Abstract
With advances in immunometabolic studies, more and more evidence has shown that metabolic changes profoundly affect the immune function of macrophages. The tricarboxylic acid cycle is a central metabolic pathway of cells. Itaconate, a byproduct of the tricarboxylic acid cycle, is an emerging metabolic small molecule that regulates macrophage inflammation and has received much attention for its potent anti-inflammatory effects in recent years. Itaconate regulates macrophage function through multiple mechanisms and has demonstrated promising therapeutic potential in a variety of immune and inflammatory diseases. New progress in the mechanism of itaconate continues to be made, but it also implies complexity in its action and a need for a more comprehensive understanding of its role in macrophages. In this article, we review the primary mechanisms and current research progress of itaconate in regulating macrophage immune metabolism, hoping to provide new insights and directions for future research and disease treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
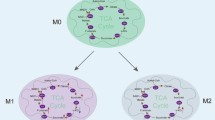
Macrophages are a crucial component of the immune system and help the host fight infection. Macrophages can change into numerous phenotypes depending on the tissue microenvironment, physiological parameters, and pathological conditions. These include traditionally activated macrophages (M1) and alternatively activated macrophages (M2). M1 macrophages are primarily responsible for starting and maintaining an inflammatory response, whereas M2 macrophages are primarily responsible for inflammation resolution and tissue repair [1,2,3]. M1 macrophages can provide a significant percentage of the energy required for their rapid multiplication by increasing aerobic glycolysis, while slow-proliferating M2 macrophages’ primary energy source is oxidative phosphorylation [4]. Numerous studies have demonstrated that the varied metabolic characteristics of macrophages control their activation status and activity. The tricarboxylic acid (TCA) cycle, also known as the Krebs cycle or the citric acid cycle, is a central metabolic pathway of cells. It has emerged as a critical regulator for guiding intracellular metabolic adaptability and starting signaling. Several metabolites of the TCA cycle such as succinate, fumarate, and the byproduct itaconate have been found to play significant roles in controlling the immunophenotype and inflammatory response of macrophages [5]. Recent research has focused a lot of interest on the novel metabolite itaconate due to the discovery that it possesses potent immunomodulatory characteristics.
Itaconate was initially utilized in the manufacturing industry, primarily for the manufacture of polymers. Later, itaconate was discovered to have antibacterial properties by inhibiting isocitrate lyase, leading researchers to explore its biological function [6,7,8]. With a better understanding of its biological role, itaconate has been shown to have a powerful immune regulatory function in macrophages in recent years. Itaconate was discovered in 2011 to be produced by decarboxylation of cis-aconitate in the TCA cycle and accumulated in large amounts in LPS-activated macrophages, implying that itaconate may play a role in macrophage immune response [9]. In 2013, Michelucci et al. [10] found a significantly upregulated gene named immune response gene 1 (IRG1) in LPS-activated macrophages. IRG1 encodes cis-aconitate decarboxylase (CAD, also known as aconitate decarboxylase 1, ACOD1), which catalyzes the production of itaconate from cis-aconitate and reveals the synthesis pathway of itaconate in macrophages (Fig. 1). In 2016, itaconate was first found to exert anti-inflammatory effects in macrophages through the inhibition of succinate dehydrogenase (SDH) [11, 12]. Subsequently, there is increasing evidence for the critical role of the IRG1-itaconate axis in macrophage immune metabolism (Fig. 1). In order to offer new options for the treatment of immune and inflammatory diseases, we review the primary mechanisms and current research progress of itaconate in regulating macrophage immune metabolism.
Itaconate is derived from the TCA cycle metabolites and regulates immune metabolism of macrophages. Itaconate is produced by the decarboxylation of cis-aconitate via the aconitate decarboxylase 1 (ACOD1) which encoded by the immune response gene 1 (IRG1). Itaconate and its derivatives have been proved to regulate macrophage immune metabolism through multiple pathways, such as inhibiting SDH and glycolysis, activating Nrf2 and ATF3, and inhibiting NLRP3 inflammasome and TET2. Other abbreviations: SDH, succinate dehydrogenase; ALDOA, fructose-bisphosphate aldolase A; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LDHA, lactate dehydrogenase A; Nrf2, nuclear transcription factor-erythroid 2-related factor 2; ATF3, activating transcription factor 3; NLRP3, NOD-, LRR-, and pyrin domain-containing protein 3; TET2, TET mehtylcytosine dioxygenase 2.
ITACONATE AND ITS DERIVATIVES
Work investigating the immunomodulatory activity of itaconate can be broadly divided into studies examining the properties of endogenous itaconate using IRG1-deficient macrophages and studies exploring the specific mechanisms by supplementing exogenous itaconate. Itaconate, a highly polar molecule, cannot easily pass through cell membranes and must be imported into the cytoplasm to exert anti-inflammatory effects. To overcome the disadvantage, researchers have synthesized membrane-permeable itaconate derivatives such as dimethyl itaconate (DI), 4-octyl itaconate (4-OI), and 4-monoethyl itaconate (4-EI). Although it has been found that itaconate can be transferred from mitochondria into salmonella-containing vacuoles through organelle interactions driven by the IRG1-Rab32-BLOC3 system, thus exerting antibacterial functions [13]. However, the mechanism of macrophages absorbing itaconate from the extracellular environment into the cytoplasm remains unclear. Therefore, whether the regulating effects mediated by the addition of exogenous itaconate and its derivatives are due to increased intracellular itaconate or extracellular mechanisms becomes a question that needs to be elucidated.
Analysis of itaconate uptake and metabolism by isotopically labeled [13C]itaconate and [13C]DI suggested that the exogenous itaconate was not absorbed into cells and DI was not metabolized into itaconate in mice bone marrow-derived macrophages [14]. In contrast, other studies showed that after being treated with native itaconate, RAW264.7 macrophages, A549 lung adenocarcinoma cells, and brown adipocytes accumulated significant amounts of itaconate. However, the immune effects resulting from this accumulation were not taken into consideration [11, 15]. The lack of a negative charge on the conjugated ester group in DI increases its reactivity to Michael addition. This makes DI a better nuclear transcription factor-erythroid 2-related factor 2 (Nrf2) activator than itaconate, similar to the Nrf2 activator dimethyl fumarate. As a result, DI is unlikely to mimic endogenous itaconate. To overcome the limitations of DI, 4-OI was synthesized, and 4-OI had a similar thiol reactivity that was far lower than that of DI, making it a suitable cell-permeable itaconate surrogate. 4-OI was hydrolyzed to itaconate by esterases in mouse myoblast C2C12 cells and LPS-activated macrophages [16]. In this regard, 4-OI may be more suitable for experiments as a simulated derivative of itaconate.
Nevertheless, itaconate derivatives do not fully mimic the effects of endogenous itaconate in vivo. Changes in their structure may produce some additional effects not related to itaconate. For this reason, Swain et al. [17] conducted a systematic comparison of the metabolic, electrophilic, and immunological properties of itaconate and its three main derivatives, DI, 4-OI, and 4-EI. It was found that none of the itaconate derivatives produced significant intracellular amounts of itaconate, while non-derived natural itaconate accumulated in unstimulated and activated macrophages. Consistent with this, He et al. found that when supplemented exogenous itaconate to the culture of resting macrophages, itaconate was able to efficiently penetrate into mouse macrophages. However, with an intracellular metabolomics analysis, no itaconate production was observed after the application of 1 mM DI and 4-OI in RAW264.7 cells [18]. In resting and LPS-activated macrophages, DI and 4-OI strongly induced the expression of the Nrf2-driven electrophilic stress markers Nqo1 and Hmox1, whereas neither itaconate nor 4-EI induced significant expression of these genes. In the absence of additional inflammatory/oxidative factors, such as prolonged LPS stimulation, itaconate itself did not induce Nrf2 protein expression [17]. These data suggest that DI and 4-OI are strongly electrophilic compounds, whereas itaconate and 4-EI are relatively less electrophilic. DI and 4-OI treatment limited the release of IFN-β in macrophages after LPS stimulation, in agreement with previous studies [16, 19]. However, natural itaconate increased the release of IFN-β, and IRG1−/− macrophages released fewer IFN-β than wild-type macrophages. RNA sequencing analysis showed that the downstream gene expression of type I IFN signaling was significantly impaired in IRG1−/− macrophages [17]. This suggests that natural itaconate behaves significantly differently from electrophilic derivatives and acts as an immunomodulator rather than a purely immunosuppressive metabolite. In addition, two recent studies have found that the two derivative isomers of itaconate, citraconate and mesaconate, also have immunomodulatory effects in macrophages. Despite the fact that all three metabolites had the ability to suppress glycolysis, citraconate and mesaconate were noticeably less effective than itaconate at inhibiting succinate dehydrogenase, TCA cycle, or oxidative phosphorylation. However, both citraconate and mesaconate, like itaconate, could restrain the production of proinflammatory cytokines, protecting against sepsis and lowering influenza replication in mouse models [18, 20]. Therefore, further thorough studies are required to comprehend the mechanism of action of these metabolites. In conclusion, there is variability between itaconate and its derivatives, further emphasizing the importance of using and differentiating between these compounds. Nonetheless, itaconate derivatives still provide a viable avenue for studying the role of itaconate in macrophage function. This is similar to dimethyl fumarate, a derivative of the TCA cycle metabolite fumarate. Dimethyl fumarate contributes to the understanding of the biochemical mechanisms of endogenous fumarate, as well as having potential immunomodulatory effects and being clinically approved for the treatment of inflammatory diseases [21, 22]. The efficacy and safety of itaconate and its derivatives as pharmacological agents need to be investigated in greater depth by researchers.
