Abstract
Knowing the relationship between size, morphological traits and swimming performance of fish is essential to understand the swimming capacity to successfully surpass these obstacles and the selective pressure that barriers in rivers and streams could exert on fish. Northern straight-mouth nase, an endemic potamodromous cyprinid fish species from the Northwest of the Iberian Peninsula, was selected to carry out volitionally swimming performance experiments in an open channel against three different flow velocities, using telemetry and video cameras. The use of thin-plate spline, on 10 landmarks, evidenced unknown patterns linked to velocity barriers. At lower flow velocity, size is the main factor explaining the swimming performance; thus, large individuals swim up more efficiently. In contrast, at high flow velocities, shape becomes the essential explanatory variable; thereby, streamlined body shapes with a higher relative position of the tail and a narrower caudal peduncle are more efficient. The obtained results show the existence of a relationship between fish morphology and swimming performance, with potential consequences due to selection pressures associated with velocity barriers and their implications on behavioural and dispersal processes. To sum up, velocity barriers could exert a selection pressure on nase populations, so the fishway design and removal should be (re)considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rivers are among the most threatened ecosystems worldwide (Grzybowski & Glińska-Lewczuk, 2019). In Europe, most rivers have been deeply modified by anthropogenic structures like dams and weirs, impacting negatively the ecological processes and aquatic species (Gough et al., 2018). Moreover, it is estimated that more than 1.2 million obstacles fragment European rivers, most of them low head barriers (< 2 m in height; Belletti et al., 2020). These structures hinder or block the movement of aquatic fauna, especially fish, which is a vital element of their life history (Strayer & Dudgeon, 2010; Lothian et al., 2020).
Often river obstacles create velocity barriers, zones of high velocity flows that may exceed the ability of some or all individuals to pass, e.g. ramped dams, gauging stations, culverts, weirs, and fishways (Sanz-Ronda et al., 2015; Bravo-Córdoba et al., 2021). Therefore, velocity barriers bring about spatio-temporal environmental variation that affects the gene flow and selection (Cooke & Hinch, 2013). This results in a variation in fish migratory behaviour and associated traits within and among individuals, reducing the phenotypic and genetic variance in populations (Tamario et al., 2019). In particular, for migratory fish, velocity barriers may cause sublethal or nonlethal nonnatural selective pressure, leading to exclusion of fish from spawning areas, which in turn impacts population level (Morita & Yamamoto, 2002; Maynard et al., 2017). Successful passage of fish through such barriers is governed by a combination of swimming capacity, behaviour, and motivation (Castro-Santos et al., 2013). Artificial selection caused by barriers could become underlying force generating this variation, by modifying their dispersal behaviour (Apgar et al., 2017; Branco et al., 2017). This variation may influence the phenotypic and genetic structure of fish populations, as much for upstream movements as for downstream ones (Silva et al., 2018).
The selection pressure on fish populations and the characterization of the evolutionary impacts of velocity barriers have barely been tackled from a phenotypic perspective and never by quantifying morphological characters. In fact, most of them have been about salmonids and take into account features like size using traditional morphometrics (Marcus, 1990), but not morphological traits by removing size effect (Maynard et al., 2017; Lothian et al., 2020). Knowing the relationship between shape (sensu Benson, 1975) and swimming capacity of fish is essential to understand the selective pressure that velocity barriers in rivers and streams can exert on these organisms. Langerhans (2008) recognized gaps to understand the effect of water flow in phenotypic diversity of fish and found that more empirical tests are required to study hypothesis about linking morphology, locomotor performance, and fitness in fish. Therefore, it is fair to draw attention to the fact that the loss of phenotypic diversity can be observed in a few decades (Haugen et al., 2008). Approaches based on traits such as morphological or physiological characters, aspects of behaviour, or genome-level features, which combine functional and evolutionary information, are used in ecology to describe populations and communities and their responses to natural or anthropogenic changes. Body shape is the main interlocutor of an organism to interact with the environment (Benson, 1975). Shape can affect the interactions with conspecifics, the responses to environmental forces, and how the organism integrates with the surrounding community (Wootton, 1990; Rincón et al., 2007). Swimming performance, as a specific body shape ability, is the main character determining fitness and survival in many fish species (Schaefer et al., 2011), and is obviously, determined by phylogenetic, interactions with habitat and other species, individual constraints, and it may strongly influence the ability of a fish to obtain food, find a mate, select suitable habitats, migrate, reduce competition, and avoid predation or unfavourable habitat conditions (Plaut, 2001; Ohlberger et al., 2006; Langerhans & Reznick, 2010; Alexandre et al., 2014). It is considered to represent an adaptation to the prevailing environmental conditions (Pakkasmaa & Piironen, 2001; Leavy & Bonner, 2009; Franssen, 2011; Franssen et al., 2013). In this kind of studies, traditional morphometric techniques have been widely used. However, these techniques are based on measures intensively dependent of size, in consequence, many patterns may remain hidden. For this purpose, quantitative traits, as provided by geometric morphometric, are more practical, objective, informative, and size independent than qualitative ones, and they usually show considerable variation within and among populations (Caillon et al., 2018; Walsh & Lynch, 2018).
Therefore, the aim of this paper is to relate morphological traits to swimming performance by using for the first time geometric morphometrics in a swimming flume to determine whether velocity barriers could exert a selection pressure on mature specimens of fish populations. The cyprinid Northern straight-mouth nase, Pseudochondrostoma duriense (Coelho, 1985), hereafter referred to as “nase”, is a potamodromous medium-bodied water column fish, which inhabits running waters (Doadrio, 2002). It is endemic to the Iberian Peninsula and categorized by International Union for Conservation of Nature (Crivelli, 2006) as vulnerable. The obtained results could be essential to detect the existence of fish morphology selection and movement limitations because of velocity barriers, to enhance conservation efforts aimed at improving fishways and culvert design and establish management strategies to recover the natural diversity of fish.
Methods
Facilities
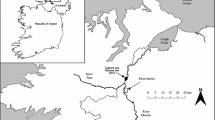
The study was carried out in a swimming flume located in the field at Vadocondes hydroelectric power plant (Burgos, Spain), on the Duero River (ETRS89 41,63,664,663 N 3,57,280,384 W). The flume was made of polished concrete to reduce turbulence, flow friction, and boundary layer effect. It had a zero slope and consisted of three modules (Fig. 1): head tank, swimming flume, and staging area. A slide gate system regulated the discharge and set the flow velocity and depth in the flume. A broader description of the flume can be seen in Sanz-Ronda et al. (2015) and Ruiz-Legazpi et al. (2018).

