Abstract
The European eel is critically endangered due to heavy impact of anthropogenic factors, such as habitat fragmentation, overexploitation and climate change. During downstream migration, silver eels may encounter hydropower plants, which often result in delay or mortality from impingement on trash-racks or turbine passage. These problems can be mitigated with downstream passage solutions, such as angled racks that guide downstream-migrating eels to safe passage routes. The importance of bar spacing and phenotypic diversity for passage performance is, however, largely unknown. In this study, we investigated how morphological parameters (body mass, eye and fin indices) and behavioral score (open field test) influenced passage rate at an experimental intake equipped with a bypass and angled racks with either 15 or 30 mm bar spacing. Both racks were efficient in guiding eels into a bypass. There was a strong positive effect of body mass and a weak positive effect of open field test score on passage rate. Other factors such as eye and fin indices played a minor role. These results demonstrate the performance of angled racks with bypasses and form a useful starting point for further research regarding the relationships between individual variation in behavior, morphology and passage solutions for silver eels.
Similar content being viewed by others
Introduction
Man-made constructions in rivers, such as hydropower dams, alter natural river systems by disrupting longitudinal connectivity and hinder the migration of riverine organisms (Righton et al., 2021). Especially for fish that move between freshwater and marine environments (i.e., diadromous species), fragmentation and destruction of riverine habitat have detrimental effects on the successful completion of their life cycles. Fish that rely on freshwater habitat are therefore today one of the most threatened groups of animals on the planet (Soulé, 1991). For example, the European eel [Anguilla anguilla (Linnaeus, 1758)] is a critically endangered catadromous fish species, and all stages in its complex life cycle are heavily affected by human activity (Pike et al., 2020). As a result of past and present effects of anthropogenic pressure, the European eel has suffered a rapid decline in abundance (Poehlmann et al., 2020). Its life cycle includes a spawning migration from the rearing grounds in freshwater to spawning areas at sea, and this habitat shift is associated with adaptive physiological and morphological processes (Tesch, 2003; Schweid, 2009). Silver eels (i.e., migratory spawners) that are ready to initiate their migration back to the Sargasso Sea for spawning, for instance, become tolerant to salt water, their eyes and pectoral fins become enlarged and the body pigmentation changes to silvery white on the ventral and dark green on the dorsal side (van Ginneken et al., 2007; Schweid, 2009). Feeding ceases, and silver eels thereby rely on accumulated fat reserves when crossing the Atlantic on their way to the spawning area (Tesch, 2003). With such complex life cycle, freshwater eels face all biodiversity threats outlined in Millennium Ecosystem Assessment: habitat fragmentation, climate change, invasive species and parasites, habitat reduction and pollution (Drouineau et al., 2018).
European eels facing hydropower stations risk mortality and injuries when encountering trash-racks and coming into direct contact with moving parts of turbines (Larinier & Travade, 2002; Calles et al., 2010). Even though threats associated with downstream passage are global, solutions to the problems are often site-specific (Fjeldstad et al., 2018), and research over the past decade, both under controlled laboratory conditions and in the field, has shown varying results (Russon et al., 2010; Calles et al., 2013, 2021; Fjeldstad et al., 2018; Økland et al., 2019). Laboratory studies indicate that both inclined (inclined plane) and angled (angled plane) bar racks may be successful in efficiently guiding eels past barriers (Amaral et al., 2003; Russon et al., 2010), which also has been demonstrated in field studies (Calles et al., 2013, 2021; Økland et al., 2019). The design features of implemented racks are typically in the range of 10–20 mm bar spacing and oriented with a 26°–45° angle to the vertical (angled racks) or inclination to the horizontal (inclined racks). In the River Sieg, for example, an angled rack (27° and 10 mm bar spacing) resulted in > 92% survival (Økland et al., 2019), and an angled rack (30° with 15 mm bar spacing) upstream of a powerhouse in the River Ätran had a 95–100% passage success (Calles et al., 2021; Kjærås et al., 2023). However, not only survival at the passage event is important for downstream migration, the duration of the passage also plays an important role.
