Abstract
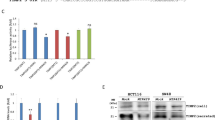
Endometriosis affects about 10-15% women for reproductive age, but it is not currently curable and the underlying etiology for this disease is still not clear. In the present study, functions and mechanisms of miR-182 and RELA in endometriosis were investigated. BAY 11-7082 was used to block NF-κB pathway. qRT-PCR, ELISA and western blot assays were employed to evaluate the expressions of miR-182 and RELA, inflammatory factors and epithelial–mesenchymal transition (EMT)-related markers, and activation of NF-κB pathway. MTT, wound healing or Transwell assays were used to evaluate the cell proliferation, migration and invasion capacities. Bioinformatic and dual-luciferase reporter assays were carried out to analyze the interaction between miR-182 and RELA. MiR-182 expression was decreased, while RELA was increased as developed from normal to eutopic and ectopic status, which was accompanied by upregulated inflammatory factors and EMT-related proteins. RELA was directly targeted by miR-182 in human endometrial stromal cells. Overexpression of RELA increased inflammation-associated and EMT-related markers expression, while miR-182 upregulation decreased the expression of these genes in a dose-dependent manner, which finally attenuated the proliferation, migration and invasion capacities of endometrial stromal cells through deactivation of NF-κB signaling pathway. Moreover, co-overexpression of RELA reversed the above effects induced by miR-182. In a word, miR-182 directly targeted RELA and inhibited proliferation, migration, invasion, EMT and inflammation of endometrial stromal cells through deactivation of NF-κB signaling pathway in endometriosis. These results provide new insights into the interaction between miR-182 and NF-κB pathway and their potential as therapeutic targets for treatment of endometriosis.







Similar content being viewed by others
Data Availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Schrager S, Falleroni J, Edgoose J (2013) Evaluation and treatment of endometriosis. Am Fam Physician 87:107–113
Lagana AS, Garzon S, Gotte M, Vigano P, Franchi M, Ghezzi F, Martin DC (2019) The pathogenesis of endometriosis: molecular and cell biology insights. Int J Mol Sci 20. https://doi.org/10.3390/ijms20225615
Baranov V, Malysheva O, Yarmolinskaya M (2018) Pathogenomics of endometriosis development. Int J Mol Sci 19. https://doi.org/10.3390/ijms19071852
Andres MP, Arcoverde FV, Souza CC, Fernandes LFC, Abrao MS, Kho RM (2019) Extra-pelvic endometriosis: a systematic review. J Minim Invasive Gynecol. https://doi.org/10.1016/j.jmig.2019.10.004
Dai Y, Li X, Shi J, Leng J (2018) A review of the risk factors, genetics and treatment of endometriosis in Chinese women: a comparative update. Reprod Health 15:82. https://doi.org/10.1186/s12978-018-0506-7
Chen FY, Wang X, Tang RY, Guo ZX, Deng YZ, Yu Q (2019) New therapeutic approaches for endometriosis besides hormonal therapy. Chin Med J. https://doi.org/10.1097/CM9.0000000000000569
Neri M, Melis GB, Giancane E, Vallerino V, Pilloni M, Piras B, Loddo A, Paoletti AM, Mais V (2019) Clinical utility of Elagolix as an Oral treatment for women with uterine fibroids: a short report on the emerging efficacy data. Int J Women's Health 11:535–546. https://doi.org/10.2147/IJWH.S185023
Sourial S, Tempest N, Hapangama DK (2014) Theories on the pathogenesis of endometriosis. Int J Reprod Med 2014:179515. https://doi.org/10.1155/2014/179515
Baranov VS, Ivaschenko TE, Liehr T, Yarmolinskaya MI (2015) Systems genetics view of endometriosis: a common complex disorder. Eur J Obstet Gynecol Reprod Biol 185:59–65. https://doi.org/10.1016/j.ejogrb.2014.11.036
Kaponis A, Iwabe T, Taniguchi F, Ito M, Deura I, Decavalas G, Terakawa N, Harada T (2012) The role of NF-kappaB in endometriosis. Front Biosci (Schol Ed) 4:1213–1234. https://doi.org/10.2741/s327
Gonzalez-Ramos R, Van Langendonckt A, Defrere S, Lousse JC, Colette S, Devoto L, Donnez J (2010) Involvement of the nuclear factor-kappaB pathway in the pathogenesis of endometriosis. Fertil Steril 94:1985–1994. https://doi.org/10.1016/j.fertnstert.2010.01.013
Gonzalez-Ramos R, Defrere S, Devoto L (2012) Nuclear factor-kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril 98:520–528. https://doi.org/10.1016/j.fertnstert.2012.06.021
Liu T, Zhang L, Joo D, Sun SC (2017) NF-kappaB signaling in inflammation. Signal Transduct Target Ther 2. https://doi.org/10.1038/sigtrans.2017.23
Napetschnig J, Wu H (2013) Molecular basis of NF-kappaB signaling. Annu Rev Biophys 42:443–468. https://doi.org/10.1146/annurev-biophys-083012-130338
Moynagh PN (2005) The NF-kappaB pathway. J Cell Sci 118:4589–4592. https://doi.org/10.1242/jcs.02579
