Abstract
We investigated the individual and combined impacts of manipulation of submerged macrophytes, large-bodied cladocerans, and nutrients on plankton communities in a tropical hypereutrophic shallow reservoir. We tested how the addition of the macrophyte Ceratophyllum demersum, the cladoceran Sarsilatona serricauda, and nutrients affected phytoplankton and zooplankton diversity, composition, and structure using mesocosms and a factorial design (3 × 3) with eight treatments. During the experiment, the reservoir experienced an intense bloom of algae (207 mg l−1 of biomass), mainly composed of cyanobacteria (> 98%). The submerged macrophytes were found to significantly reduce the biomass of cyanobacteria (by 85%), diatoms (80%), and green algae (78%), while the addition of zooplankton and nutrients led to a 96% reduction for diatoms. While both submerged macrophytes and the added cladocerans impacted the native zooplankton community, the macrophytes exerted stronger effects on phytoplankton and zooplankton diversity, composition, and structure. Intriguingly, nutrient addition did not alter the main effects of macrophytes and large cladocerans. Our findings reveal the positive potential of introducing submerged macrophytes in tropical shallow lakes, even at a low to moderate percentage of the volume inhabited, to control toxic cyanobacterial blooms. Under our experimental conditions, the method was effective even without extra zooplankton grazing and at increased nutrient input.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Harmful algal blooms have become a threat to freshwater biodiversity as they reduce water quality and ecosystem functioning and services (Reid et al., 2019; Amorim & Moura, 2021). Freshwater phytoplankton blooms are often composed of cyanobacteria that can release cyanotoxins and jeopardize aquatic communities and harm humans (Olokotum et al., 2020). Nutrient input is the most important factor triggering algal blooms, which are favored under eutrophic conditions (Griffith & Gobler, 2020). Consequently, many countries have adopted restoration measures to reduce eutrophication and algal blooms (Jeppesen et al., 2017).
An important restoration technique is biomanipulation, which involves modification of important structuring components of aquatic communities (Shapiro et al., 1975; Lammens et al., 1990; Meerhoff & Beklioğlu, 2024). The most successful cases of biomanipulation, in the form of fish removal, come from the temperate region, showing efficient reduction of nutrient concentrations, turbidity, and phytoplankton biomass (e.g., Søndergaard et al., 2008; Ha et al., 2013; Ekvall et al., 2014; Geletu, 2023). The success of this technique usually depends on the recovery of submerged vegetation, which stabilizes the clear state of the lakes (Hilt et al., 2018; Gao & Hu, 2023).
The efficiency of biomanipulation, along with the effects of top-down and bottom-up forces on phytoplankton, depends on nutrient levels (Jeppesen et al., 1997) and their critical thresholds that control the shift from turbid- to clear-water states (Scheffer et al., 1993). For instance, top-down control of phytoplankton is unlikely in (hyper)eutrophic deep lakes even after biomanipulation as phytoplankton can be inedible and phosphorus reduction may not cause nutrient limitation (Benndorf et al., 2002). In shallow lakes, the zooplankton grazing pressure is also diminished at high phosphorus concentrations in the absence of submerged macrophytes (Jeppesen et al., 1997). Stronger eutrophication might impair submerged macrophyte growth, thereby reducing the bottom-up control of phytoplankton (Søndergaard & Moss, 1998). Furthermore, nutrient levels affect the sensitivity of phytoplankton to macrophytes (Hilt & Gross, 2008) as allelopathic effects are stronger under nutrient limitation (Reigosa et al., 1999; Sim et al., 2024).
For tropical regions, the efficiency of biomanipulation is not yet fully elucidated. Some characteristics of warmer regions may impair the success of fish removal, such as dominance of fast-growing omnivorous fish, lower densities of piscivores (Jeppesen et al., 2012; Meerhoff & Beklioğlu, 2024), and predominance of small zooplankton (Jeppesen et al., 2007). Warmer conditions can also decrease the energy transfer efficiency and biomass of plankton communities, thereby affecting the structure and functioning of lake ecosystems (Barneche et al., 2021). Consequently, even a tenfold increase in zooplankton community or addition of the large cladoceran Daphnia did not control algal blooms in subtropical lakes (Lacerot et al., 2013). Similarly, stocking of piscivorous fish in subtropical lakes did not increase zooplankton grazing on phytoplankton importantly due to sustained high abundances of small zooplanktivorous fish (Liu et al., 2018; Rao et al., 2023), which in some cases may lead to increased turbidity, nutrient levels, and chlorophyll-a concentrations due to sediment disturbance (He et al., 2022) and increased amounts of fish excrements. These findings highlight the challenges of managing phytoplankton blooms in warmer lakes through enhanced zooplankton grazing and the need for more effective strategies.
Despite the low efficiency of zooplankton grazing, some studies have demonstrated that introduction of submerged macrophytes can decrease phytoplankton biomass in subtropical (e.g., Vanderstukken et al., 2011; Dong et al., 2014) and tropical lakes (e.g., Liu et al., 2018; Amorim & Moura, 2020; Portilla et al., 2023; Rao et al., 2023). Submerged macrophytes can inhibit the phytoplankton biomass through the reduction of light and nutrient availability, secretion of allelopathic substances, provision of refuges for zooplankton against fish predation, at least in temperate lakes (Meerhoff et al., 2007), and reduction of sediment resuspension (Scheffer et al., 1993; Søndergaard & Moss, 1998). Among these mechanisms, allelopathy has a strong influence on phytoplankton structure because of the different sensibilities of the main algal groups (Hilt & Gross, 2008; Amorim et al., 2019).
Although the combined influence of submerged macrophytes and large zooplankton may suppress the phytoplankton biomass (e.g., Ha et al., 2013; Rao et al., 2023), the introduction of a different species may have substantial effects on the diversity and dominance patterns of native phytoplankton and zooplankton communities (Rohwer et al., 2023). For submerged macrophytes, the effects of the introduction are dependent on the coverage (Ferreira et al., 2018). Similarly, the introduction of a non-native zooplankton can reduce the abundance of native species through competition, leading to changes in diversity and food-web interactions (Rohwer et al., 2023). Therefore, besides evaluating the efficiency of the introduction of non-native species on the control of eutrophication and algal blooms, it is crucial to elucidate their impacts on native planktonic communities.
