Abstract
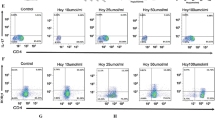
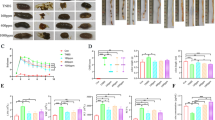
The objective was to investigate the effect of exclusive enteral nutrition (EEN) on T helper (Th) 17 cells by observing the effects of EEN on colon and serum interleukin (IL)-17A levels in juvenile inflammatory bowel disease (IBD) rat models and to reveal the potential mechanism of the therapeutic effect of EEN on IBD. ATNBS-induced IBD rat model was established. Feeding Peptison, a type of enteric nutrition (EN) for EEN-IBD group and EEN group, normal feed for IBD model group and control group for six consecutive days. Four groups of juvenile rats were sacrificed on day 7. The pathology of the intestinal mucosa was examined, the expression of IL-17A in serum was detected by ELISA, and the expression of IL-17A in intestinal tissue was detected by both western blot and real-time PCR (RT-PCR). Diarrhea, bloody stools, and weight loss were found in both the IBD group and the EEN-IBD group. After 5 days of EEN feeding, the stool characteristics, and blood in the stools of the rats in the EEN-IBD group were significantly relieved compared with those of the IBD group. There was no significant difference in the body mass growth rate between the IBD group and EEN-IBD group (P > 0.05). The growth rate of the EEN group was 51.29 ± 3.61%, which was significantly lower than that of the control group (60.17 ± 9.32%) with P < 0.05. The disease activity index (DAI) score of the EEN-IBD group was significantly lower than that of the IBD group (P < 0.05). In the IBD group, colonic congestion and edema were obvious, scattered ulcers were observed, and the intestinal mucosa had a large amount of inflammatory cell infiltration. In the EEN-IBD group, the intestinal mucosa was slightly congested and a small amount of inflammatory cell infiltrated. The serum IL-17A expression level in the IBD group was significantly higher than in the EEN-IBD group, control group, and EEN group (P < 0.05). Both the gene and protein expressions of IL-17A in the intestinal tissue of the EEN-IBD group were significantly lower than in the IBD group (P < 0.01), and it was significantly higher in the IBD group than in the control and EEN groups (P < 0.01). EEN effectively reduced the intestinal inflammation in the juvenile rats with IBD. The mechanism could be related to the regulation of Th17 cells and the expression of the corresponding cytokine, IL-17A. EEN may play a role in downregulating the expression of IL-17A in the intestinal mucosa.





Similar content being viewed by others
REFERENCES
Meijer, B., C.J. Mulder, G.J. Peters, A.A. van Bodegraven, and N.K. de Boer. 2016. Efficacy of thioguanine treatment in inflammatory bowel disease: a systematic review. World Journal of Gastroenterology 22: 9012–9021.
Gasparetto, Marco, and Graziella Guariso. 2013. Highlights in IBD epidemiology and its natural history in the paediatric age. Gastroenterology Research and Practice 2013: 829040.
Wang, X.Q., Y. Zhang, C.D. Xu, L.R. Jiang, Y. Huang, H.M. Du, and X.J. Wang. 2013. Inflammatory bowel disease in Chinese children: a multicenter analysis over a decade from Shanghai. Inflammatory Bowel Diseases 19: 423–428.
Ramos, G.P., and K.A. Papadakis. 2019. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clinic Proceedings 94: 155–165.
Globig, A.M., N. Hennecke, B. Martin, M. Seidl, G. Ruf, P. Hasselblatt, R. Thimme, and B. Bengsch. 2014. Comprehensive intestinal T helper cell profiling reveals specific accumulation of IFN-γ+IL-17+ coproducing CD4+ T cells in active inflammatory bowel disease. Inflammatory Bowel Diseases 20: 2321–2329.
Brand, S. 2009. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17cells in the pathogenesis of Crohn’s disease. Gut 58: 1152–1167.
Tani, S., R. Takano, S. Tamura, S. Oishi, M. Iwaizumi, Y. Hamaya, K. Takagaki, T. Nagata, S. Seto, T. Horii, I. Kosugi, T. Iwashita, S. Osawa, T. Furuta, H. Miyajima, and K. Sugimoto. 2017. Digoxin attenuates murine experimental colitis by downregulating Th17-related cytokines. Inflammatory Bowel Diseases 23: 728–738.