IMMUNOMODULATORY MECHANISMS OF ITACONATE
Itaconate Inhibits SDH
Succinate dehydrogenase (SDH), also known as mitochondrial complex II, is an enzyme complex located in the inner mitochondrial membrane that participates in the TCA cycle and the electron transfer process of the oxidative respiratory chain. SDH catalyzes the conversion of succinate to fumarate while transferring electrons to produce FADH2. Many investigations in recent years have demonstrated that SDH plays a significant role in the generation of reactive oxygen species (ROS) [23]. Mills et al. [24] discovered that SDH is a key regulator of the macrophage phenotype, driving the creation of mitochondrial ROS and inducing the expression of pro-inflammatory genes via boosting succinate oxidation and elevating the mitochondrial membrane potential. Inhibition of SDH exerted a corresponding anti-inflammatory effect, blocking the production of the pro-inflammatory factor IL-1β while enhancing the levels of the anti-inflammatory factors IL-1RA and IL-10 [24]. Also, in vivo experiments showed that dimethyl malonate (DMM), an SDH inhibitor, was therapeutically effective in a mouse model of LPS-induced sepsis, reducing serum IL-1β levels while increasing IL-10 levels [24]. Other studies have also found that DMM showed effectiveness in both E. coli infection [25] and ischemia–reperfusion injury [26] models. This suggests that SDH is an important regulator of inflammatory signaling and that inhibition of SDH opens up new avenues for the treatment of inflammatory diseases.
Because of its structural similarity to succinate, itaconate was found to be a competitive inhibitor of SDH decades ago [27,28,29]. However, its potential physiological role has not been explored. In 2016, itaconate was first found to exert immunomodulatory effects in macrophages by inhibiting SDH [11, 12]. It was shown that dimethyl itaconate (DI) inhibited LPS-induced inflammatory activation of macrophages and suppressed the expression of pro-inflammatory genes such as iNOS, IL-1β, IL-12p70, and IL-6. But there was no effect on TNF-α, indicating that DI treatment did not comprehensively inhibit the expression of NF-κB-dependent genes [12]. Both in vitro and in vivo experiments confirmed the inhibitory effect of itaconate on SDH. Itaconate dose-dependently blocked the activity of SDH, leading to an increase in succinate levels. Intravenous DI significantly reduced myocardial infarct size in the cardiac ischemia–reperfusion injury mouse model. Moreover, DI pretreatment attenuated hypoxia-induced ROS generation and provided dose-dependent protection against hypoxia-induced cell death in the neonatal cardiomyocyte hypoxic injury assay [12]. These results indicated that DI exerted a powerful anti-oxidative stress effect by inhibiting SDH. In IRG1−/− macrophages, lack of itaconate resulted in uninhibited SDH activity and exhibited a stronger inflammatory response under LPS stimulation compared to wild-type macrophages [12]. Other studies have found that inhibition of SDH by itaconate also ameliorated cerebral ischemia–reperfusion injury [30, 31]. In astrocytes and neurons, itaconate exerted an antioxidant stress effect by inhibiting SDH. And the addition of itaconate to the reperfusion fluid after cerebral ischemia–reperfusion injury in mice increased glutathione levels, reduced ROS production, and improved neurological function. Supplementation with itaconate significantly ameliorated hemodynamic disturbances and reduced leukocyte adhesion in the mouse cranial window surgery model [30]. These studies suggest that itaconate acts as an SDH inhibitor, regulates oxidative stress, and improves physiological outcomes associated with ischemia–reperfusion injury.
Another study found that the IRG1-itaconate-SDH axis is a central node in the regulation of immune tolerance and trained immunity [32]. In the acute phase of sepsis, excessive activation of the immune system and excessive release of cytokines can lead to infectious shock, tissue damage, hypotension, cardiovascular dysfunction, and multi-organ failure. At the same time, as the immune system initiates anti-inflammatory mechanisms, the anti-inflammatory response in the late phase of sepsis leaves the body still in a state of immune tolerance, which can easily lead to secondary infections [33]. Similar to the ability of T/B cells in acquired immunity to form memory T/B cells after the initial immune response to the antigen, certain natural immune cells such as macrophages can also form immune memory. Macrophages trigger a more intense inflammatory response upon re-exposure to the antigen through a series of metabolic and epigenetic reprogramming, which is called “trained immunity” [34]. Dominguez-Andres et al. [32] found that LPS induced itaconate synthesis through upregulation of IRG1, which inhibited SDH and subsequently mediated immune tolerance in the late phase of sepsis. In contrast, β-glucan enhanced the immune response through metabolic and epigenetically mediated trained immunity. In a human endotoxemia model, β-glucan restored SDH expression in monocytes where immune tolerance occurred. Mechanistically, β-glucan counteracted LPS-induced immune tolerance by inhibiting IRG1 and promoting SDH expression, keeping the TCA cycle intact to ensure an enhanced immune response after secondary stimulation. In contrast, the addition of the itaconate derivative DI was able to suppress trained immunity by inhibiting SDH [32]. These results demonstrate that itaconate is a key regulatory node between immune tolerance and trained immunity and may be a potential target for the treatment of sepsis.
Itaconate Activates Nrf2
Nuclear transcription factor-erythroid 2-related factor 2 (Nrf2), as a key transcription factor that regulates resistance to oxidative stress, plays an important role in the induction of antioxidant response in the body [35]. Kelch-like ECH-associated protein 1 (KEAP1) is a Cullin3-dependent substrate bridging protein of the E3 ubiquitin ligase complex that assembles with Cul3 and Rbx1 to form a functional E3 ubiquitin ligase complex (KEAP1-Cul3-E3), which in turn regulates Nrf2. Under physiological conditions, Nrf2 binds to KEAP1 and promotes ubiquitination, followed by delivery of ubiquitinated Nrf2 to the proteasome for degradation. Under oxidative stress, Nrf2 segregates from KEAP1 and subsequently translocates to the nucleus to mediate the transcription of Nrf2-dependent genes, thereby enhancing cellular defenses against oxidative stress [36]. Therefore, small molecules have been developed to reduce inflammation by disrupting the interaction of Nrf2 with KEAP1. For example, the Nrf2 inducer Tecfidera, also known as dimethyl fumarate, is used in the clinical treatment of multiple sclerosis [37]. And itaconate as an emerging Nrf2 agonist has attracted extensive research interest in recent years. In 2018, Mills et al. [16] found that itaconate was significantly increased in LPS-activated murine macrophages, and the use of itaconate derivative 4-OI significantly activated Nrf2 and promoted the expression of Nrf2 downstream antioxidant and anti-inflammatory target genes Hmox1 and Nqo1 [16]. In contrast, dimethyl malonate, a potent SDH inhibitor [24], did not activate Nrf2, suggesting that activation of Nrf2 by 4-OI is not associated with the inhibition of SDH. Since itaconate contains an electrophilic α/β-unsaturated carboxylic acid, it may alkylate protein cysteine residues via Michael addition to form 2,3-dicarboxypropyl adducts (Fig. 2). The researchers found that 4-OI prevented KEAP1-mediated degradation of Nrf2 by alkylating cysteine residues 151, 257, 288, 273, and 297 on KEAP1 in murine KEAP1-overexpressing HEK293T cells. 4-OI exerted a powerful anti-inflammatory effect by activating Nrf2, reducing levels of IL-1β, HIF-1α, ROS, nitrite, and iNOS in LPS-induced macrophages [16]. Meanwhile, 4-OI intraperitoneal injection reduced serum IL-1β and TNF-α levels, prolonged survival, reduced clinical scores, and improved thermoregulation in the mouse sepsis model [16]. However, Swain et al. found that unlike 4-OI and DI, natural itaconate has a milder electrophilicity that is not sufficient to alkylate KEAP1 and therefore unable to activate Nrf2 [17]. He et al. also demonstrated that natural itaconate still reduced IL-6 and IL-12p40 secretion and increased CXCL10 secretion in Nrf2-deficient macrophages, while the anti-inflammatory effect of 4-OI was indeed dependent on Nrf2 [18]. In summary, these findings show that more electrophilic itaconate derivatives significantly inhibit oxidative stress and inflammation by activating Nrf2.