Hesitance to enter the bypass at a fish passage solution may result in migration delay (Verhelst et al., 2018), which can have negative effects on the success of the downstream migration of silver eels. There is great individual variation in passage time when eels are provided with a fish passage solution (Behrmann-Godel & Eckmann, 2003; Pedersen et al., 2012; Calles et al., 2013), which is poorly understood but potentially associated with differences in individual traits, such as morphology, degree of maturity and behavioral type. Morphological metrics such as weight and girth have direct implications for swimming capacity and the physical passability of racks (Calles et al., 2010; Travade et al., 2010b), whereas the migrational motivation can be described by the “silver degree” calculated from the size of the eyes and pectoral fins (Durif et al., 2009). Behavioral types, i.e., behaviors that persist over time and in different contexts (e.g., bold, explorative, aggressive, timid), have been shown in laboratory studies to exist in European glass eels and elvers (Geffroy & Bardonnet, 2012; Geffroy et al., 2014, 2015), whereas such results is lacking for silver eels, potentially because silver eels are difficult to study in the laboratory. The importance of behavioral type for the downstream-migrating, adult life stage of eels remains unknown, although silver eels have been shown to perform a wide array of behaviors when facing a rack at a hydroelectric power plant. They can, for example, follow the rack, crash into or try to squeeze through it, flee upstream or perform a combination of these behaviors (Amaral et al., 2003; Behrmann-Godel & Eckmann, 2003; Russon et al., 2010; Russon & Kemp, 2011; Verhelst et al., 2018). Individual variability in behaviors is high (Calles et al., 2013, 2021), which plausibly effects passage success.
Both field and laboratory studies have shown that eels are guided into bypasses, even by inclined and angled racks with a bar spacing that does not physically prevent eels from passing racks (Amaral et al., 2003). This observation indicates that angled and inclined racks also have behavioral guidance properties. Therefore, it is possible that racks with a wide bar spacing that allow efficient operation for the hydropower company (in terms of easy cleaning and low electricity production loss) can still guide silver eels to an adjacent bypass. In this study, we investigated downstream passage performance of silver eels guided by an angled bar rack at a large-scale ecohydraulic laboratory (Älvkarleby, Vattenfall AB). We tested if passage rate was affected by bar spacing (15 vs. 30 mm), and in addition, we investigated the importance of morphological and behavioral traits for the passage rate of individual silver eels. Specifically, we tested the effects of body size, eye and pectoral fin indexes (measures of maturity) and distance traveled in an open field test (OFT; a measure of activity) in our analysis of passage rate. We hypothesized that (1) bar spacing would have minor effect on passage rates, because of the behavioral guidance properties of angled racks, and that (2) the degree of maturity would be positively related to passage rate because maturity should be linked to motivation to migrate. Further, we hypothesized that (3) the activity score (as measured by distance travelled in an OFT) would be positively related to the passage rate because activity is expected to be positively related to the ability of locating and entering a bypass opening.
Materials and methods
Experimental facility
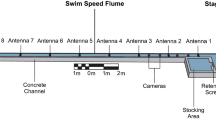
The experiment was performed at the Vattenfall Research and Development Laboratory (“Laxeleratorn”) in Älvkarleby, Sweden, between 10 October and 2 November, 2019. This experimental facility has two interconnected flumes (Fig. 1), each with the dimensions (length × width × depth) 24 × 4 × 2 m, with water supplied from the adjacent River Dalälven. Four electronically controlled pumps (Flygt N3202, Xylem, Inc., USA) provided flow with a capacity to generate velocities of up to 2 m s−1. A steel mesh (10 × 10 mm) installed at the start and end of each flume prevented eels from escaping from the experimental arenas. An angled metal bar rack (length × width = 6.8 × 2 m) was installed at a 30° angle towards the water current (i.e., a β-rack; Fig. 1). Bars in the racks were oriented horizontally facing the direction of the current. Two racks with different bar spacing were used in the experiment: one with 15 mm and one with 30 mm distance between the bars. At the downstream end of the rack a full-depth bypass entrance (width × height = 0.6 × 2.0 m) with an inclined (30°) solid steel ramp was installed to lead the fish towards the 0.5-m-deep bypass crest. The bypass was connected to a channel leading to a 1.8 m3 cage (length × width × depth = 2.0 × 0.6 × 1.5 m).