Modified from Ruiz-Legazpi et al. (2018)
Open channel flume facility in Vadocondes showing head tank, swim speed flume, and staging area as well as flow control mechanism (spillway, gates, and depth loggers) and monitoring system (PIT antennas and cameras).
During experimentation, flow monitoring was performed continuously: depth was monitored every minute (Orfeo-Mini probe, OTT Hydrome GmbH, Kempten, Germany; Accuracy: 0.002 m), flow rate every 30 min (Model 2100 speedometer, Swoffer Instruments Inc., Washington, USA; Accuracy: 0.01 m·s−1), and physicochemical parameters every hour (Multiparameter Water Quality Meter Hanna Instruments HI9829).
Fish sources
In this study, nases were engaged to carry out swimming performance experiments in an open-channel flume. We captured the specimens by electrofishing (Erreka Model; DC 300 V) and trapping in a fishway (closing water flow and collecting fish with hand nets) from the Arlanza River, a tributary of the Duero River. The captures were carried out in late autumn (28th and 29th of November 2013) when cyprinids move along the river searching winter habitats (Lucas et al., 2001).
Within 2 h of capture, fish were transported to the flume installations in 100 L aerated tanks and held in an acclimation pond at ambient water temperatures (5 °C). This pond consisted of two consecutive fish ladder pools, adjacent to the experimental flume. The pools measured 1.6 m in width, 1 m in depth, and 2.2 m in length each, and were supplied with water (50 L·s−1) directly from the Duero River.
All fish were anaesthetized with tricaine methanesulfonate 60 mg·L−1 (MS-222, Argent Chemicals, Redmond, WA), weighed (Body Mass BM ± 1 g), photographed, and surgically implanted (IP) with half-duplex PIT tags (TIRIS model RI-TRP-WRHP; Texas Instruments). These tags were 23 mm long and 3.85 mm in diameter and weighed 0.6 g, or < 1% of the body mass of the smallest tagged fish. This method has been shown to have negligible effects on growth, survival, and behaviour of many species (Brown et al., 1999; Ostrand et al., 2011; Castro-Santos & Vono, 2013). Acclimation period before trials was < 48 h, and neither fish died, nor erratic behaviour was detected. Fish were not fed during experiments, although the river water that supplied the experiment contained numerous food items that the fish could eat. Condition factor (CF = 100·BM·FL−3 in g·cm−3, where BM represents body mass and FL represents fork length) was calculated as a potential predictor of nase’s swimming performance in a swimming flume. In total, 32 nases, with FL ranging from 20.2 to 30 cm (Mean ± Standard Deviation: FL = 25.45 ± 2.75 cm; BM = 196.03 ± 67.59 g; CF = 1.29 ± 0.07 g·cm−3) participated in the experiments.
Image acquisition and shape analysis
A standardized protocol was used to acquire digital images for morphometric analyses. To avoid any damage while taking pictures, prior to photographing, all fish were anaesthetized as mentioned above. To avoid potential arching effects (Valentin et al., 2008), photographs were taken using a standardized method in which all fish were placed on their right side in a relaxed position on a flat surface and photographed from directly above using midline as a reference to linearity. A scale ruler was included in each photograph so that fork length (FL ± 0.01 cm) could be measured by using tpsDig v.2.31 (Rohlf, 2013a), and centroid size (CS), the square root of the summed square distances of each landmark from the centroid of the landmark configuration (Mean ± Standard Deviation: CS = 26.28 ± 3.02), was computed using tpsRelw version 1.70 (Rohlf, 2013b).
Morphometric analysis of body shape was carried out by means of geometric morphometrics (Bookstein, 1991; Zelditch et al., 2004). A total of 10 two-dimensional landmarks (Fig. 2) were obtained on digitized pictures by using as tpsDig v.2.31 (Rohlf, 2013a); of these, 9 landmarks had an unequivocal anatomic significance, and 1 homologous landmark (number 5) was geometrically determined but presented a clear anatomical undertone (Sánchez-González & Nicieza, 2017). Landmark configurations were superimposed, aligned, scaled, and rotated to a consensus shape. Thin-Plate Spline (TPS) analyses (Zelditch et al., 2004) were conducted by using tpsRelw version 1.70 (Rohlf, 2013b) to obtain 16 (2 k-4, where k is the number of landmarks) partial warp scores (14 uniform and 2 non-uniform components of shape), which are our geometric shape variables and used as a proxy of body size.
Collection of landmarks used for the morphometric analyses of Northern straight-mouth nase’s shape: (1) tip of upper jaw, (2) posterior supraoccipital notch, (3) anterior insertion of dorsal fin, (4) and (6) anterior junction of dorsal and ventral membrane from caudal fin, (5) intersection of lateral line and membrane of caudal fin, (7) and (8) posterior and anterior insertion of anal, (9) origin of pectoral fin, and (10) ventral insertion between operculum and the body outline
Experiments
A volitional fish swimming study was undertaken in a swimming flume. The study was configured to allow fish to swim against three different nominal flow velocities: 1.5, 2.5, and 3.0 m·s−1 (Trial1.5 [low velocity], Trial2.5 [medium velocity], and Trial3.0 [high velocity], respectively). Data dependency and the learning and fatigue dependence issues have been dealt by randomly splitting fish into the two experimental groups, and potential differences have been evaluated. The sequence of trials was selected randomly to test if learning or fatigue affect fish behaviour. The experiment was conducted between 30th November and 2nd December 2013. Fish were randomly divided into two experimental groups of 16 fish each, (n1 = 16 and n2 = 16). Each group of 16 individuals was exposed at once to the full range of flow velocity treatments, so that fish of each experimental group participated in every three trials. Each trial lasted 4 h, starting at 11:00 h (local time). Trial sequence was established to assure resting periods of > 18 h between consecutive events and fish were hold in in two resting areas placed in the fishway pools used for acclimation and in the flume-staging area (Fig. 1).
Swimming capacity was estimated with video recording and telemetry systems using the method described in Sanz-Ronda et al. (2015) and Ruiz-Legazpi et al. (2018), and the following variables were calculated: maximum distance (Dmax, maximum distance in m travelled through the flume by fish and trial), fatigue time (Ft, time in s employed in reaching Dmax from the start of the flume), and swimming speed (Us = Uf + Ug in m·s−1; where Uf is the mean flow velocity through which fish actually swam, and Ug is the ratio between Dmax and time). We considered a single attempt for each fish per trial, registering the one in which Dmax was reached, since it maximizes swimming capacity. Furthermore, we recorded this information as the binary variable response (with response [for swimming fish, i.e. when fish attempted to ascend], registering a Dmax, Ft, and Us, versus without response [for non-swimming fish, when fish did not attempt]).
