Abstract
The global spread of non-native species is leading to an increasing frequency of multiple co-occurring non-native species. We examined the co-occurrence of the bivalve mollusc Dreissena polymorpha (zebra mussel) with three Ponto-Caspian amphipods (Dikerogammarus villosus, Dikerogammarus haemobaphes, and Chelicorophium curvispinum) across England and Wales in association with in-situ substrate size. For all three amphipod species, substrate grain size where amphipods co-occurred with D. polymorpha was significantly finer than when recorded in isolation. Subsequently, we confirmed this via aquarium experiments. We examined the occurrence of D. villosus with D. polymorpha when present with cobbles, gravel, or sand from three population sources (co-location with abundant D. polymorpha populations, co-location with low populations, and naïve). Experiments demonstrated that D. villosus actively sought shelter on or near D. polymorpha, with their co-location being significantly more prevalent in finer grained substrates (sand > gravel > cobble). The strength of this co-location differed by population source, with those co-located with high D. polymorpha densities demonstrating a greater association. Our analyses and experiments indicate that D. polymorpha may facilitate Ponto-Caspian amphipod establishment in otherwise suboptimal locations, whereby mussel shells provide favourable structural habitat for the amphipods, analogous to the presence of coarse-grained benthic sediment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquatic invasive species (AIS) represent one of the leading threats to biodiversity globally (Sala et al., 2000; Doherty et al., 2016) and have also been shown to lead to geomorphic (Fei et al., 2014; Mason & Sanders, 2021) and socioeconomic impacts (Pejchar & Mooney, 2009; Diagne et al., 2023). Freshwater environments are particularly at risk from biological invasions due to their high levels of connectivity (Sala et al., 2000), with proportionally higher rates of successfully establishing invasive species (García-Berthou et al., 2005) and subsequent biodiversity losses (Dudgeon et al., 2006; Guareschi et al., 2021) comparative to other ecosystems.
A key ‘donor’ area of AIS is the Ponto-Caspian region (Cuthbert et al., 2020; Soto et al., 2023), which is characterised by heterogeneous environmental conditions, typically resulting in highly tolerant species (Reid & Orlova, 2002). Ponto-Caspian amphipod crustaceans in particular are an invasive and adaptable group and have established invasive populations across much of Europe (Copilaş-Ciocianu et al., 2023). In particular, three amphipods have increased their distribution across Great Britain (Gallardo & Aldridge, 2015): Dikerogammarus villosus (Sowinsky 1984), Dikerogammarus haemobaphes (Eichwald 1841), and Chelicorophium curvispinum (G. O. Sars 1895), first recorded in the UK in 2010, 2012, and 1935, respectively. Of particular concern are D. villosus (killer shrimp), which have been identified as one of the 100 worst invasive species in Europe (DAISIE, 2009), and one of five invasive species of special governmental concern for UK biodiversity (Gallardo & Aldridge, 2013). Dikerogammarus villosus has co-evolved with another widespread Ponto-Caspian AIS, Dreissena polymorpha (Pallas 1771) (zebra mussel), currently established through Western Europe and North America (Strayer, 2009; van de Velde et al., 2010; Sanders et al., 2022). Facilitative interactions have been cited as a key factor in the invasion success of Ponto-Caspian taxa (Ricciardi, 2001; Gallardo & Aldridge, 2015), with some evidence of facilitative association between D. villosus and D. polymorpha documented in a number of field and experimental studies (Devin et al., 2003; MacNeil et al., 2008; Tricarico et al., 2010; Kobak et al., 2014; Rolla et al., 2019). However, these observations are typically centred on observational data from small spatial scales (typically a few waterbodies) making it difficult to ascertain if this association is facilitative or just incidental co-location. The limited number of studies that have sought to quantify the association between dreissenid mussels and Ponto-Caspian amphipods have indicated that the strength of their facilitative relationship appears to vary with the substrate size and habitat complexity (MacNeil et al., 2008; Kobak et al., 2014). This is likely due to substrate preferences, with Dikerogammarus spp. observed to display preferences for coarse, complex substrates under both field and laboratory conditions (Devin et al., 2003; Kley et al., 2009; Kobak et al., 2015; Clinton et al., 2018). As such, the association of Dikerogammarus spp. with D. polymorpha may be strong where substrate composition is predominantly comprised of fine materials, but weaker in environments where coarse substrate grain sizes are dominant (Kobak et al., 2015; Mills et al., 2017; Mills, 2019). Thus, whilst the combined evidence of prior studies provides evidence for Dikerogammarus spp. preference for dreissenid mussels, as these studies have been undertaken independently, differences in the response observed may alternately be attributable to between-population variability, rather than a function of the sediments present.
Currently, species distribution modelling recognises spatial co-occurrence between Ponto-Caspian taxa (e.g. Gallardo & Aldridge 2015), but so far, such patterns have rarely been considered with respect to potentially important abiotic factors, such as bed substrate size. Understanding how the abiotic environment may influence and interact with Ponto-Caspian associations is required to better model potential invasion pathways and future invasion scenarios. A further challenge is identifying whether models of species invasions are appropriate for all populations. Invasive species have been shown to demonstrate high phenotypic plasticity (Reznick & Ghalambor, 2001; Wright et al., 2010; Davidson et al., 2011; Sol & Weis, 2019), with variability observed both within and between invasive populations (e.g. Magurran et al., 1992; Holway & Suarez, 1999; Mowery et al., 2021; Sanders et al., 2023). Populations of Ponto-Caspian amphipods that do and do not co-occur with D. polymorpha may therefore exhibit different responses to their presence if translocated to a new environment.
Here, we firstly conducted a large-scale spatial analysis of locations where three Ponto-Caspian amphipods (D. villosus, D. haemobaphes, and C. curvispinum) have been recorded to either occur in isolation or co-occur with zebra mussels (D. polymorpha) in England and Wales. We also sought to examine whether this co-occurrence differed as a function of the dominant substrate grain size present. To examine mechanistically if differences in the co-occurrence of Ponto-Caspian amphipods and D. polymorpha were structured by substrate size, laboratory experiments were subsequently undertaken. The influence of population source (co-located or naïve to D. polymorpha) was also considered. Dikerogammarus villosus were selected as the model species in the laboratory experiments due to their priority setting for UK biodiversity (Gallardo & Aldridge, 2013). Specifically, four questions were examined:
-
1.
What is the spatial extent of co-occurrence between D. polymorpha and D. villosus, D. haemobaphes, and C. curvispinum in England and Wales?
-
2.
Does the dominant substrate size differ between locations where D. polymorpha and the three Ponto-Caspian amphipods co-occur?
-
3.
Are there observable differences in the association of D. villosus and D. polymorpha as a function of substrate size when tested mechanistically in experiments?
-
4.
Does in-situ naivety of D. villosus to D. polymorpha alter the observed strength of D. villosus’ association under experimental conditions?