In this study, we experimentally evaluated the isolated and combined effects of the introduction of a submerged macrophyte, a large cladoceran, and nutrients on phytoplankton and zooplankton biomass, diversity, composition and structure in a tropical shallow reservoir with cyanobacterial blooms. We tested the following three main hypotheses: (i) submerged macrophytes effectively suppress prokaryotic algae (cyanobacteria), whereas large cladocerans are effective at reducing the biomass of edible eukaryotic algae (green algae, diatoms, and flagellates) but not cyanobacteria due to their low edibility; (ii) input of nutrients alters the response of phytoplankton and zooplankton groups to the addition of submerged macrophytes and non-native large cladocerans; (iii) presence of submerged macrophytes drives the diversity, composition, and structure of native communities of phytoplankton and zooplankton.
Materials and methods
Study site and experimental design
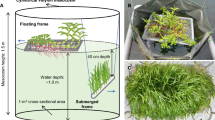
We performed a mesocosm experiment in the Tapacurá reservoir, Pernambuco, Brazil (8º2′36"S, 35º11′52"W), from February 8th to February 18th, 2019. This reservoir is located at the Ecological Station of Tapacurá, an Atlantic Rain Forest fragment, and has a water storage capacity of 105 × 106 m3 (APAC, 2019). During the experiment, Tapacurá only reached 26% of its maximum storage capacity and had a mean depth of 2 m. Although the reservoir is used for multiple purposes, including water supply, fishing, agricultural irrigation, livestock, and transportation, it is hypereutrophic and exposed to multiple stressors, such as pollution, habitat degradation, and agriculture. The reservoir has large stands of the floating macrophyte Eichhornia crassipes (Mart.) Solms and annual blooms of Microcystis spp. Rotifers and cyclopoid copepods dominate the zooplankton community, while the fish assemblage is composed of small zooplanktivorous and omnivorous species (Amorim & Moura, 2020).
A total of 32 mesocosms, constructed of PVC tubes and transparent polyethylene bags (1.25 m depth and 0.32 m width), were kept open to the atmosphere and closed at the bottom. The mesocosms were fixed to two trees in the limnetic region of the reservoir, and their buoyancy was maintained using PET bottles. Each mesocosm was filled with 100 l non-processed water from the reservoir, ensuring the same initial conditions for all mesocosms. We designated eight treatments (each with four replicates): C—control; M—macrophytes; Z—zooplankton; MZ—macrophytes + zooplankton; N—nutrients; MN—macrophytes + nutrients; ZN—zooplankton + nutrients; and MZN—macrophytes + zooplankton + nutrients. The submerged macrophyte Ceratophyllum demersum L. and the large cladoceran Sarsilatona serricauda (Sars, 1901) (mean body size > 1 mm) were added to the treatments with macrophytes and zooplankton, respectively. These organisms were collected from the Carpina reservoir, another hypereutrophic system located in the same watershed (22 km from Tapacurá reservoir) (Amorim et al., 2020). We selected these organisms due to the known allelopathic activity of C. demersum, the large size of S. serricauda, and their availability in nearby reservoirs, allowing transportation within the same day.
The submerged macrophytes were washed several times with tap and distilled water and then introduced into the mesocosms with a biomass of 3.5 g l−1 (wet weight) based on the mean biomass in the Carpina reservoir, corresponding to a plant percentage volume inhabited (PVI) of 28%. For the sampling of large cladocerans, 200 l water from the Carpina reservoir was filtered with a 320-µm mesh-opening plankton net, which allowed selection of only large organisms. The mean zooplankton biomass added to each mesocosm was 85 µg l−1 (± 10.4), composed of 92% S. serricauda and 8% Notodiaptomus cearensis (Wright, 1936), a large calanoid copepod (> 1 mm) also present in Tapacurá reservoir. In the treatments with nutrient additions, we added 0.4 and 0.5 mg l−1 of ammonium (NH4+) and phosphate (PO43−), respectively, weighted from ammonium chloride (NH4Cl) and potassium phosphate (KH2PO4) and diluted in 200 ml autoclaved distilled water. Details on the nutrient concentrations during the experiment are described in Amorim & Moura (2020). Briefly, the addition of nutrients led to an increase in ammonia (NH4: 66 µg l−1 in C and 180 µg l−1 in N), dissolved inorganic nitrogen (DIN: 86 µg l−1 in C and 194 µg l−1 in N), orthophosphate (PO4: 225 µg l−1 in C and 443 µg l−1 in N), total dissolved phosphorus (TDP: 560 µg l−1 in C and 736 µg l−1 in N), and total phosphorus (TP: 900 µg l−1 in C and 1080 µg l−1 in N) concentrations after 10 days in the N treatment. Low chlorophyll-a to DIN or TDP ratios indicate that there was no nutrient limitation.
Sampling and analyses
Phytoplankton analyses were performed based on depth-integrated samples collected with a 1-m long PVC tube at different points of the mesocosms on days 0 and 10 and then preserved with 1% acetic Lugol. Species identification was performed using a light microscope (400 × and 1000 × magnifications) and specialized literature. The density of each species was analyzed in sedimentation chambers in an inverted microscope (Utermöhl, 1958). Phytoplankton biomass (mg l−1) was determined using the geometric formulas from Hillebrand et al. (1999). The biomass was divided into cyanobacteria, green algae, diatoms, and phytoflagellates (Cryptophyta, Dinophyta, and Euglenophyta).
Zooplankton were analyzed in samples collected on days 0 and 10 by filtering the water of the mesocosms (100 l) in a 60-µm mesh-opening plankton net, followed by fixing with 4% formalin. Three subsamples per sample were analyzed in a 1 ml Sedgewick-Rafter chamber in a light microscope for species identification and quantification. Species were identified following specialized literature. Regressions between dry weight (µg l−1), length, and width of the organisms were used to estimate the biomasses of the rotifers (Ruttner-Kolisko, 1977), cladocerans, and copepods (Dumont et al., 1975).