The presence of shifts between prolonged and sprint swimming modes (Beamish, 1978) was also tested by applying the moving-point regression approach (Castro-Santos, 2005). The prolonged swimming mode corresponds to the speed that fish can maintain for around 20 s and ends in fatigue (sensu Castro-Santos et al., 2013; Sanz-Ronda et al., 2015), and sprint swimming mode corresponds to the highest speed attainable by fish and can be maintained for only short periods.
Experimentation was planned and developed under the international principles established in the Directive 2010/63 UE relative to the protection of animals used for scientific purposes.
Statistical analyses
We conducted a Principal Component Analysis (PCA) on the 16 partial warp scores (using prcomp function in stats package; R Development Core Team, 2020). This PCA was performed to obtain a multidimensional and empirical morphospace with orthogonal and, in consequence, uncorrelated independent variables (Pielou, 1977; Everitt, 2007), since original geometric shape variables are neither biologically nor statistically independent (Rohlf & Slice, 1990; Zelditch et al., 2004). PCA was performed on the covariance matrix (partial warp scores; alpha = 0) (Dryden & Mardia, 1998; McGarigal et al., 2000) to confer more weight to variables that describe global aspects of shape (Zelditch et al., 2004).
The analyses of which results are presented have been performed within trials, not between trials, so there is no data dependence between the three trials. To check for morphological and size differences (BM, FL, CS, and CF) between fish with and without response at the three different velocity trials, and to ensure there are not differences between the two 16-inviduals groups, we conducted the t student test or the nonparametric alternatives, such as Welch test and Wilcoxon rank sum test for non-homogeneous and non-normal data, to compare results between the same velocity trials. Previously and in all cases, normality was tested with the Shapiro–Wilk test, and homogeneity of variances was evaluated using the Levene’s test. These statistical analyses were conducted using R software (R Development Core Team, 2020).
In the second step, we compared shapes of the swimming reaction groups for the three different velocity trials to test the hypothesis of morphological differences between fish with and without response. With this aim, we performed three Discriminant Analyses (DA) on partial warps to assess differences between groups (McLellan & Endler, 1998), to describe the linear combination of variables that maximally discriminate between groups. To discard potential problems associated with small sample sizes, cross-validation of the discriminant functions was performed by random resampling of the experimental individuals; a subsample containing 75% of the cases was used for analysis; and the remainder 25% constituted the holdout sample in a loop of 1,000 random samples. We conducted three multivariate analysis of variance (MANOVA) tests on first three components of PCA to analyse significant morphological differences between fish groups according to their swimming response at the three trials (McGarigal et al., 2000). DA and MANOVA assumptions of homogeneity of variances and multivariate normality (McGarigal et al., 2000) were evaluated by using the Levene test and Shapiro–Wilk’s multivariate test, respectively, from the R package mvnormtest (Jarek, 2012).
After an exploratory correlation matrix analysis using chart.Correlation function from library PerformanceAnalytics (Peterson & Carl, 2020) for the three trials and cor.test and lm functions, we implemented general linear model (GLM) by using the glm and stepAIC functions in the MASS package (Venables & Ripley, 2002), with Dmax, Us, Ft, and response at Trial1.5, Trial2.5, and Trial3.0 as dependent variables, and CS, CF, BM, FL, and shape components (Principal Components from PCA; i.e. PC1, PC2, PC3, etc.) as explanatory variables; moreover, residuals were also analysed. We compared the performance of these models using the second-order Akaike Information Criterion (AICc). To assess the relative strengths of each candidate model, we used ΔAICc and calculated AICc weights and evidence ratios (ER) (Akaike, 1974; Burnham & Anderson, 2002; Richards et al., 2011). In case, Δi values were greater than 10, models were considered uninformative, and when Δi were less than 2, models were assumed to be equivalent to the best model (Burnham et al., 2011; Richards et al., 2011).
Results
Swim speed trials and parameters are included in Table 1. Moreover, distance ascent and fatigue time did not differ between the same velocity trials (Dmax: Wilcoxon rank sum test, p > 0.27; Ft: Wilcoxon rank sum test, p > 0.41). In general, fish (93%) swam in a prolonged swimming mode for Trial1.5, and in a sprint mode for Trial2.5 and Trial3 (64% and 100%, respectively). Shift in swimming mode was detected at Us = 2.6 m·s−1.
Size analysis
At Trial1.5, no significant differences were found between fish with and without swimming response (CS: t test, t30 = 1.5282, p = 0.137; BM: Wilcoxon test, W = 179, p = 0.05409; FL: t test, t30 = 1.639, p = 0.1117; CF: t test, t30 = 1.47, p = 0.152). However, it is important to point out that the BM difference was marginally significant: Fish with a lower body mass showed a higher response. At Trial2.5, swimming fish were significantly larger, longer, heavier, and had the highest CF (CS: t test, t30 = -2.5133, p = 0.01757; BM: Wilcoxon test, W = 62.5, p = 0.02649; FL: Wilcoxon test, W = 57, p = 0.01334; CF: Wilcoxon test, W = 62, p = 0.02357). Finally, at Trial3.0, non-significant differences in size were detected for BM and CF (BM: Welch test, t30 = − 1.5668, p = 0.1311; CF: t test, t30 = -0.88133, p = 0.3851) but marginally different for CS and FL (CS: Welsch test, t30 = − 1.874, p = 0.07269; FL: Welsch test, t30 = − 1.7874, p = 0.08681).
Shape analysis
The first three principal components absorbed 60.89% (PC1: 34.23%; PC2: 15.03%; PC3: 11.63%) of the whole shape variability. Specifically, PC1 arranged body shape variation on the relative head position (landmarks 1, 2, and 10; Fig. 2). Fish with a higher relative head position scored positive values of PC1, whereas fish with lower positioned head scored negative.
PC2 explained body depth or elongation; thus, shapes or morphological variation are ordered according to dorsal muscular mass development versus elongated, streamlined, or fusiform shapes. Deeper specimens, with an overdeveloped dorsal area and with a higher muscular mass in the dorsofrontal area (landmark 3), scored positive values for PC2. On the opposite side of PC2, specimens with elongated, streamlined, or fusiform shapes were placed.