In the experiment, the eels started a passage trial from a start box. The box (length × width × height = 1.00 × 0.30 × 0.35 m) was located 5 m upstream from the most upstream part of the rack, and 9 m upstream of the bypass entrance. Eels were held in the box for 5 min before we opened the hatch to allow for the eels to leave the box. Two PIT-tag antennas (passive integrated transponder; Oregon RFID, Portland, USA) were installed within the test arena to record passage times of individually tagged fish. One antenna was located at the exit of the start box to detect when the eels left the box and entered the arena. The other antenna was located at the bypass crest to detect the eels that successfully entered the bypass channel.
Eels
Silver eels (n = 108) were caught by professional fishermen in south-eastern Lake Vänern, Sweden, and the eels were transported to the experimental facility on 9 October, 2019. On arrival, eels were distributed between two circular aerated holding tanks (volume = 3.5 m3, diameter = 3 m), equipped with water coolers, pumps and UV-filters (for more details, see Harbicht et al., 2022). The holding tanks were shielded with tarpaulins to reduce external disturbance. Water temperature and oxygen saturation were controlled daily prior and during the experiment. Mean water temperature and oxygen saturation (± SD) were 10.6 ± 1.3°C and 98.9 ± 1.8%, respectively.
Between 10 and 13 October, 2019, we assessed individual behavior by scoring the eels in an open field test to quantify the total distance travelled by an individual eel in the absence of external disturbance (OFT; Mensinger et al., 2021). In an OFT trial, an individual eel was introduced into an empty holding tank (volume = 3.5 m3, diameter = 3 m) that constituted the testing arena. The eel was allowed to swim freely in the arena for 20 min during which we recorded the eel using a video camera (GoPro Hero 6; GoPro, Inc., San Mateo, USA) installed over the arena, and the last 4 min of the trial was used for analysis. After being scored in OFT, each eel was anesthetized with benzocaine (0.1 mg l−1), measured (total length TL), weighed (wet mass M), checked for injuries and tagged with a 23 mm PIT-tag (Oregon RFID, USA) in the abdominal cavity. We photographed the eel to record morphometric parameters. Four photos of each eel were taken: full body, pectoral fin, head from the left side and from the top. All photos included a ruler for scaling.
Behavioral and morphological parameters
We transformed the 4 min videos from the OFT into sequences of JPEG images using Virtual Dub (developed by Avery Lee, GNU GPL-2.0 license), and we produced 1 image s−1. Images were processed with ImageJ 1.52a (Schneider et al., 2012). We located the anterior end of the eel’s head and assigned it coordinates in each image, and we used these coordinates to calculate total distance travelled in OFT.
We calculated two morphological parameters: eye index (EI) and pectoral fin index (PF) by analyzing photos with TPS (Rohlf, 2015), where reference points were placed in pre-defined locations, uniform for all eels. For EI, we measured vertical (EDv) and horizontal (EDh) eye diameter (mm) and calculated EI using the following equation (Pankhurst, 1982; Mordenti et al., 2013)
We measured the length of the left pectoral fin (Lpf) from the insertion to the tip of the fin (mm) and calculated PF according to the following equation (Durif et al., 2005):
Passage trials
Between 21 October and 2 November, 2019, we carried out 6 replicate trials for each rack type, resulting in 12 trials conducted over 12 consecutive days, using a total of 96 eels tested in groups of 8. All eels (n = 108) were released back into the wild after the experiment, 60° 39′ 11.92″ N, 17° 20′ 41.51″ E, including the 12 individuals not taking part in the study. The two racks, 15 and 30 mm bar spacing, were alternated daily. Each trial started between 19:00 and 20:00 and lasted on average 13.5 h. Illumination during night time (mean ± SD) was 4.0 ± 0.l lux, water temperature was kept at 11.3 ± 0.4°C and water velocity was 1.0 ± 0.1 m s−1 at 0.1 m depth, 0.9 ± 0.2 m s−1 at 0.6 m depth, and 0.2 ± 0.1 m s−1 at 1.3 m depth upstream both of the racks.