Given the anti-oxidative stress and anti-inflammatory capabilities exhibited by itaconate as an Nrf2 agonist, itaconate has also shown corresponding therapeutic potential in inflammatory diseases. Oxidative stress is an important mechanism of ischemia–reperfusion injury in the liver. Knockout of the IRG1 gene was found to exacerbate hepatic ischemia–reperfusion injury and systemic inflammation. In contrast, 4-OI can significantly improve liver injury and reduce hepatocyte death by activating Nrf2 in hepatocytes [38]. 4-OI also showed significant protective effects in acute hepatic inflammatory injury induced by carbon tetrachloride, lipopolysaccharide/D-galactosamine, and Concanavalin A [39,40,41]. Furthermore, in chronic inflammation of the liver caused by obesity, Azzimato et al. [42] performed transcriptome analysis in hepatic macrophages from obese mice and identified IRG1 as a target of microRNA-144 (miR-144). Silencing of miR-144 upregulated IRG1, and accumulated itaconate in turn competitively inhibited fumarate hydratase, leading to increased fumarate. Subsequently, fumarate activates Nrf2 and attenuates hepatic inflammatory injury in obese mice [42]. This study suggests a novel mechanism for Nrf2 activation by itaconate. Another study also showed that 4-OI significantly inhibited ROS production by activating Nrf2, which in turn caused downregulation of AMPK phosphorylation levels, and improved free fatty acid-induced oxidative stress and lipid metabolism disorders in hepatocytes [43]. Itaconate has also attenuated inflammatory damage through activation of Nrf2 in other diseases such as osteoarthritis [44,45,46,47], nerve injury [48, 49], cardiovascular disease [50, 51], systemic lupus erythematosus [52], vitiligo [53], periodontitis [54], fungal keratitis [55], and diabetic wound repair [56]. Consequently, itaconate, as an Nrf2 agonist, may present new therapeutic opportunities for many inflammatory diseases.
Itaconate Inhibits NLRP3 Inflammasome
Inflammasomes, multiprotein complexes assembled with the participation of intracytoplasmic pattern recognition receptors, are capable of recognizing pathogen-associated molecular patterns or damage-associated molecular patterns and are important components of the natural immune system [57]. The NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome is one of the most characteristic inflammasomes. The NLRP3 inflammasome consists of its central protein NLRP3, the junctional protein ASC, and the mitotic kinase NIMA-associated kinase 7 (NEK7). Activation of the NLRP3 inflammasome promotes self-cleavage of pro-Caspase-1 to produce the active effector protein Caspase-1, which can cleave pro-IL-1β, pro-IL-18, and Gasdermin-D to induce inflammation and pyroptosis. The NLRP3 inflammasome is involved in the development of many diseases, such as cryopyrin-associated periodic syndrome (CAPS) caused by autosomal dominant mutations in NLRP3, Alzheimer’s disease, and rheumatoid arthritis. In 2016, it was found that DI downregulated LPS-induced IL-1β, ASC, and NLRP3 protein levels in mouse macrophages [12]. These results suggest that DI inhibits the activation of the NLRP3 inflammasome, but the exact mechanism is unclear. Hooftman et al. [58] further showed that 4-OI blocked the activation of the NLRP3 inflammasome and reduced the release of IL-1β, IL-18, and LDH. But 4-OI did not affect AIM2, NLRC4, and non-classical inflammasome-related pathways, suggesting that the action of 4-OI was targeted to NLRP3. Tandem mass spectrometry indicated that 4-OI modified the C548 site of NLRP3 and interfered with the interaction between NLRP3 and NEK7 in murine NLRP3-overexpressing HEK293T cells, thereby blocking the activation of the NLRP3 inflammasome [58]. Meanwhile, 4-OI treatment ameliorated the inflammatory response of urate-induced peritonitis in mice and inhibited the activation of the NLRP3 inflammasome in peripheral blood mononuclear cells from healthy populations and CAPS patients [58]. In addition, a study found that natural itaconate did not reduce IL-1β secretion in macrophages treated with nigericin, while both DI and 4-OI reduced IL-1β secretion after NLRP3 activation with nigericin. Concomitantly, both itaconate derivatives abrogated caspase-1 cleavage, while natural itaconate did not [18]. This may have resulted from the relatively lower cell entry rate of natural itaconate compared to DI and 4-OI designed for enhanced cellular permeability.
An additional study showed that itaconate modulated LPS-induced tolerance of late NLRP3 inflammasome activation [59]. The accumulation of itaconate under prolonged LPS stimulation prevented Caspase-1 activation and Gasdermin-D processing, and Cys77 of Gasdermin-D was found to be a possible target for itaconate modification in mice bone marrow-derived macrophages [59]. Moreover, the itaconate derivatives DI and 4-OI have been demonstrated to inhibit the activation of the NLRP3 inflammasome in mixed glia and organotypic hippocampal slice cultures, exhibiting neuroprotective effects [60]. DI also significantly attenuates ganglion macrophage infiltration and activation of central microglia in the spinal cord by inhibiting NLRP3, resulting in relief of Complete Freund’s adjuvant-induced inflammatory pain [61]. In summary, itaconate targeting the NLRP3 inflammasome has great therapeutic potential in NLRP3-driven diseases.
Itaconate Regulates ATF3-IκBζ
Activating transcription factor 3 (ATF3) is a transcription factor that belongs to the ATF/CREB family. ATF3 is expressed at low levels in resting cells but increases under stress conditions such as injury, ischemia, and chemical toxins [62]. IκBζ is a nuclear protein encoded by the Nfkbiz gene that controls the release of pro-inflammatory cytokines such as IL-6 [63]. It was found that ATF3 is a negative regulator of IκBζ and inhibits the release of targeted inflammatory factors of IκBζ [19, 64]. Bambouskova et al. [19] found that in mouse macrophages and human blood monocytes, itaconate and its derivative DI promoted ATF3 protein expression, thereby eliminating LPS-induced IκBζ protein. And the inhibitory effect of DI on IκBζ correlated with its electrophilic strength. In Nrf2−/− macrophages, DI still inhibited IκBζ and IL-6 [19]. This indicates that the inhibition of IκBζ by DI is Nrf2-independent, in agreement with the study of Swain et al. [17]. In ATF3−/− macrophages, the inhibitory effect of DI on IκBζ was impaired, suggesting that DI acts through ATF3 [19]. However, the effects of less electrophilic natural itaconate on cytokine production were unaltered in ATF3-deficient macrophages [18]. IL-17 was able to induce increased IκBζ production in epithelial cells, mediated downstream inflammatory responses [65,66,67], and was associated with immune diseases such as psoriasis [68]. Researchers found that DI inhibited IL-17-induced IκBζ in primary keratinocytes in mice and humans. Moreover, DI treatment significantly improved IL-17-IκBζ-driven skin pathology and inflammatory response in the psoriasis model [19]. In brief, itaconate induces ATF3 expression, blocks IκBζ protein, and then inhibits the expression of inflammatory genes in activated macrophages. These findings provide a focused strategy for treating autoimmune diseases like psoriasis.