Finally, PC3 summarized variation in the relative position of tail and the shape of the caudal peduncle (landmarks 4, 5, and 6; Fig. 3). Shorter peduncle and a lower position of the caudal peduncle scored negative values for PC3, whereas fish with higher-positioned, longer, and thinner peduncles had positive scores.
Scores of the principal component analysis (PCA) performed on the variances–covariances matrix for the morphological variables. First principal component (PC1; 34.2%): Positive scores are related to a higher-positioned head; second principal component (PC2; 15.1%): Positive scores correspond to deep-bodied and more robust shapes; third principal component (PC3; 13.7%): negative scores mean lower positioned tails and shorter peduncles. Red dot represents fish without any response and blue dot represents ascending fish in Trial3.0. Blue arrow indicates the observer’s perspective. This graph has been plotted using function plot_ly from plotly library (Sievert, 2020), and it has been published at https://rpubs.com/Xurde/623783
According to MANOVA results, we did not found differences in shape, either in Trial1.5 (Wilk’s Lambda = 0.94299, F3,28 = 0.56422, p = 0.6431) or in Trial2.5 (Wilk’s Lambda = 0.90668, F3,28 = 0.96067, p = 0.425), but we detected morphological differences in Trial3.0 (Wilk’s Lambda = 0.78099, F3,28 = 2.6172, p = 0.07062). These differences are mainly due to PC3 (F1,30 = 6.0906, p = 0.01951); fish with a higher relative position of their tails and a longer peduncle swam upstream during the experiments.
We conducted three DAs by calculating prior probabilities for response (with response and without response) in Trial1.5 (0.46875 and 0.53125, respectively), Trial2.5 (0.375 and 0.625, respectively), and Trial3.0 (0.21875 and 0.78125, respectively). DA functions (in a loop of 1,000 random samples) successfully classified 66.67% accuracy of fish for Trial1.5, 52.59% accuracy for Trial2.5, and 83.33% accuracy for Trial3.0.
Shape and size as swimming performance predictors
A potential positive relationship between PC3 and Us, Ft, and Dmax, was observed in Trial3.0 (Ft: S = 3418.7, ρ (Rho) = 0.3734, p = 0.03528; Dmax: S = 3176.6, ρ (Rho) = 0.4178, p = 0.01735; Us: S = 3051, ρ (Rho) = 0.4408, p = 0.01157) (Fig. 4) and marginally significant relationship between Dmax and shape in Trial1.5 (PC2: S = 302.31, ρ (Rho) = 0.4602, p = 0.0844) and Trial2.5 (PC1: S = 261.83, ρ (Rho) = 0.0845). However, even though preliminary analyses provided significant results, a detailed evaluation showed that neither PC1 nor PC2 were correlated to Dmax, Us, Ft in Trial1.5 and Trial2.5 (p > 0.05).
Linear relationship between the third principal component (PC3) and 95% confidence interval (in bluish grey), where specimens are arranged according to the relative position of their tails, shape of the caudal peduncle, and Swimming Speed (Us) in Trial3.0 (S = 3051, ρ (Rho) = 0.4408, p = 0.01157). Deformation grids (tpsSpline version 1.20 by Rohlf, 2004) show shape variation between the consensus and the individuals with the extreme PC3 values
GLM’s R-squared ranged from 0.29 to 0.39 (Online Resource). The GLM models that best explained fish response, Us, Dmax, and Ft can be partially explained by size (CS, FL, and BM) and, to a lesser extent, shape (PC2) for Trial1.5. For Trial2.5; those traits (CS, FL and, to a lesser extent, PC2) become determinant for explaining response, Us, Dmax, and Ft. For Trial3.0, shape (PC2 and PC3) became an essential descriptor of swimming performance, and size became a partial descriptor (CS and FL; Table 2 and Online Resource) (See Fig. 5, as a summary picture).
Number (n = deep + elongated) and shape of fish with a response at the three water velocity trials (1.5, 2.0, and 3.0 m·s−1). This is a conceptual scheme where numbers and distances are kept, but shapes should not be considered as binary but as gradient. Dark blue represents elongated fish with thinner and longer peduncle, and light blue represents deep-bodied fish with wider and shorter peduncles
Discussion
Predicting the viability of organisms and populations to rapid environmental changes, modifications, habitat destruction, and fragmentation is a central issue for the conservation of populations (Fagan, 2002; Hanski, 2011). Velocity barriers constitute important alterations of lotic ecosystems and cause obstructions to fish movements, such as dispersal processes and fish migration (Castro-Santos et al., 2013). Global shape aspects, such as elongation and/or streamlining, and other morphological traits, like relative tail and fin positions, mediate swimming performance and, in consequence, determine fish capability to swim up through those barriers (Beamish, 1978). In this study, we analysed those relationships and their possible consequences on nase population due to selective pressure in velocity barriers.
Despite the variance absorbed by the first 3 components (60.89%), our results suggest the presence of morphological differences, vague in Trial1.5, and conspicuous in Trial3.0. Thus, these results reveal that at the lowest tested velocities (1.5 m·s−1 and prolonged swimming mode), being large, deep-bodied, and, apparently, strong, is optimal for the individuals of the studied nase population (Fig. 5, Table 2, and Online Resource). Under higher water velocities (2.5 and 3 m·s−1 and sprint swimming mode), a change is observed and, being larger (from a lateral point of view) becomes optimal. At higher flow velocities, fusiform, elongated, and streamlined body shapes with a higher relative position of the tail and a narrower caudal peduncle are more efficient (Figs. 4, 5, Table 2, and Online Resource). This is because fish face high velocities by reducing drag forces and increasing thrust propulsion, reducing the energy expenditure required to swim up (Müller et al., 2000; Langerhans et al., 2003; Alexandre et al., 2014; Rubio-Gracia et al., 2020). Traditionally, fusiform, streamlined, and elongated shapes are linked to steady swimming in respirometer tests (Yan et al., 2013), where they develop swimming velocities close to the lowest values of prolonged speeds (Sanz-Ronda et al., 2015; Ruiz-Legazpi et al., 2018). In our case, fish did not perform prolonged swimming in Trial2.5 and Trial3.0, but sprint; under those conditions (Uf > 2.5 m·s−1), fusiform individuals could display a more efficient swimming performance than deep-bodied fish. Multimodel inference also suggested, to some extent that BM had relatively high importance at lower tested velocities, but shape gained relative weight at higher velocities. Furthermore, when flow velocity is lower, neither size nor shape is as strong a determinant as elongated shape is under high water velocity, and size cannot be considered as a plausible predictor of swimming at high flow velocities. Our results, despite multimodel constraints, show that larger deep-bodied individuals display better swimming performance at the lowest tested velocity, whereas the higher velocities could select individuals with a higher relative position of the tail, longer peduncle, and elongated shapes. This last statement is congruent with the results found by Alexandre et al. (2014) and Haas et al. (2015) in two different cyprinids species.