From the data collected by the PIT antennas during the passage trials, we calculated passage time (tP) as the difference between the last detection by the antenna at the start box exit and the first detection by the antenna located inside the bypass.
We calculated the fish guiding efficiency (FGE) to guide the eels to the bypass separately for the rack types with 15 mm and 30 mm bar spacing, using the following equation (Calles et al., 2010, 2013):
where np is the number of eels that entered the bypass during the trial time, and nt is the total number of eels released.
Statistical analyses
We verified that the eels did not differ in any of the morphological parameters between the two rack types using Mann–Whitney U tests (P > 0.05). To analyze which parameters (Table 1) affected passage rates, we used Cox-proportional hazards time-to event regression models (Castro-Santos & Haro, 2003; Hosmer et al., 2008; Castro-Santos & Perry, 2012), and to assess if the bar spacing had a significant effect on passage time, we used log-rank test on the time-to-event curves, using the survival package in R (Therneau, 2015). We used robust sandwich estimators to account for non-independence of data points (eels were released in groups of eight). The proportionality of hazards assumption was tested for all good models, as well as inspected visually by checking the Shoenfeld residuals. We tested all combinations of the parameters, using the dredge function (Harbicht et al., 2022 and references therein). Models within 2 Akaike’s information criterion units (AIC) from the best-fitting model (i.e., model with the lowest AIC value) were considered good models (Calles et al., 2021). The most parsimonious model (i.e., the model with the fewest parameters) was identified as the best model among the good ones.
Results
Total FGE was 76%, as 73 out of 96 eels successfully entered the bypass opening. For trials with the rack that had 15 mm bar spacing, FGE was 81.3% and the median passage time was 110 min, whereas the corresponding values for the rack with 30 mm bar spacing was 70.8% and 123 min. A log-rank test showed that the passage rate was not significantly different between the two rack types (P = 0.51; Fig. 2).
Non-parametric (Kaplan–Meier) time-to-event curves for PIT-tagged European eels (n = 96) guided into a bypass during flume experiments with two angled racks with different bar spacing (red = 15 mm; blue = 30 mm). Dashed lines represent 95% confidence intervals. A vertical increase in the curves indicates an event, in this case entering the bypass. The vertical marks indicate eels that were censored, i.e., the eel had not entered the bypass by the end of the trial
Body mass and length were the only explanatory parameters that were correlated (P < 0.001; all other combinations of parameters, P > 0.05). Within 2 ΔAIC of the best-fitting model, seven good models were identified (Table 2). From this subset, two models were selected for further analysis: (1) the most parsimonious model and (2) the model with the lowest AIC value (Table 3). For both of these models, we further analyzed the hazard functions (HF) to investigate how the included variables affected passage probability. HF is a value used in the time-to-event analysis that describes the effect of the chosen variable on the probability of the event, in our case successful passage through the bypass. If HF equals to 1, this indicates that the variable has no effect on the baseline probability for the event to happen (in this case a passage), whereas values above 1 indicates a positive relationship between the variable and the probability that the event happens. In the most parsimonious model, body mass had a positive significant effect on the passage rate (HF = 2.38) (Table 3). The best-fitting model with the lowest AIC value (but not the most parsimonious model) included the explanatory variables body mass and distance travelled in OFT. Both body mass and distance travelled in OFT had positive significant effects, but the effect of the mass was highly positive (HF = 2.26) while the distance travelled in OFT had a weak positive effect (HF = 1.02, Table 3).
Discussion
Our results show that the angled racks with 15 and 30 mm bar spacing, respectively, both guided eels into the bypass to a relatively high extent (FGE = 81.3 and 70.8%). These values are in line with earlier research, and for example, in a laboratory study, Amaral et al. (2003) reported guidance efficiencies of around 85–95% for 50 mm racks angled at 15° towards the current, and for racks angled at 45° with 25 and 50 mm bar spacing, the efficiencies were around 60%. Another laboratory study showed high guidance efficiencies and no impingement for 12 mm racks angled at three different inclinations (15°, 30° and 45°; Russon et al., 2010). Also field studies have demonstrated the effectiveness of inclined and angled racks for guiding silver eels past hydropower plants on the their downstream migration (Calles et al., 2013, 2021; Økland et al., 2019). In these studies, inclined and angled racks had higher guidance efficiency and less instances of impingement than conventional racks (i.e., rack with angles > 50°).