We hypothesised that there would be a high co-occurrence of Ponto-Caspian amphipods and D. polymorpha; that substrate characteristics would differ between locations where D. polymorpha and the Ponto-Caspian amphipods do and do not co-occur; that in ex-situ experiments, finer substrates would lead to a greater co-location of D. villosus and D. polymorpha; and that in-situ naivety of D. villosus to D. polymorpha would alter the observable strength of D. villosus’ association.
Materials and methods
Ponto-Caspian co-occurrence at a national scale
Environment Agency freshwater sampling locations across England and Wales where any of the four species (D. polymorpha (n = 502), D. villosus (n = 75), D. haemobaphes (n = 692), and C. curvispinum (n = 429)) were recorded to be present between 2010 and 2020 were obtained via the NBN Atlas (2023) database and subsequently cross compared to identify co-location. Dikerogammarus villosus, D. haemobaphes, and C. curvispinum were considered to co-occur if they had been recorded in the same location in the same calendar year, and D. polymorpha were considered to be present in any year from 2010 to 2020, due to the kick sampling method under-recording bivalve presence (Blackman et al., 2020). For sites where records from multiple years were present, only the most recent records were retained for analysis to prevent pseudo-replication. 8,819 sites were recorded as active between 2010 and 2020 (Environment Agency, 2023), which were used to calculate the proportion of sites at which the taxa were identified to be present at. Unconfirmed occurrences and CC-BY-NC licenced records were excluded from analyses.
To identify whether observed co-locations may be influenced by the benthic substrate size, the dominant grain size of each sampling location were quantified using the River Invertebrate Prediction and Classification System (RIVPACS) database (Centre for Ecology and Hydrology, 2023; Environment Agency, 2023). The RIVPACS database provides a record of the proportion of bed substrate via visual observations of the substrate fractions ‘boulders and cobbles’, ‘pebbles and gravel’, ‘sand’, and ‘silt and clay’ for 474 lotic reference sites across England and Wales (Fig. 1a). Spatial interpolation analysis in ArcMap 10.8 (ESRI, 2020) using Inverse Distance Weighting (Fig. 1a) was used to create a continuous raster layer across England and Wales representing the proportion of bed material characterised as coarse (defined as ‘boulders and cobbles’ or ‘pebbles and gravel’ in the RIVPACS database), with a processing extent of the UK coastline. Extrapolating reference site data to the landscape scale has been shown to provide a rapid and affordable method of river habitat mapping (Naura et al., 2016), and the substrate map produced here was validated via a visual comparison with the Channel Substrate Index mapping undertaken by Naura et al. (2016). Subsequently, the proportion of coarse material at locations where the four Ponto-Caspian species had been identified was extracted (Fig. 1b).
a Distribution of RIVPACS sites across England and Wales where substrate size has been quantified (n = 474), and coarse grain size data interpolated; b percentage of coarse bed material for each of the Ponto-Caspian sites was subsequently extracted. D. polymorpha distribution is shown here for demonstration. The distribution of datapoints is shown for the percentage of substrate characterised as ‘coarse’ at locations where D. polymorpha is shown; D. polymorpha are geographically widespread, and there is very little skew in the data distribution. Boxes show the 25th, 50th, and 75th percentiles, whiskers the 5th and 95th percentiles, and outliers are shown as dots
The spatial distribution of the dominant grain size in England and Wales is not randomly distributed (Fig. 2a), with coarser substrate prevailing mostly in the west, and finer substrate in the east. However, such a geographical bias is unlikely to affect the results with D. polymorpha being widely distributed across England and Wales and thus occupying the entire grain size distribution (Fig. 1b).
The co-location of Ponto-Caspian amphipod species is known to lead to differences in habitat occupation; for example, the more competitive D. villosus has been documented to displace its counterpart D. haemobaphes to less preferred substrate (e.g. Borza et al., 2017; Clinton et al., 2018; Mathers et al., 2023). Thus, we accounted for spatial co-location of each focal amphipod species with D. polymorpha independently, but also in the presence of another Ponto-Caspian amphipod. Locations where D. villosus co-occurred with another Ponto-Caspian amphipod were removed from the dataset prior to analysis because of low replication (Table 1), and there were no locations where all three amphipod taxa were co-located.
Subsequently, two statistical models were run: one examining D. villosus in the presence and absence of D. polymorpha, and one examining D. haemobaphes and C. curvispinum independently and co-occurring in the presence/absence of D. polymorpha, to account for the potential displacement effects of the amphipods on each other. To examine differences in the grain size observed for D. villosus with and without D. polymorpha, a Wilcoxon test was used (due to non-normality and heteroscedasticity of the data). To examine differences in grain size for D. haemobaphes and C. curvispinum, a linear model fitted with the factors of (1) D. haemobaphes, (2) C. curvispinum, (3) D. haemobaphes and C. curvispinum, (4) D. haemobaphes and D. polymorpha, (5) C. curvispinum and D. polymorpha, and (6) D. haemobaphes, C. curvispinum, and D. polymorpha was employed. Prior to analyses, proportions were logit transformed to satisfy model assumptions. Post-hoc pairwise comparisons were undertaken using estimated marginal means with p-values adjusted for multiple comparisons via Tukey tests within the ‘emmeans’ package (Lenth et al. 2020). All statistical analyses were undertaken in the R environment (version 4.3.1; R Core Team 2021).
Aquarium experiments
To observe mechanistic evidence of the possible association between D. villosus and D. polymorpha and the influence of substrate size, laboratory aquarium experiments were undertaken. Dikerogammarus villosus were selected as the model Ponto-Caspian amphipod species due to their priority setting for UK biodiversity (Gallardo & Aldridge 2013). A three × three fully factorial experimental design was employed. Experiments were undertaken using three D. villosus populations (amphipods from environments where D. polymorpha density was high, low, and absent), examined across three substrate grain sizes (sand, gravel, and cobbles). Ten experimental replicates were undertaken per substrate treatment for each amphipod population, giving a total of 90 experimental trials.
Experimental setup
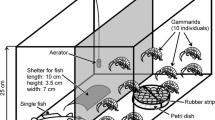
Experiments were completed in July and August 2022 using polypropylene aquariums (internal base area 290 mm × 210 mm). Substrates of fluvial origin were purchased from a local aggregate supplier, rinsed with tap water, and air dried prior to use in the experiments. Clean (non-biologically active) substrates were used in the experiments in order to remove potential differences in biofilm development. A substrate depth of ca. 40 mm was used, and each aquarium filled with 6 L of dechlorinated tap water, equivalent to 120 mm water depth. Fifteen alive, adult D. polymorpha (> 18 mm ventral length; mean = 25.1 mm ± 3.3 mm) were placed in one half (lengthways) of each aquarium (Fig. 2), representative of a density of 500 ind. m−2 of D. polymorpha, comparable with typical dreissenid densities at invaded sites (e.g. Mills, 2019; Sanders et al., 2022). Whilst D. polymorpha exhibited some minor movements during the experiments, all individuals remained within the assigned portion of the aquarium. D. polymorpha attached to the substrate in each of the experimental runs. The laboratory was illuminated for 10 h (09:00–19:00) by laboratory ceiling lights and maintained in a temperature-controlled laboratory at 20 °C (verified at the start and end of each experiment). Aquariums were prepared three hours prior to the start of the experiment to allow for water temperature to stabilise.