In general, morphometric variability reflects the adaptation to local conditions (Sánchez-González & Nicieza, 2017) and specifically to water velocity in salmonids streams (Beacham & Withler, 1985). Related to cyprinids, populations of blacktail shiner (Cyprinella venusta), living in habitats with higher mean annual run-off, exhibited, among others, characteristics, a more slender body, and caudal peduncle (Haas et al., 2015). Alexandre et al. (2014) experimented in a swimming tunnel with Iberian barbel (Luciobarbus bocagei), also a rheophilic cyprinid like nase, using individuals inhabiting both temporary river and flowing river. They found a lower critical swimming speed in temporary river fish and, as an interesting finding related to morphological traits that a combination of more fusiform body shape and a narrower caudal peduncle is better suitable for slow movement and manoeuvring than for swimming in flowing areas.
In contrast, at 2.5 and 3.0 m·s−1 flow velocities, when swimming mode changes from prolonged to sprint, elongated or fusiform shapes with thinner peduncles and a higher relative position of tails become more efficient to swim up (Figs. 4, 5, Table 2, and Online Resource). This pattern could be explained by the fact that deep-bodied shapes are related to burst swimming, territorial and hierarchical behaviour, and dominance (Monet et al., 2006; Rouleau et al., 2009; Sánchez‐González & Nicieza, 2021). In consequence, overdeveloped dorsal muscular mass, robust bodies, and caudal peduncles attain faster burst velocities (Cadrin, 2000; Pakkasmaa & Piironen, 2001), which, apparently, profit from a lower flow velocity, where burst swimming mode, short but explosive, could be more advantageous.
This study discards CF as a useful predictor of nase’s swimming performance in concordance with previous works (Reidy et al., 2000; Romão et al., 2012; Sanz-Ronda et al., 2019). However, Alexandre et al. (2016) obtained the opposite result, i.e. a significant positive linear relationship between critical swimming and CF. Since most studies used traditional analysis of morphometry, mainly with salmonids, most of the explained patterns remained unobserved so using geometric morphometric becomes essential to detect these patterns (Pakkasmaa et al., 1998; Cramon-Taubadel et al., 2005; Fraser et al., 2007). Moreover, geometric morphometric analyses are empirical, which means they are more robust than CF for hydrodynamics purposes, as their results depend on the analysed sample. Therefore, comparing results is difficult and can never be performed directly. Since the last decade, the use of geometric morphometric techniques has increased significantly, providing a plethora of contrastable outcomes.
Regarding the response variables Dmax, Us, and Ft, our study showed that they were mediated by shape and size with different patterns at different flow velocities. However, these results might be interpreted with caution due to the R-squared values, and we cannot discard the fact that other relationships were confirmed, or not, in a higher sampling size. The obtained outcomes are equally interesting since they can have important consequences for traditional management practices to deal with habitat fragmentation, dams, weirs, and other hydromorphological alterations that determine dispersal processes and connectivity. In fact, these results give reasons to doubt about the effectiveness and the effects of velocity barriers. Some of these velocity barriers permit upstream migration and dispersal processes, but then the following questions arise: which fish swim upstream? which sizes? which shapes? In this context, an important question to be explored in the future is whether these obstacles exert a selection pressure on the fish populations. Our results seem to point out that these velocity barriers, even when they are apparently working properly, can exert a selection pressure on mature specimens of this cyprinid species. In fact, we showed that ascending behaviour can be predicted by individual size and shape, which identifies correlated morphological and behavioural traits that support the pace-of-life syndrome (POLS) hypothesis. Then, these results and the found selection processes could imply substantial effects for the persistence of migratory and rheophilic fish species constrained to disperse within dendritic riverine networks with mainly unidirectional gene flow (Fagan, 2002). In consequence, velocity barriers assessment and fishways design might consider these results, by identifying the most suitable flow velocities during the nase’s migration, and, if it is required, by removing those velocity barriers of which selection pressure acts against migratory processes (Goerig et al., 2020).
To the best of our knowledge, this is one of the first times, geometric morphometrics has been used to evaluate the relationship between fish shape and swimming performance in velocity barriers, and it has been evidenced as a reliable tool for this kind of analysis, showing that at lower velocities, large individuals swim up more efficiently and that when water velocity increases, due to drag forces, streamlined body shapes with a higher relative position of the tail and a narrower caudal peduncle are more efficient. These results agree with our prediction that velocity barriers could represent a selection for certain body shapes of the individuals in a fish population, as previous studies have already confirmed (Wilkes et al., 2019; Goerig et al., 2020). This study contributes by explaining how this selection process take place at morphological level, thanks to geometrics morphometric. It could be interesting to figure out if velocity barriers by selecting shapes are causing behavioural alterations and, morphological selection, and selective fragmentation of which consequences are having an effect this selection is being produced at the genetic level, to verify the existence of a selection pressure on a migratory species within the context of a dendritic ecosystem causing an effective fragmentation of fish populations, as Goerig et al. (2020) already pointed out. In conclusion, obtained results confirm individual size and body shape as predictors of swimming performance for nase adults, with more potential consequences on fish population due to selection pressures associated with velocity barriers than might be considered by stakeholders and decision makers dealing with restoration of river connectivity and improvement of design specifications for culverts and fishways.
Availability of data and material
Data deposited in Figshare at https://doi.org/10.6084/m9.figshare.16985002.
Code availability
All codes used in this study are available through GitHub at https://github.com/JorgeRSanchez/FishMorphologyandBarriers.git or https://doi.org/10.5281/zenodo.5670135.
References
Akaike, H., 1974. A new look at the statistical model identification. IEEE Transactions on Automatic Control 19: 716–723.
Alexandre, C., R. Branca, B. R. Quintella, & P. R. Almeida, 2016. Critical swimming speed of the southern straight-mouth nase Pseudochondrostoma willkommii (Steindachner, 1866), a potamodromous cyprinid from southern Europe. Limnetica 35: 365–372.
Alexandre, C. M., B. R. Quintella, A. F. Ferreira, F. A. Romão, & P. R. Almeida, 2014. Swimming performance and ecomorphology of the Iberian barbel Luciobarbus bocagei (Steindachner, 1864) on permanent and temporary rivers. Ecology of Freshwater Fish 23: 244–258.