Data analyses
Species with relative biomasses greater than 50% were considered dominant and those with relative biomasses greater than 5% were considered abundant. Phytoplankton and zooplankton species richness (S) was defined as the number of species identified per sample. Shannon diversity (H') and Pielou evenness (J' = H'/log(S)) indexes were calculated using the vegan package of the R software (Oksanen et al., 2022).
A three-way ANOVA was used to verify significant differences between the phytoplankton and zooplankton biomass, species richness, diversity, and evenness on day 10 based on the factors macrophytes, zooplankton, and nutrients and possible interactions between them (macrophytes + zooplankton, macrophytes + nutrients, zooplankton + nutrients, and macrophytes + zooplankton + nutrients). The normality and homoscedasticity of the data were tested by Kolmogorov–Smirnov and Bartlett tests, respectively, in addition to Q-Q and residuals vs. fitted plots. When those premises were not met, we applied a variance-stabilizing transformation or tested the data using Generalized Linear Models test instead of ANOVA. PERMANOVA analyses were used to test for significant differences in phytoplankton and zooplankton composition (presence and absence matrix) and structure (biomass matrix) between treatments on day 10. Then, the data were graphically represented in non-parametric multidimensional scaling plots (NMDS) in the vegan package of the R software. All statistical analyses were performed in R (version 4.0.5) with a significance level of P < 0.05 (R Core Team, 2024).
Results
Phytoplankton
In total, 57 phytoplankton taxa were identified, composed of 24 species of cyanobacteria and 33 eukaryotic algae (21 green algae, six diatoms, and six phytoflagellates). The initial phytoplankton biomass in the control treatment was 207 mg l−1 (standard error ± 7.8), which increased to 297 mg l−1 (standard error ± 12.5) on day 10. Cyanobacteria always constituted more than 98% of the phytoplankton biomass, and Microcystis panniformis Komárek et al. dominated most of the samples (> 50%). After cyanobacteria, the second most abundant group was diatoms, followed by green algae and phytoflagellates whose relative biomasses were always lower than 2% (Fig. 1).
Boxplots showing the biomass of cyanobacteria (a), green algae (b), diatoms (c), and phytoflagellates (d) on days 0 and 10 of the experiment in the control (C), macrophyte (M), zooplankton (Z), and nutrient (N) treatments and their possible interactions. Different letters represent significant differences between the treatments (P < 0.05). Boxplots show the median (black line), 25th and 75th interquartile range (box), minimum and maximum values (whiskers), and outliers (dots)
Cyanobacteria biomass was influenced by the factors macrophytes, macrophytes + zooplankton, and zooplankton + nutrients. Higher inhibition of the cyanobacteria biomass was observed in the M (85%), MN (81%), MZN (67%), and MZ (54%) treatments. For the treatments with nutrient addition, there was a significant reduction in the cyanobacteria biomass in the ZN (26%) compared to the N treatment (Table S1; Fig. 1a). For green algae, only the factor macrophytes exhibited significant effects, with a 78% reduction of the biomass in treatment M (Table S1; Fig. 1b). The biomass of diatoms was influenced by the factors macrophytes, zooplankton, nutrients, macrophytes + zooplankton, and macrophytes + nutrients. Diatom biomass was reduced by 96% in the ZN treatment, followed by MZ (89%), MN (87%), and M (80%) (Table S1; Fig. 1c). The factors zooplankton and macrophytes + zooplankton significantly influenced the phytoflagellate biomass, but they did not differ from the control. However, in the set of mesocosms with higher eutrophication, there was a significant reduction in phytoflagellate biomass in the ZN treatment compared to N (Table S1; Fig. 1d).
Zooplankton
In total, 19 native zooplankton taxa were identified, consisting mainly of rotifers (13 spp.), copepods (3 spp.), and cladocerans (3 spp.). The initial zooplankton biomass in the control treatment was 179 µg l−1 (standard error ± 9.8), decreasing to 147 µg l−1 (standard error ± 18.1) on day 10. Copepods had the highest biomasses in most of the treatments, with the cyclopoid Thermocyclops decipiens being the dominant species in Z (96%), C (94%), N (90%), ZN (69%), MZ (55%), and MZN (52%), followed by copepod nauplii and Notodiaptomus cearensis. All the macrophyte treatments had high biomasses of rotifers, which became dominant in M (74%), MN (56%), MZN (47%), and MZ (45%), represented mainly by Lepadella patella as the dominant species, followed by Lecane bulla and Brachionus calyciflorus Pallas, 1766. Among the cladocerans, Diaphanosoma spinulosum Herbst, 1975 showed the highest abundances in the C and ZN treatments (Fig. 2).
Boxplots showing the biomass of the native zooplankton groups Rotifera (a), Cladocera (b), and Copepoda (c) besides the introduced Sarsilatona serricauda (d) on days 0 and 10 of the experiment in the control (C), macrophyte (M), zooplankton (Z), and nutrient (N) treatments and their possible interactions. Different letters represent significant differences between the treatments (P < 0.05). Boxplots show the median (black line), 25th and 75th interquartile range (box), minimum and maximum values (whiskers), and outliers (dots)
The biomass of rotifers was significantly influenced by the factors macrophytes, macrophytes + added zooplankton, and macrophytes + added zooplankton + nutrients. A remarkable increase in the biomass of rotifers compared to the control (2 µg l−1) was observed in all the macrophytes treatments: M (12,147% or 236 µg l−1), MN (8692%), MZN (8510%), and MZ (4131%), differing from the control in the M, MN, and MZN treatments (Table S2; Fig. 2a). As for the biomass of copepods, there were significant effects of the factors macrophytes, nutrients, and macrophytes + added zooplankton. However, only the Z and ZN treatments differed from the MZN (Table S2; Fig. 2b). Cladocerans were affected by all factors and interactions, with all treatments showing significantly lower biomasses than the control. Cladocerans completely disappeared (reduction of 100%) in the M, MZ, and MN treatments, followed by reductions in the Z (90%), N (85%), MZN (84%), and ZN (76%) (Table S2; Fig. 2c). The biomass of the introduced S. serricauda declined by 81.1% in treatment Z from day 0 to day 10; in the other treatments S. serricauda showed a reduction of only 12–23% (Table S2; Fig. 2d).