To examine whether D. villosus association with D. polymorpha was consistent across grain sizes (Q3), three substrate treatments were examined: (1) homogenous sand, with a grain size (median = 0.33 mm) finer than that of an average adult D. polymorpha shell (D. polymorpha mean width = 11.8 mm ± 0.3 mm; Coughlan et al., 2021), (2) fine gravel (median = 11.7 mm), with a grain size similar to that of a D. polymorpha shell; and (3) a coarse gravel and cobble mix (median 25.9 mm), with a coarser grain size to that of a D. polymorpha shell (Fig. 2).
To examine whether the association of D. villosus with D. polymorpha was consistent across populations with different levels of naivety (Q4), three populations of D. villosus were employed: (1) a sympatric population of D. villosus co-located with a high density (1775 ind. m−2) of D. polymorpha, where living D. polymorpha were the dominant substrate feature; (2) a sympatric population of D. villosus co-located with a low density of D. polymorpha (11 ind. m−2); and (3) a population of D. villosus where D. polymorpha were absent.
Organism collection
Dreissena polymorpha were collected from abundant populations on Barton Broad, Norfolk, UK (52.740, 1.501), ca. 5 m from the littoral bankside at a depth of ca. 1.2 m. Organisms were collected using a standard pond net (1 mm mesh net) and sealed in plastic storage boxes on site. A 0.2 m × 0.2 m Petit Ponar grab sampler prior to specimen collection quantified local D. polymorpha density as 1775 individuals m−2 and determined bed substrate characteristics as fine particulate organic matter (< 1 mm diameter) and empty shells/fragments of deceased D. polymorpha (2450 fragments m−2). Individuals were subsequently returned to the laboratory and placed into aquariums as described above on the day of sampling. Mussels were assessed to be alive by observing active siphoning, a lack of gaping, and resisting being opened with tweezers, as in Coughlan et al. (2020) and Sanders & Mills (2022).
Sympatric D. villosus co-located with a high density of D. polymorpha were collected using a pond net from the same location as the D. polymorpha used in the experiments (Barton Broad; 52.741, 1.500). A 0.2 m × 0.2 m Petit Ponar grab sampler quantified local D. villosus density as 350 individuals m−2. Sympatric D. villosus co-located with a low density of D. polymorpha were collected from Pitsford Water, Northamptonshire, UK (52.316, − 0.867). Amphipods were collected from the base of a large boulder berm. At this site, the dominant substrate consisted of coarse boulder fragments overlying a bed of homogenous fine particulate matter. Quadrat surveys (1 m × 1 m, n = 5) quantified local D. polymorpha density as 11 individuals m−2, and D. villous density as 47 individuals m−2, which were found exclusively on or under the coarse boulder material. A third source population that were not exposed to D. polymorpha were collected from Rollesby Broad, Norfolk, UK (52.679, 1.642). Gravel was the dominant substrate and Surber sampling (n = 5) quantified local D. villosus density as 484 individuals m−2. As with the D. polymorpha, all D. villosus individuals were sealed in a plastic storage box on site immediately after collection for transportation. All D. villosus populations examined were well established (Barton Broad first detected in 2012, Pitsford Water in 2015, and Rollesby Broad in 2020), and were expanding geographically and in density (Mathers et al. 2023; Norfolk Wildlife Trust pers. comms.), indicating that D. villosus can succeed, as well as persist, in habitats where D. polymorpha are absent.
Experimental procedure
Amphipods were kept in the laboratory for three hours prior to the start of the experiments, to allow for acclimation to the laboratory temperature. During this period, amphipods were kept in containers containing 50% dechlorinated tap water and 50% water from the transport bags. After the acclimation period, experiments commenced by placing ten individuals into each aquarium. After 24 h, a divider was placed down the centre of each aquarium, and the experiment deconstructed. First, all 15 D. polymorpha were removed by hand, and the number of D. villosus sheltering directly on the D. polymorpha recorded. Subsequently, aquarium nets were used to remove all substrate from both sides of the divider into white sorting trays, and all ten D. villosus were recovered and counted. All D. villosus and D. polymorpha were confirmed to be alive at the end of experiments. Dikerogammarus villosus survival was assessed by placing recovered amphipods into dechlorinated tap water and observing the amphipod swimming, and D. polymorpha mortality was assessed as above. At the end of the experiment, all D. villosus were preserved in 95% Industrial Methylated Spirits (IMS).
D. villosus location within the aquarium at the end of the experiment was recorded using two metrics: (1) the total number of D. villosus in the D. polymorpha half of the aquarium, and (2) the number of D. villosus sheltering directly on D. polymorpha.
Statistical analysis
All statistical analyses were undertaken in the R environment (version 4.3.1; R Core Team 2021). To analyse whether the proportion of D. villosus retrieved from the D. polymorpha half of the aquarium was greater than 50%, indicating a significant preference for the mussel substrate, a one-tailed, one-sample Wilcoxon test (due to non-normality in data) was utilised with the percentage of amphipods retrieved from the mussel half as the response variable. To analyse whether the strength of the response of D. villosus differed associated with the two factors (substrate and source population), a generalized linear model (GLM) fitted with a binomial error distribution and logit link structure using the ‘glm’ function in the ‘stats’ package was employed. Two models were employed: (1) D. villosus retrieved from the D. polymorpha half of the aquarium as the response variable, and (2) D. villosus sheltering directly on D. polymorpha (as a proportion of all individuals recovered from the D. polymorpha half of the aquarium) as the response variable. For each model, the response variable was inputted as a matrix of the proportion of amphipods that were observed with D. polymorpha and those that were not. Substrate (sand, gravel, or cobble) and source location (Barton, Pitsford, and Rollesby) were fitted as fixed interacting factors. Where significant differences occurred for location or substrate independently, post-hoc pairwise comparisons were examined using estimated marginal means with p-values adjusted for multiple comparisons via Tukey tests within the ‘emmeans’ package (Lenth et al. 2020).
Results
Ponto-Caspian co-occurrence at a national scale
The Environment Agency recorded D. polymorpha presence at 496 independent monitoring locations across England and Wales from 2010 to 2020, a proportional abundance of 5.6% of all surveyed sites during this period (n = 8,819). C. curvispinum (n = 429; 4.9%) and D. haemobaphes (n = 692; 7.8%) also displayed a widespread distribution, whereas D. villosus were recorded at 75 independent sampling locations (0.9%), which were grouped in five distinct regions/sites: The Norfolk Broads (n = 50), Grafham Water (n = 8), Pitsford Water (n = 2), the River Taff, Cardiff (n = 4), and Eglwys Nunydd Reservoir (n = 11). Of these, D. villosus were co-located with D. polymorpha at 49.3% of locations (Fig. 3a). Excluding Eglwys Nunydd Reservoir, where D. polymorpha were not recorded within the waterbody, 82% of all other D. villosus records were < 5 km from confirmed D. polymorpha records (Fig. 3h), and in each case within the same continuous water body. Therefore, sampling points where D. villosus have been recorded in the absence of D. polymorpha may reflect sampling resolution and non-detection of D. polymorpha rather than local D. polymorpha absence, and so a 49% spatial co-location can be considered a minimum estimate. Similarly, D. haemobaphes (26%) and C. curvispinum (38%) were also highly co-located with D. polymorpha throughout England and Wales (Fig. 3).