Apgar, T. M., D. E. Pearse, & E. P. Palkovacs, 2017. Evolutionary restoration potential evaluated through the use of a trait-linked genetic marker. Evolutionary Applications 10: 485–497.
Beacham, T. D., & R. E. Withler, 1985. Heterozygosity and morphological variability of chum salmon (Oncorhynchus keta) in southern British Columbia. Heredity 54: 313–322.
Beamish, F. W. H., 1978. Swimming capacity. Fish physiology. Academic Press, New York.
Belletti, B., C. Garcia de Leaniz, J. Jones, et al., 2020. More than one million barriers fragment Europe’s rivers. Nature 588(7838): 436–441. https://doi.org/10.1038/s41586-020-3005-2.
Benson, R. H., 1975. Morphological Stability in Ostracoda. Bulletin of American Paleontology 65: 13–46.
Bookstein, F. L., 1991. Morphometric Tools For Landmark Data: Geometry and Biology. Cambridge University Press, New York.
Branco, P., S. D. Amaral, M. T. Ferreira, & J. M. Santos, 2017. Do small barriers affect the movement of freshwater fish by increasing residency? Science of The Total Environment 581–582: 486–494, http://www.sciencedirect.com/science/article/pii/S0048969716328455.
Bravo-Córdoba, F. J., J. Valbuena-Castro, A. García-Vega, J. F. Fuentes-Pérez, J. Ruiz-Legazpi, & F. J. Sanz-Ronda, 2021. Fish passage assessment in stepped fishways: Passage success and transit time as standardized metrics. Ecological Engineering 162: 106172, https://www.sciencedirect.com/science/article/pii/S0925857421000264.
Brown, R. S., S. J. Cooke, W. G. Anderson, & R. S. McKinley, 1999. Evidence to Challenge the “2% Rule” for Biotelemetry. North American Journal of Fisheries Management 19: 867–871.
Burnham, K. P., & D. R. Anderson, 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer-Verlag, New York, USA.
Burnham, K. P., D. R. Anderson, & K. P. Huyvaert, 2011. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behavioral Ecology and Sociobiology 65: 23–35.
Cadrin, S. X., 2000. Advances in morphometric identification of fishery stocks. Fish Biology and Fisheries 10: 91–112.
Caillon, F., V. Bonhomme, C. Möllmann, & R. Frelat, 2018. A morphometric dive into fish diversity. Ecosphere 9: e02220.
Castro-Santos, T., 2005. Optimal swim speeds for traversing velocity barriers: an analysis of volitional high-speed swimming behavior of migratory fishes. The Journal of Experimental Biology 208: 421–432.
Castro-Santos, T., F. J. Sanz-Ronda, & J. Ruiz-Legazpi, 2012. Breaking the speed limit — comparative sprinting performance of brook trout (Salvelinus fontinalis) and brown trout (Salmo trutta). Canadian Journal of Fisheries and Aquatic Sciences 70: 280–293.
Castro-Santos, T., & V. Vono, 2013. Posthandling Survival and PIT Tag Retention by Alewives—A Comparison of Gastric and Surgical Implants. North American Journal of Fisheries Management Taylor & Francis 33: 790–794.
Coelho, M. M., 1985. The straight mouth Portuguese Chondrostoma Agassiz, 1835. II-Taxonomic position and geographic distribution of Ch. polylepis Steindachner, 1865 and Ch. willkommii Steindachner, 1866 with the description of a new subspecies —Ch. polylepis duriensis. Arquivos do Museu Bocage Serie A, 3: 13–38.
Cooke, S. J., & S. G. Hinch. 2013. Improving the reliability of fishway attraction and passage efficiency estimates to inform fishway engineering, science, and practice. Ecological Engineering 58: 123–132, http://www.sciencedirect.com/science/article/pii/S0925857413002061.
Cramon-Taubadel, N. von, E. N. Ling, D. Cotter, & N. P. Wilkins, 2005. Determination of body shape variation in Irish hatchery-reared and wild Atlantic salmon. Journal of Fish Biology 66: 1471–1482.
Crivelli, A. J., 2006. Pseudochondrostoma duriense. The IUCN Red List of Threatened Species 2006: e.T60736A12402329, https://doi.org/10.2305/IUCN.UK.2006.RLTS.T60736A12402329.en.
Doadrio, I. 2002. Atlas y Libro Rojo de los Peces Continentales de España. Consejo Superior de Investigaciones Científicas. Secretaria General de Medio Ambiente. Dirección General de Conservación de la Naturaleza. Ministerio de Medio Ambiente., Madrid. España.
Dryden, I. L., & K. V Mardia, 1998. Statistical Shape Analysis. Wiley-Blackwell.
Everitt, B., 2007. An R and S-PLUS companion to Multivariate Analysis. Springer-Verlag., London.
Fagan, W. F., 2002. Connectivity, fragmentation, and extinction risk in dendritic metapopulations. Ecology 83: 3243–3249.
Franssen, N. R., 2011. Anthropogenic habitat alteration induces rapid morphological divergence in a native stream fish. Evolutionary Applications John Wiley & Sons, Ltd 4: 791–804.
Franssen, N. R., J. Harris, S. R. Clark, J. F. Schaefer, & L. K. Stewart, 2013. Shared and unique morphological responses of stream fishes to anthropogenic habitat alteration. Proceedings of the Royal Society B: Biological Sciences Royal Society 280: 20122715.
Fraser, D. J., L. K. Weir, T. L. Darwish, J. D. Eddington, & J. A. Hutchings, 2007. Divergent compensatory growth responses within species: linked to contrasting migrations in salmon? Oecologia 153: 543–553, http://www.ncbi.nlm.nih.gov/pubmed/17541646.
Goerig, E., B. A. Wasserman, T. Castro-Santos, & E. P. Palkovacs. 2020. Body shape is related to the attempt rate and passage success of brook trout at in-stream barriers. Journal of Applied Ecology 57(1): 91–100.
Gough, P., P. Fernandez Garrido, & J. Van Herk. 2018. Dam Removal. A viable solution for the future of our European rivers. 38, https://damremoval.eu/wp-content/uploads/2018/07/Dam-Removal-Europe-Report-2018-DEF-1.pdf.
Grzybowski, M., & K. Glińska-Lewczuk, 2019. Principal threats to the conservation of freshwater habitats in the continental biogeographical region of Central Europe. Biodiversity and Conservation 28: 4065–4097.
Haas, T. C., D. C. Heins, & M. J. Blum, 2015. Predictors of body shape among populations of a stream fish (Cyprinella venusta, Cypriniformes: Cyprinidae). Biological Journal of the Linnean Society 115: 842–858.