Phytoplankton and zooplankton diversity, composition, and structure
Only the factor macrophytes influenced phytoplankton species richness, while macrophytes, macrophytes + added zooplankton, and macrophytes + nutrients impacted phytoplankton diversity and evenness. Moreover, there was a significant effect of nutrients on the Pielou evenness. None of the treatments showed significant differences from the control for species richness, while Shannon diversity and Pielou evenness were significantly lower in M, MZ, MN, ZN, and MZN than in the control (Table S1; Fig. 3a–c).
Boxplots showing the species richness, Shannon diversity index, and Pielou evenness of phytoplankton (a–c) and zooplankton (d–f) in the control (C), macrophyte (M), zooplankton (Z), and nutrient (N) treatments and their possible interactions on days 0 and 10 of the experiment. Different letters represent significant differences between the treatments (P < 0.05). Boxplots show the median (black line), 25th and 75th interquartile range (box), minimum and maximum values (whiskers), and outliers (dots)
Native zooplankton species richness was significantly influenced by the factors macrophytes, added zooplankton, nutrients, macrophytes + added zooplankton, macrophytes + nutrients, and added zooplankton + nutrients, with all treatments showing lower species richness than the control. Shannon diversity was affected by the factors added zooplankton, nutrients, macrophytes + nutrients, and added zooplankton + nutrients, with reductions in the diversity in the Z, N, and ZN treatments, while the macrophyte treatments did not differ from the control. Moreover, Pielou evenness was affected by macrophytes and macrophytes + added zooplankton + nutrients. Although none of the treatments differed from the control, MZ, MN, and MZN had higher evenness than Z and N (Table S2; Fig. 3d–f).
The presence of submerged macrophytes was the most important factor driving the composition and structure of phytoplankton and native zooplankton, and the differences between treatments with or without macrophytes were always highly significant (PERMANOVA: P < 0.001). The phytoplankton composition and structure were also influenced by the factors macrophytes + nutrients and macrophytes + added zooplankton, respectively. Besides macrophytes, the native zooplankton structure was also significantly affected by the factors nutrients, macrophytes + added zooplankton, and added zooplankton + nutrients (Table S3, Fig. 4). Cyanobacteria were typically more abundant in the treatments without macrophytes (Fig. 4b). In the zooplankton community, copepods and cladocerans were abundant in the treatments without macrophytes, whereas rotifers were more abundant in the macrophyte treatments (Fig. 4d).
Non-parametric multidimensional scaling plots (NMDS) showing the composition (a) and structure (b) of phytoplankton and the composition (c) and structure (d) of zooplankton in the control (C), macrophyte (M), zooplankton (Z), and nutrient (N) treatments and their possible interactions on day 0 (D0) and day 10 of the experiment. The shapes cluster the samples from day 0 and the treatments with or without macrophytes. Phytoplankton species with relative biomass > 5%: Miae: Microcystis aeruginosa (Kützing) Kützing; Mipa: Microcystis panniformis Komárek, Komárková-Legnerová, Sant'Anna, M.T.P.Azevedo, & P.A.C.Senna; Mipr: Microcystis protocystis W.B.Crow; Misp: Microcystis sp. (isolated cells); Rara: Raphidiopsis raciborskii (Woloszynska) Aguilera & al.: Spbr: Sphaerocavum brasiliense De Azevedo & C.L.Sant' Anna; Spap: Sphaerospermopsis aphanizomenoides (Forti) Zapomelová & al.; Woka: Woronichinia karelica Komárek & Komárková-Legnerová. Zooplankton species with relative biomass > 5%: Brca: Brachionus calyciflorus Pallas, 1766; Lebu: Lecane bulla Lepa: Lepadella patella Naup: Nauplii; Noce: Notodiaptomus cearensis (Wright, 1936); Thde: Thermocyclops decipiens Disp: Diaphanosoma spinulosum Herbst, 1975
Discussion
The introduction of the submerged macrophyte Ceratophyllum demersum and the large-bodied cladoceran Sarsilatona serricauda caused significant changes in the composition and structure of the native phytoplankton and zooplankton. The addition of C. demersum led to major changes in phytoplankton, inhibiting cyanobacteria, green algae, and diatoms, while for zooplankton, it led to an increase in rotifer biomass.
The first hypothesis was partially confirmed: Although the added cladocerans grazed upon eukaryotic algae, submerged macrophytes inhibited not only cyanobacteria (reduction of 85%) but also diatoms and green algae. The release of allelopathic substances seems to be the most plausible reason for the cyanobacterial inhibition (see Amorim & Moura, 2020 for detailed explanations). The inhibition of cyanobacteria and diatoms was expected due to their sensitivity to macrophyte allelochemicals (Hilt & Gross, 2008). Although green algae are known to be less affected (e.g., Amorim et al., 2019; Souza et al., 2021), they are considered nutritious food for zooplankton (Wilson et al., 2006) and could, in part, be grazed upon by the rotifers in the macrophyte treatments. Regarding diatoms, it is unclear if they were inhibited by the macrophytes or grazed upon by rotifers as small-sized grazable diatoms were abundant.