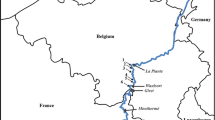
Spatial distribution of the four examined species of Ponto-Caspian AIS in England and Wales overlain on interpolated proportion of substrate typology characterised as coarse [consisting of gravel, cobble, and boulder bed substrate recorded in the RIVPACS database (Centre for Ecology and Hydrology, 2023; Environment Agency, 2023)]. a Dikerogammarus villosus, b D. haemobaphes, c C. curvispinum, and d D. haemobaphes and C. curvispinum records co-located with D. polymorpha, and e–h that were not co-located with D. polymorpha, i all D. polymorpha sites, and j a map of the River Ant, Norfolk Broads, displaying D. polymorpha and D. villosus. The Norfolk Broads is illustrated for visual comparison of spatial proximity, as this is the English region with the greatest number of recordings of D. polymorpha and D. villosus
There were also high rates of co-location between the studied amphipods. Co-occurrence of amphipods was observed at 60% of sites where C. curvispinum were recorded, 36% of sites where D. haemobaphes were recorded, and at 20% of sites where D. villosus were located (Table 1). There were no sites where all three Ponto-Caspian amphipods were co-located.
Substrate size was determined to be significantly finer at locations where D. villosus was co-located with D. polymorpha than when D. villosus was found independently (W = 185.5, P < 0.001; Fig. 4a). Considering D. haemobaphes and C. curvispinum, substrate size was found to be significantly different between factor groups (F5, 855 = 31.24, P < 0.001; Fig. 4b; Table 2). Post-hoc tests indicated that substrate size was significantly finer at locations where D. polymorpha were co-located with D. haemobaphes (P < 0.001) and C. curvispinum (P = 0.004) than when D. polymorpha were absent (Fig. 4b). There was no statistical difference in substrate size between D. polymorpha presence and absence when both D. haemobaphes and C. curvispinum were recorded together (P = 0.975). When D. polymorpha were absent, C. curvispinum inhabited significantly finer substrates than D. haemobaphes (P < 0.001).
Median percentage of substrate characterised as coarse (boulder, cobble, pebble, and gravel) for monitoring locations where D. polymorpha and the three Ponto-Caspian amphipods (Dikerogammarus villosus, D. haemobaphes, and C. curvispinum) are present in isolation, in combination, and co-located with D. polymorpha. Boxes show the 25th, 50th, and 75th percentiles, whiskers the 5th and 95th percentiles, and outliers are shown as dots. Groups with the same letter in (b) are not significantly different
Aquarium experiments
The median percentage of D. villosus recovered from the D. polymorpha half of the aquarium was significantly greater than 50% in seven of the nine treatment groups (Fig. 5; Table 3), indicating a preference for the D. polymorpha substrate in the majority of the treatments. Selection of the D. polymorpha half was greater in all substrate treatments for the D. villosus populations collected from the locations where D. polymorpha were present, but only in the sand treatment for the D. villosus that were naïve to D. polymorpha (Fig. 5; Table 3). No significant differences were recorded for the gravel and cobble treatments for naïve populations (Fig. 5; Table 3).
Proportion of D. villosus in the D. polymorpha half of the mesocosm for each of the nine tested treatments. Black dashed line indicates 50%, where no preference for either side of the aquarium is observed. Boxes show the 25th, 50th, and 75th percentiles, whiskers the 5th and 95th percentiles, and outliers are shown as dots. Asterisks indicate where the median proportion of amphipods in the D. polymorpha half of the aquarium was significantly greater than 50% (*P < 0.05, **P < 0.01; ***P < 0.001)
The Generalized Linear Model (GLM) indicated that the proportion of D. villosus recorded in the D. polymorpha half of the aquarium statistically differed as a function of the source population of D. villosus (X2,90 = 20.63, P < 0.001) and substrate treatment (X2,90 = 173.78, P < 0.001; Fig. 6a), but there was no significant interaction between source population and substrate (X4,90 = 2.85, P > 0.05). In the sand treatment, all ten individuals were recorded in the D. polymorpha half of the aquarium for every replicate, with the exception of one replicate with the D. polymorpha-naïve population (nine out of ten). Post-hoc comparisons indicated this was significantly greater than the gravel (72%) and cobble (65%) treatments (P < 0.001; Table 4; Table S1). There was no statistical difference between the gravel and cobble treatments (P > 0.05).
When the amphipod population source was considered, post-hoc comparisons indicated that the proportion of D. villosus recorded with D. polymorpha was significantly greater for amphipods collected at sites where D. polymorpha were abundant (Barton Broad; mean = 86% across all substrate treatments) compared to locations where D. polymorpha density was low (Pitsford; 78%, P = 0.008) or absent (Rollesby Broad; 73%, P < 0.001; Table 4; Table S1). There was no difference between the populations where D. polymorpha density was low and absent (P > 0.05).
When the proportion of D. villosus recorded directly on D. polymorpha (as a proportion of all amphipods in the D. polymorpha half) was considered, individuals in the sand treatment were found exclusively on the D. polymorpha (100%) with none in the substrate, and as such the treatment data were removed from subsequent analyses. Statistical examination indicated there were significant differences associated with substrate treatment (X2,90 = 33.2, P < 0.001), source population (X2,90 = 24.0, P < 0.001), and the interaction of population and substrate (X4,90 = 80.9, P < 0.001; Fig. 6). The proportion of amphipods sheltering directly on D. polymorpha in the gravel treatment was significantly greater than in the cobble treatment for all populations (high D. polymorpha density, P = 0.0014; low D. polymorpha density, P < 0.001; D. polymorpha naïve, P < 0.001; Fig. 6a; Table 5). Post-hoc tests indicated that there was no significant difference in the proportion of amphipods located on D. polymorpha taken from locations where D. polymorpha density was low and absent for the cobble treatment and between locations where D. polymorpha density was high and low for the gravel treatment (Table 5; Fig. 6b).
Discussion
Previously, spatial associations between Ponto-Caspian amphipods and dreissenid mussels have been examined experimentally in aquariums, or observed at a small number of field sites within a single lake or catchment (Devin et al., 2003; MacNeil et al., 2008; Tricarico et al., 2010; Kobak et al., 2014; Rolla et al., 2019). This study has expanded current understanding of these associations to a national scale in England and Wales and corroborated this observational finding by providing mechanistic evidence via aquarium experiments. Further, we evidence that the co-location of D. villosus with D. polymorpha was found to differ associated with substrate grain size (observational and experimental) and as a function of D. polymorpha-aware and D. polymorpha-naïve populations (experimental).