Itaconate Regulates IFN
Interferon (IFN) is a group of multifunctional active proteins with a variety of biological cellular activities that are antiviral, affect cell growth and differentiation, and regulate immune function. There are three distinct interferon families, and IFN-α/β of type I IFN are the most widely expressed and studied [69]. Mills et al. found that type I IFN could promote macrophage IRG1 expression and itaconate production. Conversely, 4-OI could limit type I IFN responses, suggesting a negative feedback loop involving interferon and itaconate [16]. Another study found that itaconate and its two isomers, mesaconate and citraconate, reduced levels of signal transducer and activator of transcription 1 (STAT1) phosphorylation levels, attenuated canonical type I IFN signaling, and reduced CXCL10 levels in influenza A virus-infected dTHP1 and A549 cells [20]. It was found that 4-OI and the Nrf2 inducer sulforaphane inhibited Stimulator of Interferon Genes (STING) expression and type I IFN production by activating Nrf2 in cells from patients with STING-dependent interferonopathies [70]. This may be part of the mechanism by which 4-OI reduces type I IFN signaling. Other studies also found that 4-OI treatment inhibited Interferon Regulatory Factor 3 (IRF3) dimerization and activation, significantly reduced the interferon response induced by the IFN agonist RIG-I, and limited the SARS-CoV2, Herpes Simplex Virus, Vaccinia virus, and Zika virus-induced inflammatory responses [71]. And 4-OI remodeled the inflammatory macrophage proteome, suppressing type I IFN and its downstream effector IFIT2 (IFN-induced protein with tetratricopeptide repeats 2) [72]. Meanwhile, the inhibitory effect of 4-OI on IFN was attenuated in Nrf2−/− macrophages [71, 72]. These results suggest that the mechanism of action of 4-OI is actually the mutual interference of Nrf2 and IFN signaling pathways.
However, some studies have also found that itaconate may not be solely a type I IFN inhibitor. In LPS-stimulated macrophages, autocrine type I IFN-driven IL-10 contributed to the inhibition of isocitrate dehydrogenase activity and itaconate synthesis [73]. Swain et al. [17] also found that itaconate derivatives DI and 4-OI could limit LPS-stimulated macrophages’ IFN-β release, while unmodified itaconate promotes LPS-induced IFN-β production. Moreover, IRG1−/− macrophages attenuated the induction of type I IFN by LPS compared to wild-type macrophages [17]. This suggests that natural itaconate and its electrophilic derivatives are differential in regulating IFN. In conclusion, itaconate has a wide application potential as an immunomodulator of IFN, but its specific mechanism needs to be further explored.
Itaconate Inhibits Glycolysis
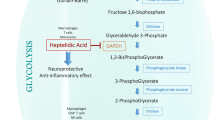
Macrophages play a key role in natural immunity and can rapidly change their morphology and state depending on the microenvironment, exhibiting extreme plasticity. In response to inflammatory stimuli, the pro-inflammatory macrophages’ glycolysis is enhanced, and oxidative phosphorylation is impaired to rapidly provide large amounts of energy, similar to the Warburg effect in tumor cells [74]. This suggests that inhibition of macrophage aerobic glycolysis is a potential target for the regulation of inflammation. As early as 2004, it was found that itaconate inhibited glycolysis in liver cells by reducing the level of fructose 2,6-bisphosphate, an activator of phosphofructokinase [75]. Recent research has shown that the itaconate derivative 4-OI inhibited the switch from oxidative phosphorylation to glycolysis in LPS-activated RAW264.7 cells by alkylating the cysteine residue 22 of the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [76]. However, GAPDH is not conserved in human, so how translatable modification by 4-OI is to human needs further research. 4-OI significantly inhibited LPS-induced IL-1β and iNOS and attenuated the inflammatory response by inhibiting macrophage glycolysis. And in a mouse model of lethal endotoxemia, 4-OI treatment significantly suppressed serum levels of inflammatory factors IL-1β and IL-6 as well as lactate and improved mouse survival [76]. These results suggest that 4-OI exerts anti-inflammatory effects by targeting GAPDH to inhibit macrophage aerobic glycolysis. Similarly, dimethyl fumarate, a derivative of fumarate, was previously reported to exert anti-inflammatory effects by modifying the cysteine of GAPDH and inhibiting glycolysis [21].
In view of the cysteine-modifying effect of itaconate, Qin et al. [77] developed a new generation of specific cysteine labeling probe 1-OH-Az. Combined with quantitative chemical proteomics techniques, the researchers successfully identified 260 itaconate-modified cysteine sites in the macrophage proteome. The analysis revealed that three key enzymes in glycolysis, fructose-bisphosphate aldolase A (ALDOA), GAPDH, and lactate dehydrogenase A (LDHA), could be modified by itaconate in RAW264.7 cells. Further experiments showed that itaconate significantly inhibited the catalytic activity of ALDOA by modifying two cysteine residues, Cys73 and Cys339, and then inhibited the glycolytic pathway in macrophages, thereby exerting anti-inflammatory activity [77]. Similarly, another study also found that both endogenous and exogenous itaconate increased the modified ALDOA levels in boar sperm, inhibited sperm glycolysis, and enhanced oxidative phosphorylation, thus maintaining the high linear motility of sperm [78]. These studies establish a negative feedback link between itaconate and glycolysis, providing new insights into the mechanisms by which itaconate regulates macrophage immune metabolism.
Antifibrotic Effect of Itaconate
Tissue fibrosis is a major cause of disability and death in many diseases, and macrophages play a key role in the development of fibrosis [79]. As an immunomodulator of macrophage function, the role of itaconate in the process of fibrosis remains unclear. Ogger et al. [80] identified itaconate as an endogenous antifibrotic factor in idiopathic pulmonary fibrosis (IPF). It was found that airway macrophages from IPF patients showed decreased IRG1 expression and itaconate levels compared with healthy subjects. In a bleomycin-induced mouse model of pulmonary fibrosis, IRG1−/− mice showed increased numbers of macrophages and neutrophils in bronchoalveolar lavage fluid and developed more severe clinical symptoms and pulmonary fibrosis [80]. Airway macrophages from IRG1−/− mice exhibited impaired metabolism and enhanced expression of profibrotic genes, whereas adoptive transfer of airway macrophages from wild-type mice into IRG1−/− mice ameliorated the bleomycin-induced pulmonary fibrosis phenotype [80]. Lung fibroblasts from IPF patients treated with exogenous itaconate impacted the metabolic phenotype and reduced both proliferation and wound healing ability, thereby limiting the severity of pulmonary fibrosis. Furthermore, treatment with inhaled itaconate in mice significantly ameliorated bleomycin-induced pulmonary fibrosis [80]. These results provide the possibility of itaconate as an antifibrotic agent, which has also aroused interest in the study of the antifibrotic mechanism of itaconate.
Fibroblast-myofibroblast differentiation (FMD) is an important cellular phenotype during the development and progression of pulmonary fibrosis. Research found that the itaconate derivative DI inhibited Thioredoxin-Interacting protein (TXNIP), which is an α-arrestin family protein that regulates the level of intracellular ROS, ameliorated FMD and pulmonary fibrosis phenotype by activating Nrf2 [81]. Consistent with this, the itaconate derivative 4-OI was also found to reduce collagen levels and inhibit the release of inflammatory factors and ROS production in systemic sclerosis dermal fibroblasts by activating Nrf2 [82]. In addition, it was found that 4-OI reduced LPS-induced signaling of p38 mitogen-activated protein kinase and promoted the expression of TGF-β, while decreased the expression of collagenase matrix metalloprotease-8. Further experiments on fibrous collagen uptake of macrophage showed that 4-OI inhibited collagen uptake by activating Nrf2, possibly promoting a stronger wound-resolving phenotype [83]. Another study found that the sodium-glucose co-transporter 2 (SGLT2) inhibitor dapagliflozin could upregulate renal cortical tissue IRG1 expression and increase itaconate levels, thereby inhibiting NLRP3 inflammasome activation and protecting the kidney from ischemia–reperfusion induced fibrosis progression [84]. In conclusion, these results suggest a regulatory relationship between itaconate or its derivatives and fibrosis, and targeting this pathway may be a viable antifibrotic therapeutic strategy.
Itaconate and M2 Macrophages
Macrophage polarization is diverse and complex. Macrophages can be divided into two typical phenotypes according to different stimulators and functions. LPS and IFN-γ stimulation can polarize macrophages into the M1 type which have pro-inflammatory effects, and IL-4 stimulation can polarize macrophages into the M2 type which have anti-inflammatory functions and promote tissue repair and regeneration [85]. Most studies have shown that itaconate inhibits the proinflammatory phenotype of M1 macrophages and limits the inflammatory response, but little is known about its role in M2 macrophages. Faas et al. [86] found that, unlike the classic IL-4-induced M2 macrophages polarization, IL-33 promoted the expression of inflammatory genes such as IL1-β, IL-6, and TNF-α in the early stage, and later triggered the delayed transition from proinflammatory phenotype to M2 macrophages phenotype. Analysis of this signaling pathway revealed that IL-33 induced metabolic reprogramming in macrophages, involving UCP2-mediated increased respiratory chain uncoupling and increased intracellular itaconate levels [86]. IL-33 strongly induced increased expression of IRG1, and IL-33-induced M2 macrophages polarization was impaired in IRG1−/− macrophages. In contrast, IL-4-induced M2 macrophages polarization was not affected in IRG1−/− macrophages. Subsequent experiments showed that itaconate mediated M2 macrophages polarization through GATA3, and GATA3 deletion accordingly abolished IL-33-induced M2 macrophages polarization and tissue repair in the muscle injury model [86]. These results suggest that the production of itaconate is essential for IL-33-induced M2 macrophages polarization. Another study found that the oxidized sodium alginate/gelatin hydrogel system loaded with 4-OI treatment promoted M2 macrophage polarization, inhibited inflammatory response, and promoted cartilage regeneration in a rat knee joint cartilage defect model [87].