The introduction of the large cladoceran, S. serricauda, had a smaller effect on cyanobacteria biomass than the effects of the submerged macrophytes. Besides being less edible, cyanobacteria also possess adaptive strategies to avoid zooplankton grazing, such as large size, toxicity, and poor nutritional quality (Moustaka-Gouni & Sommer, 2020). The addition of S. serricauda, however, decreased the biomass of diatoms and phytoflagellates in the ZN treatment compared to N, both groups being susceptible to zooplankton grazing. The selectivity of zooplankton for these small-sized phytoplankton may also have stimulated cyanobacterial growth through the exclusion of their main competitors (Ger et al., 2019). The decline in the biomass of the introduced S. serricauda, however, reflects its poor capacity to coexist with dense cyanobacterial blooms. Reduced grazing effects of large-bodied cladocerans on phytoplankton have been found also in other tropical and subtropical lakes (e.g., Lacerot et al., 2013; Portilla et al., 2023).
The effects of combined addition of macrophytes and zooplankton, or of macrophytes and nutrients, did not differ from those of the macrophyte-only treatment, emphasizing the crucial role of submerged plants in structuring phytoplankton communities in shallow lakes. Similarly, Liu et al. (2018) observed that restoration of submerged macrophytes was more important than top-down effects of fish manipulation for phytoplankton abundance in a tropical shallow lake. A follow-up study in the same restored lake (Rao et al., 2023) showed a higher proportion of large herbivorous calanoid copepods, though in low abundances, in macrophyte basins, while basins with higher algal biomass did not experience changes in their zooplankton community, maintaining higher abundances of omnivorous cyclopoid copepods.
To better understand the effects of macrophytes on phytoplankton structure, it is important to consider the number of added plants and their percentage of volume inhabited (PVI). A macrophyte PVI > 15% has been shown to be enough to decrease phytoplankton biomass and thus the potentially harmful cyanobacteria in tropical lakes (Portilla et al., 2023). In temperate lakes, a macrophyte PVI of 15–20% can boost zooplankton abundance, consequently reducing phytoplankton biovolume and increasing water transparency, provided that planktivorous fish density is low (Schriver et al., 1995). In our study (PVI of 28%), macrophytes did not change the relative proportion of phytoplankton groups, perhaps due to the short duration of the experiment. Longer periods of coexistence of submerged macrophytes and the natural phytoplankton might result in a shift from cyanobacterial to green algae dominance as observed in other studies from subtropical lakes with similar macrophyte PVIs (35% or 3.3 g l−1) (e.g., Vanderstukken et al., 2011).
Different from what we expected (hypothesis 2), higher nutrient input did not affect the main response of phytoplankton and zooplankton groups to the additions of submerged macrophytes and added large cladocerans. The weak effect of nutrients can be attributed to the overall high nutrient concentrations in both the control and the treatments, as also emphasized in the low chlorophyll-a to DIN or TDP ratios in all treatments (see Materials and Methods as well as Amorim & Moura, 2020).
Despite the low effect of the isolated additions of the large cladocerans in the Z-only treatment, they lowered the cyanobacterial biomass when combined with nutrients in the ZN treatment. Shurin et al. (2012) and Kratina et al. (2012) found that eutrophication increased zooplankton biomass and size, which consequently favored the grazing on phytoplankton in the absence of fish predation, as in our experiment. Large cladocerans, however, are unable to reduce the cyanobacteria biomass in the blooming phase, even in absence of fish (Gliwicz, 1990), as also shown in the present study, and they may completely disappear when the blooms become dense (Gliwicz, 1990). This is critical for warmer regions where the classical biomanipulation model, through trophic cascades, already has serious limitations due to high fish predation on zooplankton and the fact that cyanobacteria thrive under eutrophic conditions (Jeppesen et al., 2012). Moreover, the introduction of S. serricauda apparently had negative effects on the abundance of copepods and cladocerans (though only marginally significant for copepods), likely due to food shortage. It is well established that non-native species may have serious impacts on native organisms by changing the interactions between different components of the food web and by competing for resources (e.g. Papa et al., 2012; Havel et al., 2015; Rohwer et al., 2023).
It is evident from our results that the presence of submerged macrophytes was the most important driving factor for phytoplankton and zooplankton diversity, composition, and structure, corroborating the third hypothesis. The reduction of phytoplankton species diversity and evenness induced by submerged macrophytes aligns with the findings by Ferreira et al. (2018), who compared areas with stands of submerged macrophyte and the pelagic zone of a subtropical lake. However, the degree of influence usually depends on fish abundance, macrophyte coverage, and trophic interactions (Søndergaard & Moss, 1998). The most noticeable impact of submerged macrophytes on zooplankton structure was the increase in rotifer biomass in all treatments with C. demersum. This finding is consistent with previous studies, which have shown that plants with higher structural complexity, such as C. demersum, significantly impact rotifer life conditions and community structure. Such plants provide greater habitat heterogeneity, increased surface area, higher food availability through biofilms, and a favorable environment for rotifer survival and reproduction (Kuczyńska-Kippen, 2007). Consequently, higher biomasses of rotifers may perhaps have contributed to the disappearance of cladocerans in macrophyte treatments due to competition for resources.
Conclusions
We found that the submerged macrophytes (at a low or moderate percentage of the volume inhabited) decreased the biomass of different phytoplankton groups in tropical shallow reservoir mesocosms, likely reflecting allelopathy. The addition of macrophytes was the main driver of phytoplankton and zooplankton community composition and structure, even at higher eutrophication levels. We also tested the effects of introducing a large-bodied cladoceran commonly occurring in the same watershed, which grazed upon eukaryotic algae. Given its only modest effect on the cyanobacteria biomass and negative effects on the native zooplankton in our experiment, as well as the fact that fish predation will likely eliminate them quickly in the real world (Jeppesen et al., 2012), addition of zooplankton is not recommended for (sub)tropical lakes. We tested only the short-term effects of macrophyte addition, so longer-term mesocosm and field studies are needed to verify if the effects of the introduced plants and the plants themselves will persist and help to attain a clear-water state.
Data availability
Additional data may be obtained from the first author (alvescihelio@gmail.com, cihelio.amorim@wcl.ac.at) upon reasonable request.
References
Amorim, C. A. & A. N. Moura, 2020. Effects of the manipulation of submerged macrophytes, large zooplankton, and nutrients on a cyanobacterial bloom: a mesocosm study in a tropical shallow reservoir. Environmental Pollution 265: 114997. https://doi.org/10.1016/j.envpol.2020.114997.