Hanski, I., 2011. Habitat Loss, the Dynamics of Biodiversity, and a Perspective on Conservation. AMBIO 40: 248–255.
Haugen, T. O., P. Aass, N. C. Stenseth, & L. A. Vøllestad, 2008. Changes in selection and evolutionary responses in migratory brown trout following the construction of a fish ladder. Evolutionary Applications 1: 319–335.
Jarek, S. 2012. mvnormtest: Normality test for multivariate variables. R project package: mvnormtest v. 0.1–9. R Foundation for Statistical Computing., https://cran.r-project.org/package=mvnormtest.
Langerhans, R. B., 2008. Predictability of phenotypic differentiation across flow regimes in fishes. Integrative and Comparative Biology 48: 750–768.
Langerhans, R. B., C. A. Layman, & T. J. Dewitt, 2003. Habitat-associated morphological divergence in two Neotropical fish species. Biological Journal of the Linnean Society John Wiley & Sons, Ltd 80: 689–698.
Langerhans, R., & D. Reznick, 2010. Ecology and Evolution of Swimming Performance in Fishes: Predicting Evolution with Biomechanics In Domenici, P., & B. G. Kapoor (eds), Fish Locomotion. An Eco-ethological Perspective. Science Publishers: 200–248.
Leavy, T. R., & T. H. Bonner, 2009. Relationships among Swimming Ability, Current Velocity Association, and Morphology for Freshwater Lotic Fishes. North American Journal of Fisheries Managements 29: 72–83.
Lothian, A. J., M. Schwinn, A. H. Anton, C. E. Adams, M. Newton, A. Koed, & M. C. Lucas. 2020. Are we designing fishways for diversity? Potential selection on alternative phenotypes resulting from differential passage in brown trout. Journal of Environmental Management 262: 110317, http://www.sciencedirect.com/science/article/pii/S0301479720302528.
Lucas, M. C., E. Baras, T. J. Thom, A. Duncan, & O. Slavik, 2001. Migration of freshwater fishes. Blackwell Science Ltd, Oxford.
Marcus, L. 1990. Tradicional morphometrics. In Rohlf, F. J., & F. L. Bookstein (eds), Proceedings of the Michigan Morphometrics Workshop. Special publication 2. The University of Michigan Museum of Zoology., Ann Arbor, Michigan, pp. 77–122.
Maynard, G. A., M. T. Kinnison, & J. D. Zydlewski, 2017. Size selection from fishways and potential evolutionary responses in a threatened Atlantic salmon population. River Research and Applications John Wiley & Sons, Ltd 33: 1004–1015.
McGarigal, K., S. Cushman, & S. Stafford, 2000. Multivariate Statistics for Wildlife and Ecology Research. Springer, New York, NY.
McLellan, T., & J. A. Endler, 1998. The relative success of some methods for measuring and describing the shape of complex objects. Systematic Zoology 47: 264–281.
Monet, G., A. Uyanik, & A. Champigneulle, 2006. Geometric morphometrics reveals sexual and genotypic dimorphisms in the brown trout. Aquatic Living Resources 19: 47–57.
Morita, K., & S. Yamamoto, 2002. Effects of habitat fragmentation by damming on the persistence of stream-dwelling Charr populations. Conservation Biology 16: 1318–1323. https://doi.org/10.1111/ecog.04985
Müller, U. K., E. J. Stamhuis, & J. J. Videler, 2000. Hydrodynamics of unsteady fish swimming and the effects of body size: comparing the flow fields of fish larvae and adults. Journal of Experimental Biology 203: 193–206.
Ohlberger, J., G. Staaks, & F. Hölker, 2006. Swimming efficiency and the influence of morphology on swimming costs in fishes. Journal of Comparative Physiology B 176: 17–25.
Ostrand, K. G., G. B. Zydlewski, W. L. Gale, & J. D. Zydlewski, 2011. Long term retention, survival, growth, and physiological indicators of salmonids marked with passive integrated transponder tags. American Fisheries Society Symposium 76: 1–11, http://pubs.er.usgs.gov/publication/70193177.
Pakkasmaa, S., & J. Piironen, 2001. Morphological differentiation among local trout (Salmo trutta) populations. Biological Journal of the Linnean Society 72: 231–239, http://linkinghub.elsevier.com/retrieve/pii/S0024406600904887.
Pakkasmaa, S., E. Ranta, & J. Piironen, 1998. A morphometric study on four land-locked salmonid species. Annales Zoologici Fennici 35: 131–140.
Peterson, B. G., & P. Carl. 2020. PerformanceAnalytics: Econometric tools for performance and risk analysis. R package version 2.0.4. https://cran.r-project.org/package=PerformanceAnalytics.
Pielou, E. C., 1977. Mathematical Ecology. Harper & Row., New York.
Plaut, I. 2001. Critical swimming speed: its ecological relevance. Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology 131: 41–50. http://www.sciencedirect.com/science/article/B6VNH-44HXSF8-5/2/63a13defb4f7f87da3900339b61258f0.
R Development Core Team. 2020. R: A Language and Environment for Statistical Computing. R version 4.0.2 - “Taking Off Again.” R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/.
Reidy, S. P., S. R. Kerr, & J. A. Nelson, 2000. Aerobic and anaerobic swimming performance of individual Atlantic cod. Journal of Experimental Biology The Company of Biologists Ltd 203: 347–357.
Richards, S. A., M. J. Whittingham, & P. A. Stephens, 2011. Model selection and model averaging in behavioural ecology: the utility of the IT-AIC framework. Behavioral Ecology and Sociobiology 65: 77–89.
Rincón, P. A., M. Bastir, & G. D. Grossman, 2007. Form and performance: body shape and prey-capture success in four drift-feeding minnows. Oecologia 152: 345–355.
Rohlf, F. J., 2013a. tpsDig2: a program to digitize landmarks and outlines. Department of Ecology and Evolution, State University of New York, Stony Brook, http://life.bio.sunysb.edu/morph/.
Rohlf, F. J., 2013b. tpsRelw. Relative warps analysis. Department of Ecology and Evolution, State University of New York, Stony Brook, http://life.bio.sunysb.edu/morph/.
Rohlf, F. J., & D. Slice, 1990. Extensions of the procrustes method for the optimal superimposition of landmarks. Systematic Zoology 39: 40–59.