However, the role of itaconate in macrophage polarization may be more complex. MicroRNA93 was discovered to suppress interferon regulatory factor-9, which in turn reduced IRG1-itaconate synthesis, induced M2 macrophages polarization in ischemic muscle, and improved angiogenesis, arteriogenesis, and perfusion recovery in experimental peripheral artery disease [88]. In this study, the reduction of itaconate allowed M2 macrophages polarization, probably because low levels of itaconate increased oxidative phosphorylation, a hallmark of M2 macrophages. Another study found that the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) was required for IL4-dependent gene expression and has been implicated in M2 macrophages. However, PPARγ deficiency resulted in enhanced IRG1 expression and increased itaconate levels [89]. These studies suggest that itaconate may be an M2 macrophages inhibitor.
A recent study identified a mechanism by which itaconate inhibits M2 macrophages polarization. Runtsch et al. [90] found that pretreatment with itaconate and its derivative 4-OI significantly inhibited IL-4-induced M2 macrophages polarization. It was shown that itaconate and 4-OI blocked M2 macrophages polarization by inhibiting the JAK1-STAT6 signaling pathway downstream of IL-4. In vitro assays of JAK1 kinase activity showed that itaconate and 4-OI were able to reduce JAK1 activity. JAK1 was directly modified by 4-OI at multiple cysteine residues by using the ITalk itaconate probe in rat JAK1-overexpressing HEK293T cells and THP-1 cells [90]. The researchers found that the expression of M2 macrophages-related genes and the phosphorylation of JAK1 and STAT6 were significantly inhibited in peritoneal exudate cells from 4-OI treated mice in the IL-4C-indued M2 macrophages activation mouse model. In the severe steroid-resistant asthma disease model established by ovalbumin and Chlamydia muridarum, 4-OI treatment also inhibited M2 macrophages polarization in alveolar lavage fluid and JAK1 phosphorylation levels in lung tissue [90]. These results suggest that itaconate acts as a JAK1 inhibitor to block M2 macrophages polarization, which may be a potential strategy to treat diseases driven by M2 macrophages such as allergic asthma. In addition, the JAK/STAT signaling pathway is important in systemic lupus erythematosus [91]. A recent study found that 4-OI inhibited JAK1 activation and played beneficial roles in reducing immune dysregulation and organ damage in murine lupus [92]. In conclusion, the mechanism of itaconate in macrophage polarization is still complex, and further profound studies are needed to better understand the immunomodulatory role of itaconate in macrophages.
Itaconate Inhibits TET2
Deletion of the IRG1 gene or treatment with exogenous itaconate or its derivatives altered LPS-induced inflammatory transcriptional features [93]. Itaconate achieves transcriptional regulation of numerous inflammatory genes through a variety of mechanisms. Recently, Chen et al. [94] reported for the first time that itaconate is a novel metabolite molecule that regulates epigenetic modification. By analyzing IRG1 expression and itaconate accumulation on global histone and DNA de/methylation in transfected HEK293T cells, it was found that TET mehtylcytosine dioxygenase 2 (TET2)-mediated 5-hydroxymethylcytosine global levels were significantly decreased [94]. TET2 is an enzyme capable of oxidizing methylated cytosine in DNA to 5-hydroxymethylcytosine, mediating DNA demethylation to regulate gene expression. Mutations and dysregulation of TET2 result in clonal hematopoiesis, altered responses to immunotherapy, atherosclerosis, and hematopoietic malignancy [95]. Transcriptome analysis revealed that TET2 was a major target for itaconate to inhibit LPS-induced genes, including genes regulated by NF-κB and STAT signaling pathways. Itaconate was able to non-covalently bind to the catalytic center of TET2 protein and inhibited the activity of the TET2 enzyme by competitive binding to α-ketoglutarate, leading to elevated DNA methylation levels and down-regulation of the expression of TET2-regulated inflammatory genes [94]. In the mouse model of endotoxemia, itaconate treatment inhibited cytokine storm, alleviated liver and lung injury, and prolonged survival. Moreover, this protective effect occurred only in wild-type mice and was not present in mice carrying inactivating mutants of TET2, providing strong evidence that TET2 was an important functional target of itaconate for anti-inflammation [94]. Itaconate has stronger inhibition and selectivity of the epigenetic modification enzyme TET2 than known carcinogenic metabolites, which will provide new ideas for the development of TET2-specific inhibitors.
Antibacterial Function of Itaconate
As early as nearly half a century ago, itaconate was discovered to have antibacterial properties. Isocitrate lyase (ICL) is a key enzyme required for glyoxylate shunting during bacterial infections, and ICL inhibition by itaconate limited the growth of Pseudomonas Indigofera which depends on ICL activity [6,7,8]. After that, many studies have shown that itaconate can effectively inhibit the growth of a variety of bacteria, such as Salmonella, Mycobacterium tuberculosis, and Legionella pneumophila [10, 93, 96, 97]. Recently, several important studies demonstrated novel mechanisms of itaconate regulating macrophage antibacterial immunity [13, 98, 99]. Rab32 is a GTPase, and BLOC3 is an exchange factor required for the function of Rab32, coordinating intracellular host defense mechanisms and limiting the replication of intracellular pathogens such as Salmonella [100]. Chen et al. found that this antibacterial mechanism required the synthesis of itaconate by IRG1 [13]. During Salmonella infection, itaconate could be transferred from mitochondria into Salmonella-containing vacuoles through organelle interactions driven by the IRG1-Rab32-BLOC3 system, thus exposing the pathogen to elevated itaconate levels and limiting the growth of Salmonella. Moreover, the researchers used a virulence defect mutant strain of Salmonella that was unable to resist the Rab32 defense mechanism and exhibited a reduced ability to replicate in wild-type macrophages. The replication-deficient phenotype was rescued in IRG1−/− macrophages, allowing the replication of the Salmonella mutant to levels almost equivalent to those of wild-type bacteria [13]. These findings highlight the importance of itaconate in the control of bacterial infections and establish a link between itaconate and the cell-autonomous defense mechanism Rab32 pathway.
Consistent with this, Schuster et al. recently further identified the lysosomal biogenesis factor transcription factor EB (TFEB) as a regulator for phago-lysosome-mitochondria crosstalk in macrophages [98]. TFEB activation in response to bacterial stimulation promoted IRG1 transcription and itaconate production. Subsequently, TFEB-driven itaconate was transferred into Salmonella-containing vacuoles via the IRG1-Rab32-BLOC3 system, restricting Salmonella proliferation [98]. Interestingly, another study found that itaconate promoted lysosome biogenesis by activating TFEB [99]. Zhang et al. found that itaconate alkylated human TFEB at cysteine 212 (Cys270 in mice), which prevented protein kinase mTOR-mediated Ser211 phosphorylation and disrupted the combination of phosphorylated TFEB and 14–3-3 regulatory proteins, resulting in TFEB translocation from the cytosol to the nucleus, activating lysosomal biogenesis and improving the ability of macrophages to defend against bacterial invasion. Furthermore, knockin mice harboring an alkylation-deficient TFEB mutant showed increased susceptibility to Salmonella typhimurium infection, and in vivo treatment of 4-OI significantly extended the survival time of Salmonella typhimurium-infected mice, indicating that itaconate-mediated TFEB alkylation is an important pathway for maintaining the body’s ability to resist bacterial infection [99]. These studies suggest a possible interaction between itaconate and TFEB that warrants further exploration. Together, itaconate plays a key role in the cooperation of mitochondria and lysosomes to resist pathogens in macrophages, implying a potential therapeutic value of itaconate in antibacterial.