Amorim, C. A. & A. N. Moura, 2021. Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Science of the Total Environment 758: 143605. https://doi.org/10.1016/j.scitotenv.2020.143605.
Amorim, C. A., Ê. W. Dantas & A. N. Moura, 2020. Modeling cyanobacterial blooms in tropical reservoirs: the role of physicochemical variables and trophic interactions. Science of the Total Environment 744: 140659. https://doi.org/10.1016/j.scitotenv.2020.140659.
Amorim, C. A., R. H. Moura-Falcão, C. R. Valença, V. R. de Souza & A. D. N. Moura, 2019. Allelopathic effects of the aquatic macrophyte Ceratophyllum demersum L. on phytoplankton species: contrasting effects between cyanobacteria and chlorophytes. Acta Limnologica Brasiliensia 31: e21. https://doi.org/10.1590/s2179-975x1419.
APAC, Agência Pernambucana de Águas e Clima, 2019. http://www.apac.pe.gov.br/ (accessed 27 March 2019).
Barneche, D. R., C. J. Hulatt, M. Dossena, D. Padfield, G. Woodward, M. Trimmer & G. Yvon-Durocher, 2021. Warming impairs trophic transfer efficiency in a long-term field experiment. Nature 592: 76–79. https://doi.org/10.1038/s41586-021-03352-2.
Benndorf, J., W. Böing, J. Koop & I. Neubauer, 2002. Top-down control of phytoplankton: the role of time scale, lake depth and trophic state. Freshwater Biology 47: 2282–2295. https://doi.org/10.1046/j.1365-2427.2002.00989.x.
Dong, J., K. Yang, S. Li, G. Li & L. Song, 2014. Submerged vegetation removal promotes shift of dominant phytoplankton functional groups in a eutrophic lake. Journal of Environmental Science 26: 1699–1707. https://doi.org/10.1016/j.jes.2014.06.010.
Dumont, H. J., I. Van de Velde & S. Dumont, 1975. The dry weight estimate of biomass in a selection of Cladocera, Copepoda and Rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 19: 75–97. https://doi.org/10.1007/BF00377592.
Ekvall, M. K., P. Urrutia-Cordero & L.-A. Hansson, 2014. Linking cascading effects of fish predation and zooplankton grazing to reduced cyanobacterial biomass and toxin levels following biomanipulation. PLoS One 9: e112956. https://doi.org/10.1371/journal.pone.0112956.
Ferreira, T. F., L. O. Crossetti, D. M. L. Motta Marques, L. Cardoso, C. R. Fragoso & E. H. van Nes, 2018. The structuring role of submerged macrophytes in a large subtropical shallow lake: clear effects on water chemistry and phytoplankton structure community along a vegetated-pelagic gradient. Limnologica 69: 142–154. https://doi.org/10.1016/j.limno.2017.12.003.
Gao, J. & W. Hu, 2023. A bibliometric analysis of lake restoration with submerged macrophytes. Water 15: 2411. https://doi.org/10.3390/w15132411.
Geletu, T. T., 2023. Lake eutrophication: control of phytoplankton overgrowth and invasive aquatic weeds. Lakes & Reservoirs: Research and Management 28: e12425. https://doi.org/10.1111/lre.12425.
Ger, K. A., S. Naus-Wiezer, L. De Meester & M. Lürling, 2019. Zooplankton grazing selectivity regulates herbivory and dominance of toxic phytoplankton over multiple prey generations. Limnology and Oceanography 64: 1214–1227. https://doi.org/10.1002/lno.11108.
Gliwicz, Z. M., 1990. Why do cladocerans fail to control algal blooms? Hydrobiologia 200(201): 83–97. https://doi.org/10.1007/978-94-017-0924-8_8.
Griffith, A. W. & C. J. Gobler, 2020. Harmful algal blooms: a climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 91: 101590. https://doi.org/10.1016/j.hal.2019.03.008.
Ha, J.-Y., M. Saneyoshi, H.-D. Park, H. Toda, S. Kitano, T. Homma, T. Shiina, Y. Moriyama, K.-H. Chang & T. Hanazato, 2013. Lake restoration by biomanipulation using piscivore and Daphnia stocking; results of the biomanipulation in Japan. Limnology 14: 19–30. https://doi.org/10.1007/s10201-012-0381-9.
Havel, J. E., K. E. Kovalenko, S. M. Thomaz, S. Amalfitano & L. B. Kats, 2015. Aquatic invasive species: challenges for the future. Hydrobiologia 750: 147–170. https://doi.org/10.1007/s10750-014-2166-0.
He, H., T. Qian, R. Shen, J. Yu, K. Li, Z. Liu & E. Jeppesen, 2022. Piscivore stocking significantly suppresses small fish but does not facilitate a clear-water state in subtropical shallow mesocosms: a biomanipulation experiment. Science of the Total Environment 842: 156967. https://doi.org/10.1016/j.scitotenv.2022.156967.
Hillebrand, H., C.-D. Dürselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424. https://doi.org/10.1046/j.1529-8817.1999.3520403.x.
Hilt, S. & E. M. Gross, 2008. Can allelopathically active submerged macrophytes stabilise clear-water states in shallow lakes? Basic and Applied Ecology 9: 422–432. https://doi.org/10.1016/j.baae.2007.04.003.
Hilt, S., M. M. Alirangues Nuñez, E. S. Bakker, I. Blindow, T. A. Davidson, M. Gillefalk, L.-A. Hansson, J. H. Janse, A. B. G. Janssen, E. Jeppesen, T. Kabus, A. Kelly, J. Köhler, T. L. Lauridsen, W. M. Mooij, R. Noordhuis, G. Phillips, J. Rücker, H.-H. Schuster, M. Søndergaard, S. Teurlincx, K. van de Weyer, E. van Donk, A. Waterstraat, N. Willby, C. D. Sayer, 2018. Response of submerged macrophyte communities to external and internal restoration measures in North temperate shallow lakes. Frontiers in Plant Science 9: 194. https://doi.org/10.3389/fpls.2018.00194.