Romão, F., B. R. Quintella, T. J. Pereira, & P. R. Almeida, 2012. Swimming performance of two Iberian cyprinids: the Tagus nase Pseudochondrostoma polylepis (Steindachner, 1864) and the bordallo Squalius carolitertii (Doadrio, 1988). Journal of Applied Ichthyology John Wiley & Sons, Ltd 28: 26–30.
Rouleau, S., H. Glémet, & P. Magnan, 2009. Effects of morphology on swimming performance in wild and laboratory crosses of brook trout ecotypes. Functional Ecology Blackwell Science 24: 1–12.
Rubio-Gracia, F., E. García-Berthou, H. Guasch, L. Zamora, & A. Vila-Gispert, 2020. Size-related effects and the influence of metabolic traits and morphology on swimming performance in fish. Current Zoology 66: 493–503.
Ruiz-Legazpi, J., F. J. Sanz-Ronda, F. J. Bravo-Córdoba, J. F. Fuentes-Pérez, & T. Castro-Santos, 2018. Influencia de factores ambientales y biométricos en la capacidad de nado del barbo ibérico (Luciobarbus bocagei Steindachner, 1864), un ciprínido potamódromo endémico de la Península Ibérica. Limnetica 37: 251–265.
Sánchez-González, J.-R., & A. G. Nicieza, 2017. Phenotypic convergence of artificially reared and wild trout is mediated by shape plasticity. Ecology and Evolution 7: 5922–5929.
Sánchez‐González, J.-R., & A. G. Nicieza, 2021. Individual differences in dominance-related traits drive dispersal and settlement in hatchery-reared juvenile brown trout. Scientific Reports 14. Accepted.
Sanz-Ronda, F. J., F. J. Bravo-Córdoba, A. Sánchez-Pérez, A. García-Vega, J. Valbuena-Castro, L. Fernandes-Celestino, M. Torralva, & F. J. Oliva-Paterna, 2019. Passage performance of technical pool-type fishways for potamodromous cyprinids: Novel experiences in semiarid environments. Water (Switzerland) 11: 1–14.
Sanz-Ronda, F. J., J. Ruiz-Legazpi, F. J. Bravo-Córdoba, S. Makrakis, & T. Castro-Santos, 2015. Sprinting performance of two Iberian fish: Luciobarbus bocagei and Pseudochondrostoma duriense in an open channel flume. Ecological Engineering 83: 61–70, http://www.sciencedirect.com/science/article/pii/S0925857415300513.
Schaefer, J., D. Duvernell, & B. Kreiser, 2011. Shape variability in topminnows (Fundulus notatus species complex) along the river continuum. Biological Journal of the Linnean Society.
Sievert, C. 2020. Interactive Web-Based Data Visualization with R, plotly, and shiny. Chapman and Hall/CRC. https://plotly-r.com.
Silva, A. T., M. C. Lucas, T. Castro-Santos, C. Katopodis, L. J. Baumgartner, J. D. Thiem, K. Aarestrup, P. S. Pompeu, G. C. O’Brien, D. C. Braun, N. J. Burnett, D. Z. Zhu, H.-P. Fjeldstad, T. Forseth, N. Rajaratnam, J. G. Williams, & S. J. Cooke, 2018. The future of fish passage science, engineering, and practice. Fish and Fisheries 19: 340–362.
Strayer, D. L., & D. Dudgeon, 2010. Freshwater biodiversity conservation: recent progress and future challenges. Journal of the North American Benthological Society 29: 344–358.
Tamario, C., J. Sunde, E. Petersson, P. Tibblin, & A. Forsman, 2019. Ecological and Evolutionary Consequences of Environmental Change and Management Actions for Migrating Fish. Frontiers in Ecology and Evolution 7: 1–24.
Valentin, A. E., X. Penin, J.-P. P. Chanut, J.-M. Sevigny, F. J. Rohlf, J. M. Sévigny, & F. J. Rohlf, 2008. Arching effect on fish body shape in geometric morphometric studies. Journal of Fish Biology 73: 623–638.
Venables, W. N., & B. D. Ripley, 2002. Modern Applied Statistics with S. Springer-Verlag New York, New York, NY.
Walsh, B., & M. Lynch, 2018. Evolution and Selection of Quantitative Traits. Oxford University Press, Oxford.
Wilkes, M. A., J. A. Webb, P. S. Pompeu, L. G. M. Silva, A. S. Vowles, C. F. Baker, P. Franklin, O. Link, E. Habit, & P. S. Kemp, 2019. Not just a migration problem: Metapopulations, habitat shifts, and gene flow are also important for fishway science and management. River Research and Applications 35: 1688–1696. https://doi.org/10.1002/rra.3320.
Wootton, R. J., 1990. Ecology of teleost Fishes. Chapman & Hall, London.
Yan, G. J., X. K. He, Z. D. Cao, & S. J. Fu, 2013. An interspecific comparison between morphology and swimming performance in cyprinids. Journal of Evolutionary Biology 26: 1802–1815.
Zelditch, M. L., D. L. Swiderski, H. D. Sheets, & W. L. Fink, 2004. Geometric Morphometrics for Biologists: A Primer. Elsevier. Elsevier Academic Press.
Acknowledgements
We specifically thank Juan Carlos Romeral de la Puente (SAVASA) for letting us use his installations in Vadocondes. This research was supported by Castilla and León Regional Government (Project VA299B11-2). We would also like to thank Itagra.ct technological centre for logistical support.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research has been supported by Castilla and León Regional Government: Project VA299B11-2: “Swimming capacity evaluation in Iberian fish” and Itagra.ct technological center.
Author information
Authors and Affiliations
Contributions
FJSR and JRL planned the work and conducted the trials. JRSG processed the images, generated the morphometric, and analysed the data. FM and FJSR contributed to statistical analyses. JRSG and FM led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
The authors provide formal written consent to publish before the publication of the work.
Ethical approval
Fish were treated in accordance with the European Union Directive 2010/63/UE on the protection of animals used for scientific purposes, and following the ethical guidelines of Valladolid University, code CEA ES471860000033, and the Government of Castille and Leon Region, under authorization 7904309. All efforts were made to minimize stress, and fish were released after the experiments.
Additional information
Guest editors: Javier Sánchez-Hernández & Rufino Vieira-Lanero / Insights and Advances in Iberian Ichthyology
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sánchez-González, J.R., Morcillo, F., Ruiz-Legazpi, J. et al. Fish morphology and passage through velocity barriers. Experience with northern straight-mouth nase (Pseudochondrostoma duriense Coelho, 1985) in an open channel flume. Hydrobiologia 849, 1351–1366 (2022). https://doi.org/10.1007/s10750-021-04712-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04712-9