Ponto-Caspian co-occurrence at a national scale
Analysis of a large national dataset indicated that 49%, 26%, and 38% of locations where D. villosus, D. haemobaphes, and C. curvispinum have been recorded, respectively, also supported D. polymorpha (RQ1). Such co-occurrence is notable because of the comparatively low proportion of total possible sites where these taxa were recorded. For instance, two species occurring at 80% of all recorded sites would be expected to have a high co-location rate due to their high abundance (with a minimum co-location of 75% of sites that contain species A also containing species B). However, the low total proportion of sites that D. villosus were present at (0.9%) combined with D. polymorpha being present at 5.6% of all possible locations indicates such co-location is high.
Previously, meta-analysis studies (Ricciardi, 2001; Gallardo & Aldridge, 2015), experimental aquarium (e.g. Kobak & Żytkowicz, 2007; MacNeil et al., 2008; Kobak et al., 2013; Kobak et al., 2014; Rolla et al., 2019), and observational studies at a small number of field sites (e.g. Devin et al., 2003; MacNeil et al., 2008; Tricarico et al., 2010) have evidenced a degree of co-location between these species; however, our analysis of a single large national dataset suggests such patterns may also occur at large spatial scales. This finding suggests that co-occurrence of Ponto-Caspian AIS is a frequent phenomenon across invaded lotic systems, which may be driven by the facilitative effects of the more widespread D. polymorpha. Such results are concerning with respect to the potential spread and establishment of D. villosus, which is currently limited to five focal locations in the UK (75 sampling locations). Given that D. polymorpha represents a widely distributed species, it is plausible that populations of D. polymorpha could support the further geographic spread of D. villosus and other AIS (Devin et al., 2003; MacNeil et al., 2008; Tricarico et al., 2010; Gallardo & Aldridge, 2013, 2015).
There are many mechanisms via which dreissenid mussels are hypothesised to facilitate benthic taxa: by increasing habitat complexity (Burlakova et al., 2012), with the complex interstices providing refugia from predation (Botts et al., 1996); by providing a solid medium for oviposition (Stewart et al., 1999) and a hard surface for tube attachment in relation to C. curvispinum (Nakano & Strayer, 2014); by facilitating other macroinvertebrate biomass (DeVanna et al., 2011) which can be predated on; and by providing a build-up of shell biofilm and mussel pseudofaeces as food resources (Stewart et al. 1998). Such habitats are likely actively selected as documented in observational experiments examining utilisation of live vs dead mussel shells (Kobak et al., 2009, 2013).
Such facilitation may also extend the range of invasive gammarids with regard to substrate type. Our analysis showed that, on average, co-location of D. polymorpha with the amphipods occurred in water bodies where substrate size was finer than where the amphipods were found in the absence of D. polymorpha, with pairwise tests demonstrating this difference for all three tested amphipod species (RQ2). Dikerogammarus species have been observed to display preferences for coarse, complex substrates in both field and laboratory conditions (Devin et al., 2003; Kley et al., 2009; Kobak et al., 2015; Clinton et al., 2018), and so the presence of D. polymorpha may facilitate Dikerogammarus spp. establishment in otherwise unfavourable habitats. Gammarid utilisation of dreissenid mussels for habitat in environments characterised by fine-grained substrate has been observed in the North American Great Lakes (Ricciardi, 2001; González & Burkart, 2004), and our results suggest this may also occur in lotic systems.
Previous research has documented antagonistic interactions between Ponto-Caspian amphipods, with the less dominant amphipod being forced to a less favourable substrate, or being completely displaced (Borza et al., 2017; Clinton et al., 2018; Mathers et al., 2023). In the absence of D. polymorpha, D. haemobaphes and C. curvispinum inhabited waterbodies with significantly different substrate grain sizes, with C. curvispinum preferring fine substrate. However, co-location of D. haemobaphes and C. curvispinum with D. polymorpha was recorded at coarser locations. This result suggests the potential overlap of these species may represent the grain size overlap at the fine and coarse substrate extents of D. haemobaphes and C. curvispinum, respectively, and that these two amphipods may be able to co-exist together. Whilst Ponto-Caspian amphipods can co-exist via niche partitioning (e.g. Kley & Maier, 2005; Borza et al., 2017, 2018), the boom-and-bust dynamics of invasions can drive changes between invaders (Mathers et al., 2023), and so increased high-resolution monitoring of invaded systems and further aquarium experiments are required to fully examine potential interactions between Ponto-Caspian taxa across the habitat template.
Dikerogammarus villosus aquarium experiments
Dikerogammarus villosus were found to be associated with D. polymorpha across all three substrate treatments, but this association was strongest in the sand treatment where 99% of amphipods were recorded from the D. polymorpha half of the aquariums, and 100% were directly sheltering on the D. polymorpha (RQ3). This association was significantly stronger than in the gravel and cobble treatments, respectively. Moreover, in the gravel and cobble treatments, we observed that the majority of D. villosus individuals were located in the substrate rather than directly on the mussels, further demonstrating that the relation of amphipods with D. polymorpha with coarse substrates is weaker than with sand. Given the coarse substrate preference of both D. villosus and D. haemobaphes (Clinton et al., 2018), it is possible this substrate-dependent utilisation of D. polymorpha habitat is present for both members of the Dikerogammarus genus.
Dikerogammarus villosus have been documented to use chemical cues to locate D. polymorpha (Rolla et al., 2019). Our experiments were undertaken in lentic aquariums, where the strength and detectability of chemical cues were likely to be consistent across treatments. Therefore, the selection of substrate other than D. polymorpha is probably an active selection, rather than a random selection following amphipod non-detection of D. polymorpha.
Considering population source, D. villosus were found to be associated with D. polymorpha across all three tested populations, but the strength of this response differed between populations (RQ4). D. villosus previously co-located with high densities of D. polymorpha in situ displayed the strongest association in our experiments. Inter-specific population differences have not been examined previously, although one study did observe no differences in the movement of D. villosus towards the chemical cues of D. polymorpha when considering D. villosus from sympatric and allopatric populations (Rolla et al., 2019). In contrast, our study is the first to our knowledge that has observed differences in the association between D. villosus populations that have varying degrees of previous exposure to D. polymorpha. This is notable, because D. villosus previously exposed to high densities of D. polymorpha displayed stronger responses than those exposed to low densities of D. polymorpha. However, it should be noted that the substrate characteristics of the sites where D. villosus were collected from differed (Barton Broad, fine sediment; Pitsford Water, fine sediment with large boulders; Rollesby Broad, gravels), which could have affected our results. However, whilst there was a difference in the magnitude of the response between D. villosus populations with varying previous exposure to D. polymorpha, the direction of the response was consistent across all tested populations. Therefore, whilst care is required when considering behavioural naivety, the notion that dreissenid mussels may facilitate the establishment of D. villosus can still be supported. Previously, burrowing Hexagenia mayflies (DeVanna et al. 2011) and Physidae snails (Stewart et al. 1999) have rapidly adapted to the presence of dreissenid mussels, utilising their shell interstices as refugia, demonstrating there is broad adaptability within the aquatic invertebrate community to dreissenid invasion.