CONCLUSION AND PERSPECTIVE
In recent years, itaconate has received much attention as a star molecule in macrophage immune metabolism for its potent anti-inflammatory effects. Itaconate and its derivatives modulate macrophage immune responses through multiple mechanisms and show promising therapeutic potential in a range of inflammatory diseases. However, itaconate does not always exert a beneficial side to the organism. For example, it was found that itaconate is one of the metabolites produced by proinflammatory subtypes of tumor-associated macrophages and can promote tumor development [101]. Itaconate can also competitively inhibit erythroid-specific 5-aminolevulinate synthase, the first and rate-limiting step in heme synthesis, thereby inhibiting erythropoietic heme synthesis and promoting anemia during an inflammatory response in the erythroid compartment [102]. Moreover, the regulatory mechanisms of itaconate remain complex, such as the regulation of IFN and M2 macrophages polarization, so more studies are needed to elucidate the mechanisms and expand the applicable disease models. In addition, among the various regulatory mechanisms of itaconate, which mechanism is the most important, and whether there is interaction and influence among these mechanisms, it is unclear and worth further exploration. On the other hand, most studies have used exogenous itaconate derivatives, and the results of these studies may not reflect the effects of endogenous production of itaconate. Therefore, given the differences between itaconate and its derivatives and the biosafety of exogenous supplementation, it is necessary to develop more appropriate compounds or IRG1 agonists and inhibitors to help us better understand the regulatory effects of itaconate on macrophages. In conclusion, itaconate has been identified as an important immunomodulator of macrophages. Perfecting the role of itaconate in regulating macrophage inflammatory responses will provide new insights and directions for the prevention and treatment of a variety of diseases.
AVAILABILITY OF DATA AND MATERIALS
No data and materials were collected or produced in this study.
References
Mosser, D.M., and J.P. Edwards. 2008. Exploring the full spectrum of macrophage activation. Nature Reviews Immunology. https://doi.org/10.1038/nri2448.
Saha, S., I.N. Shalova, and S.K. Biswas. 2017. Metabolic regulation of macrophage phenotype and function. Immunological Reviews. https://doi.org/10.1111/imr.12603.
Funes, S.C., M. Rios, J. Escobar-Vera, et al. 2018. Implications of macrophage polarization in autoimmunity. Immunology. https://doi.org/10.1111/imm.12910.
Mills, C.D. 2015. Anatomy of a discovery: M1 and m2 macrophages. Frontiers in Immunology. https://doi.org/10.3389/fimmu.2015.00212.
O’Neill, L.A.J., and M.N. Artyomov. 2019. Itaconate: The poster child of metabolic reprogramming in macrophage function. Nature Reviews Immunology. https://doi.org/10.1038/s41577-019-0128-5.
Williams, J.O., T.E. Roche, and B.A. McFadden. 1971. Mechanism of action of isocitrate lyase from Pseudomonas indigofera. Biochemistry. https://doi.org/10.1021/bi00784a017.
Rittenhouse, J.W., and B.A. McFadden. 1974. Inhibition of isocitrate lyase from Pseudomonas indigofera by itaconate. Archives of Biochemistry and Biophysics. https://doi.org/10.1016/0003-9861(74)90456-1.
McFadden, B.A., and S. Purohit. 1977. Itaconate, an isocitrate lyase-directed inhibitor in Pseudomonas indigofera. Journal of Bacteriology. https://doi.org/10.1128/jb.131.1.136-144.1977.
Strelko, C.L., W. Lu, F.J. Dufort, et al. 2011. Itaconic acid is a mammalian metabolite induced during macrophage activation. Journal of the American Chemical Society. https://doi.org/10.1021/ja2070889.
Michelucci, A., T. Cordes, J. Ghelfi, et al. 2013. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proceedings of the National Academy of Sciences USA. https://doi.org/10.1073/pnas.1218599110.
Cordes, T., M. Wallace, A. Michelucci, et al. 2016. Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. Journal of Biological Chemistry. https://doi.org/10.1074/jbc.M115.685792.
Lampropoulou, V., A. Sergushichev, M. Bambouskova, et al. 2016. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metabolism. https://doi.org/10.1016/j.cmet.2016.06.004.
Chen, M., H. Sun, M. Boot, et al. 2020. Itaconate is an effector of a Rab GTPase cell-autonomous host defense pathway against Salmonella. Science. https://doi.org/10.1126/science.aaz1333.
ElAzzouny, M., C.T. Tom, C.R. Evans, et al. 2017. Dimethyl itaconate is not metabolized into itaconate intracellularly. Journal of Biological Chemistry. https://doi.org/10.1074/jbc.C117.775270.
Shen, H., G.C. Campanello, D. Flicker, et al. 2017. The human knockout gene CLYBL connects itaconate to vitamin B(12). Cell. https://doi.org/10.1016/j.cell.2017.09.051.
Mills, E.L., D.G. Ryan, H.A. Prag, et al. 2018. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. https://doi.org/10.1038/nature25986.
Swain, A., M. Bambouskova, H. Kim, et al. 2020. Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nature Metabolism. https://doi.org/10.1038/s42255-020-0210-0.
He, W., A. Henne, M. Lauterbach, et al. 2022. Mesaconate is synthesized from itaconate and exerts immunomodulatory effects in macrophages. Nature Metabolism. https://doi.org/10.1038/s42255-022-00565-1.
Bambouskova, M., L. Gorvel, V. Lampropoulou, et al. 2018. Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature. https://doi.org/10.1038/s41586-018-0052-z.
Chen, F., W.A.M. Elgaher, M. Winterhoff, et al. 2022. Citraconate inhibits ACOD1 (IRG1) catalysis, reduces interferon responses and oxidative stress, and modulates inflammation and cell metabolism. Nature Metabolism. https://doi.org/10.1038/s42255-022-00577-x.
Kornberg, M.D., P. Bhargava, P.M. Kim, et al. 2018. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science. https://doi.org/10.1126/science.aan4665.
Ryan, D.G., M.P. Murphy, C. Frezza, et al. 2019. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nature Metabolism. https://doi.org/10.1038/s42255-018-0014-7.
Hadrava Vanova, K., M. Kraus, J. Neuzil, et al. 2020. Mitochondrial complex II and reactive oxygen species in disease and therapy. Redox Report. https://doi.org/10.1080/13510002.2020.1752002.
Mills, E.L., B. Kelly, A. Logan, et al. 2016. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. https://doi.org/10.1016/j.cell.2016.08.064.
Garaude, J., R. Acín-Pérez, S. Martínez-Cano, et al. 2016. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nature Immunology. https://doi.org/10.1038/ni.3509.
Chouchani, E.T., V.R. Pell, A.M. James, et al. 2016. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metabolism. https://doi.org/10.1016/j.cmet.2015.12.009.
Ackermann, W.W., and V.R. Potter. 1949. Enzyme inhibition in relation to chemotherapy. Proceedings of the Society for Experimental Biology and Medicine. https://doi.org/10.3181/00379727-72-17313.
Booth, A. N., J. Taylor, R. H. Wilson, et al. 1952. The inhibitory effects of itaconic acid in vitro and in vivo. Journal of Biological Chemistry.
Dervartanian, D. V., and C. Veeger. 1964. Studies on succinate dehydrogenase. I. Spectral properties of the purified enzyme and formation of enzyme-competitive inhibitor complexes. Biochim Biophys Acta.
Cordes, T., A. Lucas, A.S. Divakaruni, et al. 2020. Itaconate modulates tricarboxylic acid and redox metabolism to mitigate reperfusion injury. Mol Metab. https://doi.org/10.1016/j.molmet.2019.11.019.
Zhang, D., Z. Lu, Z. Zhang, et al. 2019. A likely protective effect of dimethyl itaconate on cerebral ischemia/reperfusion injury. International Immunopharmacology. https://doi.org/10.1016/j.intimp.2019.105924.
Domínguez-Andrés, J., B. Novakovic, Y. Li, et al. 2019. The itaconate pathway is a central regulatory node linking innate immune tolerance and trained immunity. Cell Metabolism. https://doi.org/10.1016/j.cmet.2018.09.003.
Medzhitov, R., D.S. Schneider, and M.P. Soares. 2012. Disease tolerance as a defense strategy. Science. https://doi.org/10.1126/science.1214935.
Netea, M.G., L.A. Joosten, E. Latz, et al. 2016. Trained immunity: A program of innate immune memory in health and disease. Science. https://doi.org/10.1126/science.aaf1098.
Battino, M., F. Giampieri, F. Pistollato, et al. 2018. Nrf2 as regulator of innate immunity: A molecular Swiss army knife! Biotechnology Advances. https://doi.org/10.1016/j.biotechadv.2017.12.012.
