCRR. This study was presented in part at the Research Centers in Minority Institutions (RCMI) 2019, Collaborative Solutions to Improve Minority Health and Reduce Health Disparities, Bethesda, MD, USA (December 15–16, 2019); 53rd Annual Meeting of the Society for the Study of Reproduction (Virtual), Washington DC, USA (March 28–31, 2020); and ENDO 2022, Endocrine Society, Atlanta, GA, USA (June 11–14, 2022).
Author information
Authors and Affiliations
Contributions
I.C. and S.B. contributed to the study concept and design, acquisition, analysis and interpretation of data, statistical analysis, and drafting of the manuscript. W.X. and A.D. contributed experimental support. C.N. and N.S. contributed patient samples. W.E.T., R.N.T., N.S., and A.D. critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Banerjee, S., Xu, W., Doctor, A. et al. TNFα-Induced Altered miRNA Expression Links to NF-κB Signaling Pathway in Endometriosis. Inflammation 46, 2055–2070 (2023). https://doi.org/10.1007/s10753-023-01862-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01862-x