Invasional meltdown hypothesis
Where established AIS have been evidenced to facilitate additional species invasions, with accumulative deleterious impacts on native species (Simberloff, 2006; Guareschi et al. 2021), it has been described as ‘invasional meltdown’ (sensu Simberloff & von Holle, 1999). It has been suggested that UK freshwater environments are on the verge of widespread invasional meltdown (Gallardo & Aldridge, 2015), with the establishment of co-occurring AIS populations being recorded at an accelerating frequency (Keller et al., 2009). Within this context, our study provides some evidence for facilitative associations between AIS in UK freshwaters but also suggests that bed substrate typology and the biotic context of expanding populations may alter the strength of facilitative mechanisms in some environments. For example, whilst D. villosus were recorded more frequently with D. polymorpha in aquarium experiments in coarse-grained substrate, such habitat selection was not observed for D. polymorpha-naïve D. villosus. Nevertheless, there were high levels of Ponto-Caspian amphipod and D. polymorpha co-location across England and Wales, which differed by substrate grain size, with greater co-location in finer grained environments. It follows that contribution to an ‘invasional meltdown’ in UK rivers by D. polymorpha may not be spatially uniform, but contingent on biotic histories and local environmental conditions.
More broadly, experiments examining interactions between invasive species, including those examining invasional meltdown hypothesis, have often presented contradictory results (e.g. see Jeschke et al., 2012; Braga et al., 2018). The results presented in this study—that interactions between the same invasive species differ depending on both biotic (population source) and abiotic (sediment size) factors—may help explain contradictory observations from studies examining the same species interactions but from independent populations and environments. Combining experimental approaches to form a process-based understanding with analyses of large-scale field datasets will help to further our understanding of the variability of interactions between invasive species, and help inform large-scale and location-nuanced management of biological invasions.
Conclusion
Our study demonstrates that, at a large spatial level (England and Wales), there is a high degree of co-location between Ponto-Caspian amphipods and D. polymorpha, and that this is mostly evident at locations characterised by finer grained substrates. Aquarium experiments supported this mechanistic association with finer substrates. Further, they suggested the strength of this co-location differed between amphipod populations, with D. villosus individuals that occurred with high D. polymorpha densities in situ demonstrating greater co-location than D. polymorpha-naïve amphipods. Overall, this study has provided evidence that D. polymorpha may be facilitating the geographical spread of Ponto-Caspian amphipods into less optimal substrate compositions, but such facilitation is dependent on both biotic histories and abiotic environmental context. As the number of translocated non-native species continues to grow and co-occurrences become more common, understanding the potential role of abiotic factors (substrate composition) in contributing to distribution patterns is vital.
Data availability
Data are available from the lead author upon reasonable request.
References
Blackman, R. C., K. K. S. Ling, L. R. Harper, P. Shum, B. Hänfling & L. Lawson-Handley, 2020. Targeted and passive environmental DNA approaches outperform established methods for detection of quagga mussels, Dreissena rostriformis bugensis in flowing water. Ecology and Evolution 10(23): 13248–13259.
Borza, P., T. Huber, P. Leitner, N. Remund & W. Graf, 2017. Current velocity shapes co-existence patterns among invasive Dikerogammarus species. Freshwater Biology 62(2): 317–328. https://doi.org/10.1111/fwb.12869.
Borza, P., T. Huber, P. Leitner, N. Remund, & W. Graf. 2018. How to coexist with the ‘killer shrimp’Dikerogammarus villosus? Lessons from other invasive Ponto-Caspian peracarids. Aquatic Conservation: Marine and Freshwater Ecosystems 28(6): 1441–1450. https://doi.org/10.1002/aqc.2985.
Botts, P.S., B.A. Patterson, & D.W. Schloesser. 1996. Zebra mussel effects on benthic invertebrates: physical or biotic? Journal of the North American Benthological Society 15(2): 179–184. https://doi.org/10.2307/1467947.
Braga, R. R., L. Gómez-Aparicio, T. Heger, J. R. S. Vitule & J. M. Jeschke, 2018. Structuring evidence for invasional meltdown: broad support but with biases and gaps. Biological Invasions 20(4): 923–936.
Burlakova, L. E., A. Y. Karatayev & V. A. Karatayev, 2012. Invasive mussels induce community changes by increasing habitat complexity. Hydrobiologia 685: 121–134. https://doi.org/10.1007/s10750-011-0791-4.
Centre for Ecology and Hydrology, 2023. RIVPACS reference database website. https://www.ceh.ac.uk/services/rivpacs-reference-database.
Clinton, K. E., K. L. Mathers, D. Constable, C. Gerrard & P. J. Wood, 2018. Substrate preferences of coexisting invasive amphipods, Dikerogammarus villosus and Dikerogammarus haemobaphes, under field and laboratory conditions. Biological Invasions 20: 2187–2196. https://doi.org/10.1007/s10530-018-1695-2.
Copilaş-Ciocianu, D., D. Sidorov & E. Šidagytė-Copilas, 2023. Global distribution and diversity of alien Ponto-Caspian amphipods. Biological Invasions 25: 179–195. https://doi.org/10.1007/s10530-022-02908-1.
Coughlan, N. E., E. M. Cunningham, S. Potts, D. McSweeney, E. Healey, J. T. Dick, G. Y. Vong, K. Crane, J. M. Caffrey, F. E. Lucy & E. Davis, 2020. Steam and flame applications as novel methods of population control for invasive Asian Clam (Corbicula fluminea) and Zebra Mussel (Dreissena polymorpha). Environmental Management 66: 654–663. https://doi.org/10.1007/s00267-020-01325-1.
Coughlan, N. E., E. M. Cunningham, R. N. Cuthbert, P. W. Joyce, P. Anastácio, F. Banha, N. Bonel, S. J. Bradbeer, E. Briski, V. L. Butitta & Z. Čadková, 2021. Biometric conversion factors as a unifying platform for comparative assessment of invasive freshwater bivalves. Journal of Applied Ecology 58: 1945–1956. https://doi.org/10.1111/1365-2664.13941.
Cuthbert, R. N., S. G. Kotronaki, J. T. Dick & E. Briski, 2020. Salinity tolerance and geographical origin predict global alien amphipod invasions. Biology Letters 16: 20200354. https://doi.org/10.1098/rsbl.2020.0354.
DAISIE, 2009. Handbook of Alien Species in Europe, Springer, Knoxville.
Davidson, A. M., M. Jennions & A. B. Nicotra, 2011. Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A Meta-Analysis. Ecology Letters 14(4): 419–431.