Itoh, K., N. Wakabayashi, Y. Katoh, et al. 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes & Development. https://doi.org/10.1101/gad.13.1.76.
Burness, C.B., and E.D. Deeks. 2014. Dimethyl fumarate: A review of its use in patients with relapsing-remitting multiple sclerosis. CNS Drugs. https://doi.org/10.1007/s40263-014-0155-5.
Yi, Z., M. Deng, M.J. Scott, et al. 2020. Immune-responsive gene 1/itaconate activates nuclear factor erythroid 2-related factor 2 in hepatocytes to protect against liver ischemia-reperfusion injury. Hepatology. https://doi.org/10.1002/hep.31147.
Li, R., W. Yang, Y. Yin, et al. 2021. 4-OI attenuates carbon tetrachloride-induced hepatic injury via regulating oxidative stress and the inflammatory response. Frontiers in Pharmacology. https://doi.org/10.3389/fphar.2021.651444.
Li, R., W. Yang, Y. Yin, et al. 2021. Protective role of 4-octyl itaconate in murine LPS/D-GalN-induced acute liver failure via inhibiting inflammation, oxidative stress, and apoptosis. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2021/9932099.
Yang, W., Y. Wang, P. Zhang, et al. 2022. Immune-responsive gene 1 protects against liver injury caused by concanavalin A via the activation Nrf2/HO-1 pathway and inhibition of ROS activation pathways. Free Radical Biology & Medicine. https://doi.org/10.1016/j.freeradbiomed.2022.02.030.
Azzimato, V., P. Chen, E. Barreby, et al. 2021. Hepatic miR-144 drives fumarase activity preventing NRF2 activation during obesity. Gastroenterology. https://doi.org/10.1053/j.gastro.2021.08.030.
Chu, X., L. Li, W. Yan, et al. 2022. 4-octyl itaconate prevents free fatty acid-induced lipid metabolism disorder through activating Nrf2-AMPK signaling pathway in hepatocytes. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2022/5180242.
Chen, X., C. Li, X. Cao, et al. 2022. Mitochondria-targeted supramolecular coordination container encapsulated with exogenous itaconate for synergistic therapy of joint inflammation. Theranostics. https://doi.org/10.7150/thno.70623.
Ni, L., Z. Lin, S. Hu, et al. 2022. Itaconate attenuates osteoarthritis by inhibiting STING/NF-κB axis in chondrocytes and promoting M2 polarization in macrophages. Biochemical Pharmacology. https://doi.org/10.1016/j.bcp.2022.114935.
Zhang, P., X. Wang, Q. Peng, et al. 2022. Four-octyl itaconate protects chondrocytes against H(2)O(2)-induced oxidative injury and attenuates osteoarthritis progression by activating Nrf2 signaling. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2022/2206167.
Zhang, Q., X. Bai, R. Wang, et al. 2022. 4-octyl Itaconate inhibits lipopolysaccharide (LPS)-induced osteoarthritis via activating Nrf2 signalling pathway. Journal of Cellular and Molecular Medicine. https://doi.org/10.1111/jcmm.17185.
Ni, L., J. Xiao, D. Zhang, et al. 2022. Immune-responsive gene 1/itaconate activates nuclear factor erythroid 2-related factor 2 in microglia to protect against spinal cord injury in mice. Cell Death & Disease. https://doi.org/10.1038/s41419-022-04592-4.
Liu, H., Y. Feng, M. Xu, et al. 2018. Four-octyl itaconate activates Keap1-Nrf2 signaling to protect neuronal cells from hydrogen peroxide. Cell Communication and Signaling: CCS. https://doi.org/10.1186/s12964-018-0294-2.
Song, H., T. Xu, X. Feng, et al. 2020. Itaconate prevents abdominal aortic aneurysm formation through inhibiting inflammation via activation of Nrf2. eBioMedicine. https://doi.org/10.1016/j.ebiom.2020.102832.
Nakkala, J.R., Y. Yao, Z. Zhai, et al. 2021. Dimethyl itaconate-loaded nanofibers rewrite macrophage polarization, reduce inflammation, and enhance repair of myocardic infarction. Small (Weinheim an der Bergstrasse, Germany). https://doi.org/10.1002/smll.202006992.
Tang, C., X. Wang, Y. Xie, et al. 2018. 4-octyl itaconate activates Nrf2 signaling to inhibit pro-inflammatory cytokine production in peripheral blood mononuclear cells of systemic lupus erythematosus patients. Cellular Physiology and Biochemistry. https://doi.org/10.1159/000495400.
Xie, Y., Z. Chen, and Z. Wu. 2022. Four-octyl itaconate attenuates UVB-induced melanocytes and keratinocytes apoptosis by Nrf2 activation-dependent ROS inhibition. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2022/9897442.
Xin, L., F. Zhou, C. Zhang, et al. 2022. Four-octyl itaconate ameliorates periodontal destruction via Nrf2-dependent antioxidant system. International Journal of Oral Science. https://doi.org/10.1038/s41368-022-00177-1.
Gu, L., J. Lin, Q. Wang, et al. 2020. Dimethyl itaconate protects against fungal keratitis by activating the Nrf2/HO-1 signaling pathway. Immunology and Cell Biology. https://doi.org/10.1111/imcb.12316.
Ding, Q., X. Jing, S. Yao, et al. 2022. Multifunctional hydrogel loaded with 4-octyl itaconate exerts antibacterial, antioxidant and angiogenic properties for diabetic wound repair. Biomater Adv. https://doi.org/10.1016/j.bioadv.2022.212979.
Swanson, K.V., M. Deng, and J.P. Ting. 2019. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nature Reviews Immunology. https://doi.org/10.1038/s41577-019-0165-0.
Hooftman, A., S. Angiari, S. Hester, et al. 2020. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metabolism. https://doi.org/10.1016/j.cmet.2020.07.016.
Bambouskova, M., L. Potuckova, T. Paulenda, et al. 2021. Itaconate confers tolerance to late NLRP3 inflammasome activation. Cell Reports. https://doi.org/10.1016/j.celrep.2021.108756.
HoyMahmoud ElAzzounyle, C., J.P. Green, S.M. Allan, et al. 2022. Itaconate and fumarate derivatives inhibit priming and activation of the canonical NLRP3 inflammasome in macrophages. Immunology. https://doi.org/10.1111/imm.13454.
Lin, J., J. Ren, B. Zhu, et al. 2022. Dimethyl itaconate attenuates CFA-induced inflammatory pain via the NLRP3/ IL-1β signaling pathway. Frontiers in Pharmacology. https://doi.org/10.3389/fphar.2022.938979.
Zhou, H., N. Li, Y. Yuan, et al. 2018. Activating transcription factor 3 in cardiovascular diseases: A potential therapeutic target. Basic Research in Cardiology. https://doi.org/10.1007/s00395-018-0698-6.
Ghosh, S., and M.S. Hayden. 2008. New regulators of NF-kappaB in inflammation. Nature Reviews Immunology. https://doi.org/10.1038/nri2423.
Kim, E.Y., H.Y. Shin, J.Y. Kim, et al. 2010. ATF3 plays a key role in Kdo2-lipid A-induced TLR4-dependent gene expression via NF-κB activation. PLoS ONE. https://doi.org/10.1371/journal.pone.0014181.
Muromoto, R., T. Hirao, K. Tawa, et al. 2016. IL-17A plays a central role in the expression of psoriasis signature genes through the induction of IκB-ζ in keratinocytes. International Immunology. https://doi.org/10.1093/intimm/dxw011.
Johansen, C., M. Mose, P. Ommen, et al. 2015. IκBζ is a key driver in the development of psoriasis. Proceedings of the National Academy of Sciences USA. https://doi.org/10.1073/pnas.1509971112.
Okuma, A., K. Hoshino, T. Ohba, et al. 2013. Enhanced apoptosis by disruption of the STAT3-IκB-ζ signaling pathway in epithelial cells induces Sjögren’s syndrome-like autoimmune disease. Immunity. https://doi.org/10.1016/j.immuni.2012.11.016.
Tsoi, L.C., S.L. Spain, E. Ellinghaus, et al. 2015. Enhanced meta-analysis and replication studies identify five new psoriasis susceptibility loci. Nature Communications. https://doi.org/10.1038/ncomms8001.
McNab, F., K. Mayer-Barber, A. Sher, et al. 2015. Type I interferons in infectious disease. Nature Reviews Immunology. https://doi.org/10.1038/nri3787.