Guariso, G., M. Gasparetto, L. Visona Dalla Pozza, R. D’Incà, L. Zancan, G. Sturniolo, F. Brotto, and P. Facchin. 2010. Inflammatory bowel disease developing in paediatric and adult age. Journal of Pediatric Gastroenterology and Nutrition 5l (6): 698–707.
Hamamoto, N., K. Maemura, I. Hirata, M. Murano, S. Sasaki, and K. Katsu. 1999. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules (endothelial leucocyte adhesion molecule-1(ELAM- 1) or intercellular adhesion molecule-1(ICAM-1)). Clinical and Experimental Immunology 117: 462–468.
Hundorfean, G., M.F. Neurath, and J. Mudter. 2012. Functional relevance of T helper 17 (Th17) cells and the IL-17cytokine family in inflammatory bowel disease. Inflammatory Bowel Diseases 18: 180–186.
van den Brink, G., L. Stapersma, L.E. Vlug, D. Rizopolous, A.G. Bodelier, H. van Wering, P.C.W.M. Hurkmans, R.J.L. Stuyt, D.M. Hendriks, J.A.T. van der Burg, E.M.W.J. Utens, and J.C. Escher. 2018. Clinical disease activity is associated with anxiety and depressive symptoms in adolescents and young adults with inflammatory bowel disease. Alimentary Pharmacology & Therapeutics 48: 358–369.
Neurath, M.F., and S.P. Travis. 2012. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 61: 1619–1635.
Michail, S., M. Ramsy, and E. Soliman. 2012. Advances in inflammatory bowel diseases in children. Minerva Pediatrica 64 (3): 257–270.
Chinese Society of Gastroenterology inflammatory bowel disease group. 2015. Consensus among nutritional support therapists for inflammatory bowel disease (2013·Shenzhen). Chinese Journal of Gastrointestinal Surgery 20: 97–105.
Yu, Y., K.C. Chen, and J. Chen. 2019. Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn’s disease: a meta-analysis. World Journal of Pediatrics 15: 26–36.
Chinese Society for Parenteral and Enteral Nutrition, Chinese Society of Gastroenterology inflammatory bowel disease group. 2013. Consensus on nutritional support therapy for inflammatory bowel disease. Chinese Journal of Integrative Medicine 52: 1082–1087.
Ungaro, R., S. Mehandru, P.B. Allen, L. Peyrin-Biroulet, and J.F. Colombel. 2017. Ulcerative colitis. Lance 389: 1756–1770.
Kmieć, Z., M. Cyman, and T.J. Ślebioda. 2017. Cells of the innate and adaptive immunity and their interactions in inflammatory bowel disease. Advances in Medical Sciences 62: 1–16.
Khan, D., and Ahmed S. Ansar. 2015. Regulation of IL-17 in autoimmune diseases by transcriptional factors and microRNAs. Frontiers in Genetics 6: 236.
Fujino, S., A. Andoh, S. Bamba, A. Ogawa, K. Hata, Y. Araki, T. Bamba, and Y. Fujiyama. 2003. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52: 65–70.
Li, L., Q.G. Shi, F. Lin, Y.G. Liang, L.J. Sun, J.S. Mu, Y.G. Wang, H.B. Su, B. Xu, C.C. Ji, et al. 2014. Cytokine IL-6 is required in Citrobacter rodentium infection induced intestinal Th17 responses and promotes IL-22 expression in inflammatory bowel disease. Molecular Medicine Reports 9: 831–836.
Rubio, A., B. Pigneur, H. Gamier-Lengling, C. Talbotec, J. Schmitz, D. Canioni, O. Goulet, and F.M. Ruemmele. 2011. The efficacy of exclusive nutritional therapy in paediatric Crohn’s disease, comparing fractionated oral vs. continuous enteral feeding. Alimentary Pharmacology & Therapeutics 33: 1332–1339.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Teng, X., Qi, Y., Li, J. et al. Effect of Exclusive Enteral Nutrition on Th17 Cells in Juvenile Rats with Inflammatory Bowel Disease. Inflammation 44, 261–269 (2021). https://doi.org/10.1007/s10753-020-01328-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01328-4