Devin, S., C. Piscart, J. N. Beisel & J. C. Moreteau, 2003. Ecological traits of the amphipod invader Dikerogammarus villosus on a mesohabitat scale. Archiv Für Hydrobiologie 158: 43–56. https://doi.org/10.1127/0003-9136/2003/0158-0043.
DeVanna, K.M., P.M. Armenio, C.A. Barrett, & C.M. Mayer. 2011. Invasive ecosystem engineers on soft sediment change the habitat preferences of native mayflies and their availability to predators. Freshwater Biology 56(12): 2448–2458. https://doi.org/10.1111/j.1365-2427.2011.02668.
Diagne, C., L. Ballesteros-Mejia, R. N. Cuthbert, T. W. Bodey, J. Fantle-Lepczyk, E. Angulo, A. Bang, G. Dobigny & F. Courchamp, 2023. Economic costs of invasive rodents worldwide: the tip of the iceberg. PeerJ 11: e14935. https://doi.org/10.7717/peerj.14935.
Doherty, T. S., A. S. Glen, D. G. Nimmo, E. G. Ritchie & C. R. Dickman, 2016. Invasive predators and global biodiversity loss. Proceedings of the National Academy of Sciences of the United States of America 113: 11261–11265. https://doi.org/10.1073/pnas.1602480113.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z. I. Kawabata, D. J. Knowler, C. Lévêque, R. J. Naiman, A. H. Prieur-Richard, D. Soto, M. L. Stiassny & C. A. Sullivan, 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81: 163–182. https://doi.org/10.1017/S1464793105006950.
Environment Agency, 2023. Environment Agency Ecology & Fish Data Explorer website. https://environment.data.gov.uk/ecology/explorer/.
ESRI, 2020. ArcGIS desktop: Version 10.8. Environmental Systems Research Institute, Redlands.
Fei, S., J. Phillips & M. Shouse, 2014. Biogeomorphic impacts of invasive species. Annual Review of Ecology, Evolution, and Systematics 45: 69–87. https://doi.org/10.1146/annurev-ecolsys-120213-091928.
Gallardo, B. & D. C. Aldridge, 2013. The ‘dirty dozen’: socio-economic factors amplify the invasion potential of 12 high-risk aquatic invasive species in Great Britain and Ireland. Journal of Applied Ecology 50: 757–766. https://doi.org/10.1111/1365-2664.12079.
Gallardo, B. & D. C. Aldridge, 2015. Is Great Britain heading for a Ponto-Caspian invasional meltdown? Journal of Applied Ecology 52: 41–49. https://doi.org/10.1111/1365-2664.12348.
García-Berthou, E., C. Alcaraz, Q. Pou-Rovira, L. Zamora, G. Coenders & C. Feo, 2005. Introduction pathways and establishment rates of invasive aquatic species in Europe. Canadian Journal of Fisheries and Aquatic Sciences 62: 453–463. https://doi.org/10.1139/f05-017.
González, M.J., & G.A. Burkart. 2004. Effects of food type, habitat, and fish predation on the relative abundance of two amphipod species, Gammarus fasciatus and Echinogammarus ischnus. Journal of Great Lakes Research 30(1): 100–113. https://doi.org/10.1016/S0380-1330(04)70333-0.
Guareschi, S., A. Laini, J. England, J. Barrett & P. J. Wood, 2021. Multiple co-occurrent alien invaders constrain aquatic biodiversity in rivers. Ecological Applications 31(6): e02385.
Holway, D. A. & A. V. Suarez, 1999. Animal behavior: an essential component of invasion biology. Trends in Ecology and Evolution 14: 328–330. https://doi.org/10.1016/S0169-5347(99)01636-5.
Jeschke, J., L. G. Aparicio, S. Haider, T. Heger, C. Lortie, P. Pyšek & D. Strayer, 2012. Support for major hypotheses in invasion biology is uneven and declining. NeoBiota 14: 1.
Keller, R. P., P. S. Zu Ermgassen & D. C. Aldridge, 2009. Vectors and timing of freshwater invasions in Great Britain. Conservation Biology 23(6): 1526–1534. https://doi.org/10.1111/j.1523-1739.2009.01249.x.
Kley, A. & G. Maier, 2005. An example of niche partitioning between Dikerogammarus villosus and other invasive and native gammarids: a field study. Journal of Limnology 64(1): 85–88.
Kley, A., W. Kinzler, Y. Schank, G. Mayer, D. Waloszek & G. Maier, 2009. Influence of substrate preference and complexity on co-existence of two non-native gammarideans (Crustacea: Amphipoda). Aquatic Ecology 43: 1047–1059. https://doi.org/10.1007/s10452-009-9242-y.
Kobak, J. & J. Żytkowicz, 2007. Preferences of invasive Ponto-Caspian and native European gammarids for zebra mussel (Dreissena polymorpha, Bivalvia) shell habitat. Hydrobiologia 589: 43–54. https://doi.org/10.1007/s10750-007-0716-4.
Kobak, J., T. Kakareko, Ł Jermacz & M. Poznańska, 2013. The impact of zebra mussel (Dreissena polymorpha) periostracum and biofilm cues on habitat selection by a Ponto-Caspian amphipod Dikerogammarus haemobaphes. Hydrobiologia 702: 215–226. https://doi.org/10.1007/s10750-012-1322-7.
Kobak, J., Ł Jermacz & D. Płąchocki, 2014. Effectiveness of zebra mussels to act as shelters from fish predators differs between native and invasive amphipod prey. Aquatic Ecology 48: 397–408. https://doi.org/10.1007/s10452-014-9492-1.
Kobak, J., Ł Jermacz & A. Dzierżyńska-Białończyk, 2015. Substratum preferences of the invasive killer shrimp Dikerogammarus villosus. Journal of Zoology 297: 66–76. https://doi.org/10.1111/jzo.12252.
Kobak, J., T. Kakareko, M. Poznańska, & J. Żbikowski. 2009. Preferences of the Ponto-Caspian amphipod Dikerogammarus haemobaphes for living zebra mussels. Journal of Zoology 279(3): 229–235. https://doi.org/10.1111/j.1469-7998.2009.00610.x.
Lenth, R., P. Buerkner, M. Herve, J. Love, H. Riebl, & H. Singmann, 2020. emmeans. https://CRAN.Rprojectorg/package=emmean.
MacNeil, C., D. Platvoet & J. T. Dick, 2008. Potential roles for differential body size and microhabitat complexity in mediating biotic interactions within invasive freshwater amphipod assemblages. Fundamental and Applied Limnology 172: 175. https://doi.org/10.1127/1863-9135/2008/0172-0175.
Magurran, A. E., B. H. Seghers, G. R. Carvalho & P. W. Shaw, 1992. Behavioural consequences of an artificial introduction of guppies (Poecilia reticulata) in N. Trinidad: evidence for the evolution of anti-predator behaviour in the wild. Proceedings of the Royal Society of London. Series B: Biological Sciences 248: 117–122. https://doi.org/10.1098/rspb.1992.0050.