Our time-to-event analysis indicated that body mass was positively related to the probability of passage. Large eels may be stronger swimmers, having higher muscular mass, and it is possible that this will allow them to move faster along the racks and locate the bypass opening faster. Furthermore, since the majority of silver eels > 500 mm are female, due to the documented sexual dimorphism in European eels (Tesch, 2003), angled racks with bypasses are expected to increase survival of large migrating highly fecund female eels. If this holds true it would have implications for conservation, and could also balance the observed reverse pattern for silver eel turbine passage, i.e., size being negatively related to turbine passage survival (Calles et al., 2010). The two morphometrical variables, eye and pectoral fin indexes, did not influence passage rate, and thus we did not find evidence that stage of maturity influenced passage rate. In previous studies, these indexes have generally been used descriptively (Durif & Elie, 2008; Calles et al., 2013; Mordenti et al., 2013) and have not been included as explanatory variables in an analysis of passage rate. Perhaps all individuals in our study were roughly equally motivated to migrate, and potential effects of differences in eye and pectoral fin indexes would only be detected if we also had included eels with a low degree of maturity.
In previous studies, migratory silver eels have shown a great variety of behaviors when facing barriers (Amaral et al., 2003; Russon et al., 2010; Calles et al., 2013, 2021; Verhelst et al., 2018). We hypothesized that individual differences in activity, measured as distance travelled in OFT, is linked to passage performance. Earlier behavioral studies on variation in European eel individual behavior have focused on the juvenile life stage, and they have for example reported a connection between behavioral type and growth (Geffroy & Bardonnet, 2012; Geffroy et al., 2014). Further, in a study on upstream migrating juvenile eels, Mensinger et al. (2021) showed that individuals that were classified as exploratory in an OFT had a high success rate when climbing an experimental fishway. In our study, we found some (albeit weak) evidence that OFT also can predict passage rate of downstream-migrating silver eels. This result adds to the growing body of literature that associate fish passage solutions and individual variation in behavior (Silva et al., 2020), and future fish passage studies should perhaps consider the potential mechanism that fishways may act as selective agents for behavioral traits (Calles et al., 2021; Mensinger et al., 2021).
We did not measure activity in OFT at night. Both silver eels (Aarestrup et al., 2010) and elvers (Watz et al., 2019) are mainly nocturnal, and the use of low-light video cameras in our study would have provided additional information of individual variation in exploratory behavior and activity. Variation in passage performance could potentially be better explained by nocturnal than diurnal behavioral metrics. On the other hand, OFT carried out in daylight has been shown to relate to passage performance in juvenile eels (Mensinger et al., 2021).
Angled racks have shown to be an effective measure for facilitating downstream migration of eels, both in the laboratory experiments and in the field. In addition to preventing eels from entering the turbines, inclined and angled racks likely also provide behavioral guidance. Furthermore, our study showed that the eel size plays an important role for successful entering of a bypass at a downstream fish passage solution, with heavier fish, predominantly female, having higher chances of performing this action. Our results showed that individual variation in activity scored, potentially also has a small effect on passage rates. Given the status as critically endangered, the European eel should be offered safe and timely routes past barriers on their downstream migration. In river systems where silver eels have to pass multiple hydropower plants on their way from rearing to spawning grounds, passage rates of close to 100% are required for each facility to resolve the bottleneck of low cumulative survival and avoiding artificial selection for certain phenotypes. Studies aimed at unraveling links between phenotypic diversity and passage success may be important for optimizing fish passage solution design for all phenotypes.
Data availability
The dataset generated and analysed during the current study is available as a Supplementary File.
References
Aarestrup, K., E. Thorstad, A. Koed, N. Jepsen, M. Pedersen & F. Økland, 2010. Survival and progression rates of large European silver eel Anguilla anguilla in late freshwater and early marine phase. Aquatic Biology 9: 263–270. https://doi.org/10.3354/ab00260.