Brasier AR (2010) The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res 86:211–218. https://doi.org/10.1093/cvr/cvq076
Cobellis L, Razzi S, De Simone S, Sartini A, Fava A, Danero S, Gioffre W, Mazzini M, Petraglia F (2004) The treatment with a COX-2 specific inhibitor is effective in the management of pain related to endometriosis. Eur J Obstet Gynecol Reprod Biol 116:100–102. https://doi.org/10.1016/j.ejogrb.2004.02.007
Ma X, Becker Buscaglia LE, Barker JR, Li Y (2011) MicroRNAs in NF-kappaB signaling. J Mol Cell Biol 3:159–166. https://doi.org/10.1093/jmcb/mjr007
Wu J, Ding J, Yang J, Guo X, Zheng Y (2018) MicroRNA roles in the nuclear factor kappa B signaling pathway in Cancer. Front Immunol 9:546. https://doi.org/10.3389/fimmu.2018.00546
Panir K, Schjenken JE, Robertson SA, Hull ML (2018) Non-coding RNAs in endometriosis: a narrative review. Hum Reprod Update 24:497–515. https://doi.org/10.1093/humupd/dmy014
Filigheddu N, Gregnanin I, Porporato PE, Surico D, Perego B, Galli L, Patrignani C, Graziani A, Surico N (2010) Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J Biomed Biotechnol 2010:369549. https://doi.org/10.1155/2010/369549
Masuda A, Katoh N, Nakabayashi K, Kato K, Sonoda K, Kitade M, Takeda S, Hata K, Tomikawa J (2016) An improved method for isolation of epithelial and stromal cells from the human endometrium. J Reprod Dev 62:213–218. https://doi.org/10.1262/jrd.2015-137
Arnold JT, Kaufman DG, Seppala M, Lessey BA (2001) Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod 16:836–845. https://doi.org/10.1093/humrep/16.5.836
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2:329–333. https://doi.org/10.1038/nprot.2007.30
Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C (2006) Clonogenic assay of cells in vitro. Nat Protoc 1:2315–2319. https://doi.org/10.1038/nprot.2006.339
Yang Y, Leonard M, Zhang Y, Zhao D, Mahmoud C, Khan S, Wang J, Lower EE, Zhang X (2018) HER2-driven breast tumorigenesis relies upon interactions of the estrogen receptor with coactivator MED1. Cancer Res 78:422–435. https://doi.org/10.1158/0008-5472.CAN-17-1533
Gao W, Weng T, Wang L, Shi B, Meng W, Wang X, Wu Y, Jin L, Fei L (2019) Long noncoding RNA NORAD promotes cell proliferation and glycolysis in nonsmall cell lung cancer by acting as a sponge for miR1365p. Mol Med Rep 19:5397–5405. https://doi.org/10.3892/mmr.2019.10210
Yang YM, Yang WX (2017) Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 8:41679–41689. https://doi.org/10.18632/oncotarget.16472
Kong X, Qian X, Duan L, Liu H, Zhu Y, Qi J (2016) microRNA-372 suppresses migration and invasion by targeting p65 in human prostate Cancer cells. DNA Cell Biol 35:828–835. https://doi.org/10.1089/dna.2015.3186
Parasar P, Ozcan P, Terry KL (2017) Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep 6:34–41. https://doi.org/10.1007/s13669-017-0187-1
Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, Print CG, Hull LM (2009) MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol 23:265–275. https://doi.org/10.1210/me.2008-0387
Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM (2011) Functional microRNA involved in endometriosis. Mol Endocrinol 25:821–832. https://doi.org/10.1210/me.2010-0371
Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P (2018) Endometriosis. Nat Rev Dis Primers 4:9. https://doi.org/10.1038/s41572-018-0008-5
Zeisberg M, Neilson EG (2009) Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119:1429–1437. https://doi.org/10.1172/JCI36183
Funding
This work was supported by the grant from Health Commission of Hunan Province (No. B2017125).
Author information
Authors and Affiliations
Contributions
Guarantor of integrity of the entire study: MW. Study concepts: MW. Study design: YZ. Definition of intellectual content: MW. Literature research: MW. Clinical studies: MW. Experimental studies: MW. Data acquisition: MW. Data analysis: MW. Statistical analysis: MW. Manuscript preparation: MW. Manuscript editing: MW. Manuscript review: YZ. All the authors approved for the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study has been conducted strictly following the ethical standards of Ethics Committee of Hunan Provincial Maternal and Child Health Care Hospital (Changsha, Hunan, China). Written informed consent was obtained from each patient and healthy participant before the collection of any specimen.
Informed Consent
The informed consent obtained from study participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, M., Zhang, Y. MiR-182 inhibits proliferation, migration, invasion and inflammation of endometrial stromal cells through deactivation of NF-κB signaling pathway in endometriosis. Mol Cell Biochem 476, 1575–1588 (2021). https://doi.org/10.1007/s11010-020-03986-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03986-2