Jeppesen, E., J. P. Jensen, M. Søndergaard, T. Lauridsen, L. J. Pedersen & L. Jensen, 1997. Top-down control in freshwater lakes: the role of nutrient state, submerged macrophytes and water depth. Hydrobiologia 342(343): 151–164. https://doi.org/10.1007/978-94-011-5648-6_17.
Jeppesen, E., M. Meerhoff, B. A. Jacobsen, R. S. Hansen, M. Søndergaard, J. P. Jensen, T. L. Lauridsen, N. Mazzeo, C. W. C. Branco, et al., 2007. Restoration of shallow lakes by nutrient control and biomanipulation – the successful strategy varies with lake size and climate. Hydrobiologia 581: 269–285. https://doi.org/10.1007/s10750-006-0507-3.
Jeppesen, E., M. Søndergaard, T. L. Lauridsen, T. A. Davidson, Z. Liu, N. Mazzeo, C. Trochine, K. Özkan, H. S. Jensen, et al., 2012. Biomanipulation as a restoration tool to combat eutrophication: recent advances and future challenges. Advances in Ecological Research 47: 411–488. https://doi.org/10.1016/B978-0-12-398315-2.00006-5.
Jeppesen, E., M. Søndergaard & Z. Liu, 2017. Lake restoration and management in a climate change perspective: an introduction. Water 9: 122. https://doi.org/10.3390/w9020122.
Kratina, P., H. S. Greig, P. L. Thompson, T. S. Carvalho-Pereira & J. B. Shurin, 2012. Warming modifies trophic cascades and eutrophication in experimental freshwater communities. Ecology 93: 1421–1430. https://doi.org/10.1890/11-1595.1.
Kuczyńska-Kippen, N., 2007. Habitat choice in Rotifera communities of three shallow lakes: impact of macrophyte substratum and season. Hydrobiologia 593: 27–37. https://doi.org/10.1007/s10750-007-9073-6.
Lacerot, G., C. Kruk, M. Lürling & M. Scheffer, 2013. The role of subtropical zooplankton as grazers of phytoplankton under different predation levels. Freshwater Biology 58: 494–503. https://doi.org/10.1111/fwb.12075.
Lammens, E. H. R. R., R. D. Gulati, M. L. Meijer & E. van Donk, 1990. The first biomanipulation conference: a synthesis. Hydrobiologia 200: 619–627. https://doi.org/10.1007/BF02530378.
Liu, Z., J. Hu, P. Zhong, X. Zhang, J. Ning, S. E. Larsen, D. Chen, Y. Gao, H. He & E. Jeppesen, 2018. Successful restoration of a tropical shallow eutrophic lake: strong bottom-up but weak top-down effects recorded. Water Research 146: 88–97. https://doi.org/10.1016/j.watres.2018.09.007.
Meerhoff, M., J. M. Clemente, F. Teixeira de Mello, C. Iglesias, A. R. Pedersen & E. Jeppesen, 2007. Can warm climate-related structure of littoral predator assemblies weaken the clear water state in shallow lakes? Global Change Biology 13: 1888–1897. https://doi.org/10.1111/j.1365-2486.2007.01408.x.
Meerhoff, M. & M. Beklioğlu, 2024. Shallow lakes and ponds. In Wetzel's Limnology (fourth edition). Academic Press. 859–892. https://doi.org/10.1016/B978-0-12-822701-5.00026-4.
Moustaka-Gouni, M. & U. Sommer, 2020. Effects of harmful blooms of large-sized and colonial cyanobacteria on aquatic food webs. Water 12: 1587. https://doi.org/10.3390/w12061587.
Oksanen, J., G. L. Simpson, F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O'Hara, P. Solymos, M. H. H. Stevens, E. Szoecs, H. Wagner, M. Barbour, M. Bedward, B. Bolker, D. Borcard, G. Carvalho, M. Chirico, M. De Caceres, S. Durand, H. B. A. Evangelista, R. FitzJohn, M. Friendly, B. Furneaux, G. Hannigan, M. O. Hill, L. Lahti, D. McGlinn, M. -H. Ouellette, E. R. Cunha, T. Smith, A. Stier, C. J. F. Ter Braak & J. Weedon, 2022. vegan: community Ecology Package (Version 2.6–4). https://CRAN.R-project.org/package=vegan.
Olokotum, M., V. Mitroi, M. Troussellier, R. Semyalo, C. Bernard, B. Montuelle, W. Okello, C. Quiblier & J.-F. Humbert, 2020. A review of the socioecological causes and consequences of cyanobacterial blooms in Lake Victoria. Harmful Algae 96: 101829. https://doi.org/10.1016/j.hal.2020.101829.
Papa, R. D. S., H. Li, D. T. Tordesillas, B. Han & H. J. Dumont, 2012. Massive invasion of Arctodiaptomus dorsalis (Copepoda, Calanoida, Diaptomidae) in Philippine lakes: a threat to Asian zooplankton biodiversity? Biological Invasions 14: 2471–2478. https://doi.org/10.1007/s10530-012-0250-9.
Portilla, K., E. Velarde, E. Decaestecker, F. Teixeira de Mello & K. Muylaert, 2023. Potential submerged macrophytes to mitigate eutrophication in a high-elevation tropical shallow lake – a mesocosm experiment in the Andes. Water 15: 75. https://doi.org/10.3390/w15010075.
R Core Team, 2024. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
Rao, X., J. Lu, P. Zhong, X. Zhang, Y. Tang, J. Yu, H. He, E. Jeppesen & Z. Liu, 2023. Do submerged macrophytes facilitate the development of large crustacean zooplankton in tropical shallow lakes? Hydrobiologia 850: 4763–4778. https://doi.org/10.1007/s10750-023-05277-5.