Amaral, S. V., F. C. Winchell, B. J. McMahon & D. A. Dixon, 2003. Evaluation of angled bar racks and louvers for guiding silver phase American eel. In Dixon, D. A. (ed.), Biology, Management and Protection of Catadromous Eel. Symposium 33. American Fisheries Society, Bethesda: 367–376.
Behrmann-Godel, J. & R. Eckmann, 2003. A preliminary telemetry study of the migration of silver European eel (Anguilla anguilla L.) in the River Mosel, Germany. Ecology of Freshwater Fish 12: 196–202. https://doi.org/10.1034/j.1600-0633.2003.00015.x.
Calles, O., I. C. Olsson, C. Comoglio, P. S. Kemp, L. Blunden, M. Schmitz & L. A. Greenberg, 2010. Size-dependent mortality of migratory silver eel at a hydropower plant, and implications for escapement to the sea. Freshwater Biology 55: 2167–2180. https://doi.org/10.1111/j.1365-2427.2010.02459.x.
Calles, O., S. Karlsson, P. Vezza, C. Comoglio & J. Tielman, 2013. Success of a low-sloping rack for improving downstream passage of silver eel at a hydroelectric plant. Freshwater Biology 58: 2168–2179. https://doi.org/10.1111/fwb.12199.
Calles, O., J. Elghagen, D. Nyqvist, A. Harbicht & P. A. Nilsson, 2021. Efficient and timely downstream passage solutions for European silver eel at hydropower dams. Ecological Engineering 170: 106350. https://doi.org/10.1016/j.ecoleng.2021.106350.
Castro-Santos, T. & A. Haro, 2003. Quantifying migratory delay: a new application of survival analysis methods. Canadian Journal of Fisheries and Aquatic Sciences 60: 986–996. https://doi.org/10.1139/f03-086.
Castro-Santos, T. & R. W. Perry, 2012. Time-to-event analysis as a framework for quantifying fish passage performance. In Telemetry Techniques: A User Guide for Fisheries Research. American Fisheries Society, Bethesda: 427–452.
Drouineau, H., C. Durif, M. Castonguay, M. Mateo, E. Rochard, G. Verreault, K. Yokouchi & P. Lambert, 2018. Freshwater eel: a symbol of the effects of global change. Fish and Fisheries 19: 903–930. https://doi.org/10.1111/faf.12300.
Durif, C., S. Dufour & P. Elie, 2005. The silvering process of Anguilla anguilla: a new classification from the yellow resident to the silver migrating stage. Journal of Fish Biology 66: 1025–1043. https://doi.org/10.1111/j.0022-1112.2005.00662.x
Durif, C. M. F. & P. Elie, 2008. Predicting downstream migration of silver eel in a large river catchment based on commercial fishery data. Fisheries Management and Ecology 15: 127–137. https://doi.org/10.1111/j.1365-2400.2008.00593.x.
Durif, C.M.F., A. Guibert & P. Elie, 2009. Morphological discrimination of the silvering stages of the European eel. Page 449. In: Eels at the Edge: Science, Status, and Conservation Concerns. American Fisheries Society.
Fjeldstad, H. P., U. Pulg & T. Forseth, 2018. Safe two-way migration for salmonids and eel past hydropower structures in Europe: a review and recommendations for best-practice solutions. Marine and Freshwater Research 69: 1834–1847. https://doi.org/10.1071/MF18120.
Geffroy, B. & A. Bardonnet, 2012. Differential effects of behaviour, propensity to migrate and recruitment season on glass eel and elvers’ growing performance. Ecology of Freshwater Fish 21: 469–482. https://doi.org/10.1111/j.1600-0633.2012.00566.x.
Geffroy, B., N. Bru, S. Dossou-Gbété, C. Tentelier & A. Bardonnet, 2014. The link between social network density and rank-order consistency of aggressiveness in juvenile eel. Behavioral Ecology and Sociobiology 68: 1073–1083. https://doi.org/10.1007/s00265-014-1719-6.
Geffroy, B., B. Sadoul & A. Bardonnet, 2015. Behavioural syndrome in juvenile eel and its ecological implications. Behaviour 152: 147–166. https://doi.org/10.1163/1568539X-00003236.