Olagnier, D., A.M. Brandtoft, C. Gunderstofte, et al. 2018. Nrf2 negatively regulates STING indicating a link between antiviral sensing and metabolic reprogramming. Nature Communications. https://doi.org/10.1038/s41467-018-05861-7.
Olagnier, D., E. Farahani, J. Thyrsted, et al. 2020. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nature Communications. https://doi.org/10.1038/s41467-020-18764-3.
Ryan, D. G., E. V. Knatko, A. M. Casey, et al. 2022. Nrf2 activation reprograms macrophage intermediary metabolism and suppresses the type I interferon response. iScience. https://doi.org/10.1016/j.isci.2022.103827.
De Souza, D.P., A. Achuthan, M.K. Lee, et al. 2019. Autocrine IFN-I inhibits isocitrate dehydrogenase in the TCA cycle of LPS-stimulated macrophages. The Journal of Clinical Investigation. https://doi.org/10.1172/jci127597.
Russell, D.G., L. Huang, and B.C. VanderVen. 2019. Immunometabolism at the interface between macrophages and pathogens. Nature Reviews Immunology. https://doi.org/10.1038/s41577-019-0124-9.
Sakai, A., A. Kusumoto, Y. Kiso, et al. 2004. Itaconate reduces visceral fat by inhibiting fructose 2,6-bisphosphate synthesis in rat liver. Nutrition. https://doi.org/10.1016/j.nut.2004.08.007.
Liao, S.T., C. Han, D.Q. Xu, et al. 2019. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nature Communications. https://doi.org/10.1038/s41467-019-13078-5.
Qin, W., K. Qin, Y. Zhang, et al. 2019. S-glycosylation-based cysteine profiling reveals regulation of glycolysis by itaconate. Nature Chemical Biology. https://doi.org/10.1038/s41589-019-0323-5.
Zhu, Z., T. Umehara, N. Tsujita, et al. 2020. Itaconate regulates the glycolysis/pentose phosphate pathway transition to maintain boar sperm linear motility by regulating redox homeostasis. Free Radical Biology & Medicine. https://doi.org/10.1016/j.freeradbiomed.2020.07.008.
Wynn, T.A., and K.M. Vannella. 2016. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. https://doi.org/10.1016/j.immuni.2016.02.015.
Ogger, P.P., G.J. Albers, R.J. Hewitt, et al. 2020. Itaconate controls the severity of pulmonary fibrosis. Sci Immunol. https://doi.org/10.1126/sciimmunol.abc1884.
Han, Y.Y., X. Gu, C.Y. Yang, et al. 2021. Protective effect of dimethyl itaconate against fibroblast-myofibroblast differentiation during pulmonary fibrosis by inhibiting TXNIP. Journal of Cellular Physiology. https://doi.org/10.1002/jcp.30456.
Henderson, J., S. Dayalan Naidu, A.T. Dinkova-Kostova, et al. 2021. The cell-permeable derivative of the immunoregulatory metabolite itaconate, 4-octyl itaconate, is anti-fibrotic in systemic sclerosis. Cells. https://doi.org/10.3390/cells10082053.
Maassen, S., B. Coenen, M. Ioannidis, et al. 2023. Itaconate promotes a wound resolving phenotype in pro-inflammatory macrophages. Redox Biology. https://doi.org/10.1016/j.redox.2022.102591.
Ke, Q., C. Shi, Y. Lv, et al. 2022. SGLT2 inhibitor counteracts NLRP3 inflammasome via tubular metabolite itaconate in fibrosis kidney. The FASEB Journal. https://doi.org/10.1096/fj.202100909RR.
Italiani, P., and D. Boraschi. 2014. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Frontiers in Immunology. https://doi.org/10.3389/fimmu.2014.00514.
Faas, M., N. Ipseiz, J. Ackermann, et al. 2021. IL-33-induced metabolic reprogramming controls the differentiation of alternatively activated macrophages and the resolution of inflammation. Immunity. https://doi.org/10.1016/j.immuni.2021.09.010.
Xiao, H., Y. Dong, D. Wan, et al. 2023. Injectable hydrogel loaded with 4-octyl itaconate enhances cartilage regeneration by regulating macrophage polarization. Biomater Sci. https://doi.org/10.1039/d2bm01894b.
Ganta, V.C., M.H. Choi, A. Kutateladze, et al. 2017. A MicroRNA93-interferon regulatory factor-9-immunoresponsive gene-1-itaconic acid pathway modulates M2-like macrophage polarization to revascularize ischemic muscle. Circulation. https://doi.org/10.1161/circulationaha.116.025490.
Nelson, V.L., H.C.B. Nguyen, J.C. Garcìa-Cañaveras, et al. 2018. PPARγ is a nexus controlling alternative activation of macrophages via glutamine metabolism. Genes & Development. https://doi.org/10.1101/gad.312355.118.
Runtsch, M.C., S. Angiari, A. Hooftman, et al. 2022. Itaconate and itaconate derivatives target JAK1 to suppress alternative activation of macrophages. Cell Metabolism. https://doi.org/10.1016/j.cmet.2022.02.002.
Hedrich, C.M., T. Rauen, S.A. Apostolidis, et al. 2014. Stat3 promotes IL-10 expression in lupus T cells through trans-activation and chromatin remodeling. Proceedings of the National Academy of Sciences USA. https://doi.org/10.1073/pnas.1408023111.
Blanco, L.P., E. Patino-Martinez, S. Nakabo, et al. 2022. Modulation of the itaconate pathway attenuates murine lupus. Arthritis & Rhematology. https://doi.org/10.1002/art.42284.
Nair, S., J. P. Huynh, V. Lampropoulou, et al. 2018. Irg1 expression in myeloid cells prevents immunopathology during M. tuberculosis infection. Journal of Experimental Medicine. https://doi.org/10.1084/jem.20180118.
Chen, L.L., C. Morcelle, Z.L. Cheng, et al. 2022. Itaconate inhibits TET DNA dioxygenases to dampen inflammatory responses. Nature Cell Biology. https://doi.org/10.1038/s41556-022-00853-8.
Cong, B., Q. Zhang, and X. Cao. 2021. The function and regulation of TET2 in innate immunity and inflammation. Protein & Cell. https://doi.org/10.1007/s13238-020-00796-6.
Naujoks, J., C. Tabeling, B.D. Dill, et al. 2016. IFNs modify the proteome of legionella-containing vacuoles and restrict infection via IRG1-derived itaconic acid. PLoS Pathogens. https://doi.org/10.1371/journal.ppat.1005408.
Ruetz, M., G.C. Campanello, M. Purchal, et al. 2019. Itaconyl-CoA forms a stable biradical in methylmalonyl-CoA mutase and derails its activity and repair. Science. https://doi.org/10.1126/science.aay0934.
Schuster, E.M., M.W. Epple, K.M. Glaser, et al. 2022. TFEB induces mitochondrial itaconate synthesis to suppress bacterial growth in macrophages. Nature Metabolism. https://doi.org/10.1038/s42255-022-00605-w.
Zhang, Z., C. Chen, F. Yang, et al. 2022. Itaconate is a lysosomal inducer that promotes antibacterial innate immunity. Molecular Cell. https://doi.org/10.1016/j.molcel.2022.05.009.
Spanò, S., and J.E. Galán. 2012. A Rab32-dependent pathway contributes to Salmonella typhi host restriction. Science. https://doi.org/10.1126/science.1229224.
Scheurlen, K.M., A.T. Billeter, S.J. O’Brien, et al. 2020. Metabolic dysfunction and early-onset colorectal cancer - how macrophages build the bridge. Cancer Medicine. https://doi.org/10.1002/cam4.3315.
Marcero, J.R., J.E. Cox, H.A. Bergonia, et al. 2021. The immunometabolite itaconate inhibits heme synthesis and remodels cellular metabolism in erythroid precursors. Blood Advances. https://doi.org/10.1182/bloodadvances.2021004750.
ACKNOWLEDGEMENTS
We acknowledge support from the Department of Endocrinology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC), Tianshu Zeng, 82270909 and Wen Kong, 81974107.
Author information
Authors and Affiliations
Contributions
ZL for the writing, review, and editing; WZ, WK, and TZ revised the manuscript. All authors approve the final version of the manuscript and agree to be accountable for the content of the work.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Zheng, W., Kong, W. et al. Itaconate: A Potent Macrophage Immunomodulator. Inflammation 46, 1177–1191 (2023). https://doi.org/10.1007/s10753-023-01819-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01819-0