Reid, A. J., A. K. Carlson, I. F. Creed, E. J. Eliason, P. A. Gell, P. T. J. Johnson, K. A. Kidd, T. J. MacCormack, J. D. Olden, S. J. Ormerod, J. P. Smol, W. W. Taylor, K. Tockner, J. C. Vermaire, D. Dudgeon & S. J. Cooke, 2019. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews 94: 849–873. https://doi.org/10.1111/brv.12480.
Reigosa, M. J., A. Sánchez-Moreiras & L. González, 1999. Ecophysiological approach in allelopathy. Critical Reviews in Plant Sciences 18: 577–608. https://doi.org/10.1080/07352689991309405.
Rohwer, R. R., R. J. Hale, M. J. Vander Zanden, T. R. Miller & K. D. McMahon, 2023. Species invasions shift microbial phenology in a two-decade freshwater time series. Proceedings of the National Academy of Sciences 120: e2211796120. https://doi.org/10.1073/pnas.2211796120.
Ruttner-Kolisko, A., 1977. Suggestions for biomass calculation of planktonic rotifers. Archiv Für Hydrobiologie 8: 71–76.
Scheffer, M., S. H. Hosper, M.-L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology & Evolution 8: 275–279. https://doi.org/10.1016/0169-5347(93)90254-M.
Schriver, P., J. Bøgestrand, E. Jeppesen & M. Søndergaard, 1995. Impact of submerged macrophytes on fish-zooplanlankton-phytoplankton interactions: large-scale enclosure experiments in a shallow eutrophic lake. Freshwater Biology 33: 255–270. https://doi.org/10.1111/j.1365-2427.1995.tb01166.x.
Shapiro, J., V. Lamarra, M. Lynch, 1975. Biomanipulation: an ecosystem approach to lake restoration. In Brezonik, P. L. & J. L. Fox (eds), Proceedings from the Symposium on Water Quality Management Through Biological Control. University of Florida: 85–96.
Shurin, J. B., J. L. Clasen, H. S. Greig, P. Kratina & P. L. Thompson, 2012. Warming shifts top-down and bottom-up control of pond food web structure and function. Philosophical Transactions of the Royal Society b: Biological Sciences 367: 3008–3017. https://doi.org/10.1098/rstb.2012.0243.
Sim, D. Z., M. A. Mowe, S. M. Mitrovic, N. K. Tulsian, G. S. Anand & D. C. Yeo, 2024. Nutrient conditions influence allelopathic capabilities of Ludwigia adscendens and other tropical macrophytes against Microcystis aeruginosa. Freshwater Biology in Press. https://doi.org/10.1111/fwb.14227.
Søndergaard, M., L. Liboriussen, A. R. Pedersen & E. Jeppesen, 2008. Lake restoration by fish removal: short- and long-term effects in 36 Danish lakes. Ecosystems 11: 1291–1305. https://doi.org/10.1007/s10021-008-9193-5.
Søndergaard, M., B. Moss, 1998. Impact of Submerged Macrophytes on Phytoplankton in Shallow Freshwater Lakes. In Jeppesen, E., M. Søndergaard, M. Søndergaard, K. Christoffersen (Eds) The structuring Role of Submerged Macrophytes in Lakes. Ecological Studies, vol. 131. Springer, New York, 115–132. https://doi.org/10.1007/978-1-4612-0695-8_6.
Souza, V. R., C. Amorim, A. D. N. Moura, 2021. Effects of the submerged macrophyte Ceratophyllum demersum (Ceratophyllaceae) and the cladoceran Moina micrura (Cladocera: Moinidae) on microalgal interactions. Revista de Biología Tropical 69: 1276–1288. https://doi.org/10.15517/rbt.v69i4.42589.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitteilungen Internationale Vereinigung Für Theoretische und Angewandte Limnologie 9: 1–38. https://doi.org/10.1080/05384680.1958.11904091.
Vanderstukken, M., N. Mazzeo, W. Van Colen, S. A. J. Declerck & K. Muylaert, 2011. Biological control of phytoplankton by the subtropical submerged macrophytes Egeria densa and Potamogeton illinoensis: a mesocosm study. Freshwater Biology 56: 1837–1849. https://doi.org/10.1111/j.1365-2427.2011.02624.x.
Wilson, A. E., O. Sarnelle & A. R. Tillmanns, 2006. Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: meta-analyses of laboratory experiments. Limnology and Oceanography 51: 1915–1924. https://doi.org/10.4319/lo.2006.51.4.1915.
Acknowledgements
This work was supported by the Brazilian National Council of Technological and Scientific Development (CNPq) (grant number 305829/2019-0 to ANM), Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) (grant number IBPG-0308-2.03/17 to CAA), and Cordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) (Finance Code 001 to CAA). CAA and EJ were supported by the TÜBITAK program BIDEB2232 (No. 118C250). We thank the Limnology Laboratory and Department of Fisheries and Aquaculture of the Federal Rural University of Pernambuco for supporting nutrient analysis and Anne Mette Poulsen for valuable linguistic improvements of the manuscript.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The funding was provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Grant No. GPCT-88881.846401/2023-01), Conselho Nacional de Desenvolvimento Científico e Tecnológico (Grant No. 305829/2019-0), Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (Grant No. IBPG-0308-2.03/17) and Türkiye Bilimsel ve Teknolojik Araştırma Kurumu (Grant No. BIDEB2232-118C250).
Author information
Authors and Affiliations
Contributions
Cihelio A. Amorim: contributed to conceptualization, methodology, validation, data curation, formal analysis, writing—original draft, and writing—review & editing. Erik Jeppesen helped in writing—review & editing. Ariadne N. Moura contributed to conceptualization, supervision, methodology, and writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
Declaration of conflicts of interest: none.
Additional information
Handling editor: Sidinei M. Thomaz
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Priit Zingel, Helen Agasild, Maria Brigida Boveri & Erik Jeppesen / Secrets of Shallow Lakes: Insights from Research
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amorim, C.A., Jeppesen, E. & Moura, A.N. How do additions of submerged macrophytes, large-bodied cladocerans and nutrients impact tropical plankton communities? A mesocosm experiment. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05646-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05646-8