Harbicht, A. B., J. Watz, D. Nyqvist, T. Virmaja, N. Carlsson, D. Aldvén, P. A. Nilsson & O. Calles, 2022. Guiding migrating salmonid smolts: experimentally assessing the performance of angled and inclined screens with varying gap widths. Ecological Engineering 174: 106438. https://doi.org/10.1016/j.ecoleng.2021.106438.
Hosmer D.W., S. Lemeshow & S. May, 2008. Applied Survival Analysis: Regression Modeling of Time-to-Event Data, 2nd edition. Wiley.
Kjærås, H., H. Baktoft, A. T. Silva, K. Ø. Gjelland, F. Økland, T. Forseth, M. Szabo-Meszaros & O. Calles, 2023. Three-dimensional migratory behaviour of European silver eels (Anguilla anguilla) approaching a hydropower plant. Journal of Fish Biology 102: 465–478. https://doi.org/10.1111/jfb.15278.
Larinier, M. & F. Travade, 2002. Downstream migration: problems and facilities. Bulletin Francais De La Peche Et De La Pisciculture 364: 181–207. https://doi.org/10.1051/kmae/2002102.
Mensinger, M. A., A. M. Brehm, A. Mortelliti, E. J. Blomberg & J. D. Zydlewski, 2021. American eel personality and body length influence passage success in an experimental fishway. Journal of Applied Ecology 58: 2760–2769. https://doi.org/10.1111/1365-2664.14009.
Mordenti, O., A. D. Biase, G. Bastone, R. Sirri, A. Zaccaroni & A. Parmeggiani, 2013. Controlled reproduction in the wild European eel (Anguilla anguilla): two populations compared. Aquaculture International 21: 1045–1063. https://doi.org/10.1007/s10499-012-9611-8.
Økland, F., T. B. Havn, E. B. Thorstad, L. Heermann, S. A. Sæther, M. Tambets, M. A. K. Teichert & J. Borcherding, 2019. Mortality of downstream migrating European eel at power stations can be low when turbine mortality is eliminated by protection measures and safe bypass routes are available. International Review of Hydrobiology 104: 68–79. https://doi.org/10.1002/iroh.201801975.
Pankhurst, N. W., 1982. Relation of visual changes to the onset of sexual maturation in the European eel Anguilla anguilla (L.). Journal of Fish Biology 21: 127–140. https://doi.org/10.1111/j.1095-8649.1982.tb03994.x.
Pedersen, M. I., N. Jepsen, K. Aarestrup, A. Koed, S. Pedersen & F. Økland, 2012. Loss of European silver eel passing a hydropower station. Journal of Applied Ichthyology 28: 189–193. https://doi.org/10.1111/j.1439-0426.2011.01913.x.
Pike, C., V. Crook & M. Gollock, 2020. Anguilla anguilla. The IUCN Red List of Threatened Species 2020: e.T60344A152845178 [available on internet at https://www.iucnredlist.org/species/60344/152845178]. Accessed 5 May 2021.
Poehlmann, J.-D., K. Sims, E. Amilhat, J. Bajinskis, L. Beaulaton, C. Belpaire, P. Bernotas, C. Boulenger, U. Brämick, C. Briand, A. Bryhn, M. Christoffersen, E. Ciccotti, W. Dekker, E. Díaz, I. Domingos, H. Drouineau, C. Durif, D. Evans & K. Wysujack, 2020. 2020 Report of the EIFAAC/ICES/GFCM Working Group on Eel (WGEEL).
Righton, D., A. Piper, K. Aarestrup, E. Amilhat, C. Belpaire, J. Casselman, M. Castonguay, E. Díaz, H. Dörner, E. Faliex, E. Feunteun, N. Fukuda, R. Hanel, C. Hanzen, D. Jellyman, K. Kaifu, K. McCarthy, M. J. Miller, T. Pratt, et al., 2021. Important questions to progress science and sustainable management of anguillid eel. Fish and Fisheries 22: 762–788. https://doi.org/10.1111/faf.12549.
Rohlf, F. J., 2015. The Tps Series of Software. Hystrix 26: 1–4. https://doi.org/10.4404/hystrix-26.1-11264.
Russon, I. J. & P. S. Kemp, 2011. Advancing provision of multi-species fish passage: behaviour of adult European eel (Anguilla anguilla) and brown trout (Salmo trutta) in response to accelerating flow. Ecological Engineering 37: 2018–2024. https://doi.org/10.1016/j.ecoleng.2011.08.005.
Russon, I. J., P. S. Kemp & O. Calles, 2010. Response of downstream migrating adult European eel (Anguilla anguilla) to bar racks under experimental conditions. Ecology of Freshwater Fish 19: 197–205. https://doi.org/10.1111/j.1600-0633.2009.00404.x.
Schneider, C. A., W. S. Rasband & K. W. Eliceiri, 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. https://doi.org/10.1038/nmeth.2089.
Schweid, R., 2009. Eel, Reaktion Books, London:
Silva, A. T., K. M. Bærum, R. D. Hedger, H. Baktoft, H. P. Fjeldstad, K. Ø. Gjelland, F. Økland & T. Forseth, 2020. The effects of hydrodynamics on the three-dimensional downstream migratory movement of Atlantic salmon. Science of the Total Environment 705: 135773. https://doi.org/10.1016/j.scitotenv.2019.135773.
Soulé, M. E., 1991. Conservation: tactics for a constant crisis. Science 253: 744–750. https://doi.org/10.1126/science.253.5021.744.
Tesch, F. W., 2003. The Eel, Blackwell Publishing, Hoboken:
Therneau, T., 2015. A package for survival analysis in R. R package version 2.38 [available on internet at https://CRAN.R-project.org/packages=survial].
Travade F., M. Larinier, S. Subra, P. Gomes & E. De-Oliveira, 2010. Downstream passage of silver eels at hydroeletric plants. Study of the pathways at the hydroelectric plant Baigts-de-Béarn (64). Test of downstream bypasses and small spacing trashrack. EDF R&D - Laboratoire National d’Hydraulique et Environnement (in French).
van Ginneken, V., C. Durif, S. P. Balm, R. Boot, M. W. A. Verstegen, E. Antonissen & G. van den Thillart, 2007. Silvering of European eel (Anguilla anguilla L.): seasonal changes of morphological and metabolic parameters. Animal Biology 57: 63–77. https://doi.org/10.1163/157075607780002014.
Verhelst, P., D. Buysse, J. Reubens, I. Pauwels, B. Aelterman, S. Van Hoey, P. Goethals, J. Coeck, T. Moens & A. Mouton, 2018. Downstream migration of European eel (Anguilla anguilla L.) in an anthropogenically regulated freshwater system: Implications for management. Fisheries Research 199: 252–262. https://doi.org/10.1016/j.fishres.2017.10.018.
Watz, J., P. A. Nilsson, E. Degerman, C. Tamario & O. Calles, 2019. Climbing the ladder: an evaluation of three different anguillid eel climbing substrata and placement of upstream passage solutions at migration barriers. Animal Conservation 22: 452–462. https://doi.org/10.1111/acv.12485.
Acknowledgements
We would also like to express our gratitude to the staff of the Vattenfall R&D Laboratory for their help with operating the Laxeleratorn flume and setting up the experimental arena.
Funding
Open access funding provided by Karlstad University. This work was funded by the Faculty of Health, Science and Technology, Karlstad University, the Swedish Energy Agency (Grant P52096-1) and in-kind support from Vattenfall AB.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by Roman Motyka, Olle Calles and Johan Watz. Data collection was performed by all authors. Analysis was performed by Roman Motyka, Johan Watz and Andrew Harbicht. The first draft of the manuscript was written by Roman Motyka and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The experiments were carried out with ethical permission issued by the Animal Ethical Board of Sweden (5.8.18-13184/2017 and 5.8.18-03390/2019).
Additional information
Handling editor: Nicholas R. Bond
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Motyka, R., Watz, J., Aldvén, D. et al. Downstream passage performance of silver eel at an angled rack: effects of behavior and morphology. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05530-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05530-5