Mason, R. J. & H. Sanders, 2021. Invertebrate zoogeomorphology: a review and conceptual framework for rivers. Wiley Interdisciplinary Reviews: Water 8: e1540. https://doi.org/10.1002/wat2.1540.
Mathers, K. L., K. Clinton, D. Constable, C. Gerrard, C. Patel & P. J. Wood, 2023. Invasion dynamics of Ponto-Caspian amphipods leads to changes in invertebrate community structure and function. Ecosphere 14(7): e4593. https://doi.org/10.1002/ecs2.4593.
Mills, D. N., 2019. Ecological Impacts of a New Invasive Species in UK Rivers: The Quagga Mussel, Dreissena Rostriformis Bugensis (Bivalva: Dreissenidae; Andrusov 1897). Doctoral dissertation, King's College London.
Mills, D. N., M. A. Chadwick & R. A. Francis, 2017. Impact of invasive quagga mussel (Dreissena rostriformis bugensis, Bivalva: Dreissenidae) on the macroinvertebrate community structure of a UK river. Aquatic Invasions 4: 509–521. https://doi.org/10.3391/ai.2017.12.4.08.
Mowery, M. A., C. Vink, A. C. Mason & M. C. Andrade, 2021. Behavioural, morphological, and life history shifts during invasive spread. Biological Invasions 23: 3497–3511. https://doi.org/10.1007/s10530-021-02593-6.
Nakano, D. & D. L. Strayer, 2014. Biofouling animals in fresh water: biology, impacts, and ecosystem engineering. Frontiers in Ecology and the Environment 12(3): 167–175. https://doi.org/10.1890/130071.
Naura, M., M. J. Clark, D. A. Sear, P. M. Atkinson, D. D. Hornby, P. Kemp, J. England, G. Peirson, C. Bromley & M. G. Carter, 2016. Mapping habitat indices across river networks using spatial statistical modelling of River Habitat Survey data. Ecological Indicators 66: 20–29. https://doi.org/10.1016/j.ecolind.2016.01.019.
NBN Atlas, 2023. National Biodiversity Network Atlas website. https://nbnatlas.org/.
Pejchar, L. & H. A. Mooney, 2009. Invasive species, ecosystem services and human well-being. Trends in Ecology & Evolution 24: 497–504. https://doi.org/10.1016/j.tree.2009.03.016.
R Core Team, 2021. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
Reid, D. F. & M. I. Orlova, 2002. Geological and evolutionary underpinnings for the success of Ponto-Caspian species invasions in the Baltic Sea and North American Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 59: 1144–1158. https://doi.org/10.1139/f02-099.
Reznick, D. N. & C. K. Ghalambor, 2001. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. In Hendry, A. P. & M. T. Kinnison (eds), Microevolution Rate, Pattern, Process. Contemporary Issues in Genetics and Evolution, Vol. 8. Springer, Dordrecht: 183–198. https://doi.org/10.1007/978-94-010-0585-2_12.
Ricciardi, A., 2001. Facilitative interactions among aquatic invaders: is an “invasional meltdown” occurring in the Great Lakes? Canadian Journal of Fisheries and Aquatic Sciences 58: 2513–2525. https://doi.org/10.1139/f01-178.
Rolla, M., S. Consuegra & C. G. de Leaniz, 2019. Predator recognition and anti-predatory behaviour in a recent aquatic invader, the killer shrimp (Dikerogammarus villosus). Aquatic Invasion. https://doi.org/10.1101/636100.
Sala, O. E., F. I. I. I. Stuart Chapin, J. J. Armesto, E. Berlow, J. Bloomfield, R. Dirzo, E. Huber-Sanwald, L. F. Huenneke, R. B. Jackson, A. Kinzig & R. Leemans, 2000. Global biodiversity scenarios for the year 2100. Science 287: 1770–1774. https://doi.org/10.1126/science.287.5459.1770.
Sanders, H. & D. N. Mills, 2022. Predation preference of signal crayfish (Pacifastacus leniusculus) on native and invasive bivalve species. River Research and Applications 38: 1469–1480. https://doi.org/10.1002/rra.4023.
Sanders, H., R. J. Mason, D. N. Mills & S. P. Rice, 2022. Stabilization of fluvial bed sediments by invasive quagga mussels (Dreissena bugensis). Earth Surface Processes and Landforms 47: 3259–3275. https://doi.org/10.1002/esp.5455.
Sanders, C. H., S. P. Rice, P. J. Wood & L. K. Albertson, 2023. River bank burrowing is innate in native and invasive signal crayfish (Pacifastacus leniusculus) and is driven by biotic and abiotic cues. Biological Invasions 25: 3425–3442. https://doi.org/10.1007/s10530-023-03115-2.
Simberloff, D., 2006. Invasional meltdown 6 years later: important phenomenon, unfortunate metaphor, or both? Ecology Letters 9(8): 912–919. https://doi.org/10.1111/j.1461-0248.2006.00939.x.
Simberloff, D. & B. Von Holle, 1999. Positive interactions of nonindigenous species: invasional meltdown? Biological Invasions 1(1): 21–32. https://doi.org/10.1023/A:1010086329619.
Sol, D. & J. S. Weis, 2019. Highlights and insights from “Biological Invasions and Animal Behaviour.” Aquatic Invasions. https://doi.org/10.3391/ai.2019.14.3.12.
Soto, I., R. N. Cuthbert, A. Ricciardi, D. A. Ahmed, F. Altermatt, R. B. Schäfer, G. Archambaud-Suard, N. Bonada, M. Cañedo-Argüelles, Z. Csabai & T. Datry, 2023. The faunal Ponto-Caspianization of central and western European waterways. Biological Invasions. https://doi.org/10.1007/s10530-023-03060-0.
Stewart, T.W., J.G. Miner, & R.L. Lowe. 1998. Quantifying mechanisms for zebra mussel effects on benthic macroinvertebrates: organic matter production and shell-generated habitat. Journal of the North American Benthological Society 17(1): 81–94. https://doi.org/10.2307/1468053.
Stewart, T.W., J.C. Gafford, J.G. Miner, & R.L. Lowe. 1999. Dreissena-shell habitat and antipredator behavior: combined effects on survivorship of snails co-occurring with molluscivorous fish. Journal of the North American Benthological Society 18(2): 274–283. https://doi.org/10.2307/1468465.
Strayer, D. L., 2009. Twenty years of zebra mussels: lessons from the mollusk that made headlines. Frontiers in Ecology and the Environment 7: 135–141. https://doi.org/10.1890/080020.
Tricarico, E., G. Mazza, G. Orioli, C. Rossano, F. Scapini & F. Gherardi, 2010. The killer shrimp, Dikerogammarus villosus (Sowinsky, 1894), is spreading in Italy. Aquatic Invasions 5: 211–214. https://doi.org/10.3391/ai.2010.5.2.14.
van der Velde, G., Rajagopal, S. & bij de Vaate, A. eds., 2010. The Zebra Mussel in Europe. Backhuys, Leiden.
Wright, T. F., J. R. Eberhard, E. A. Hobson, M. L. Avery & M. A. Russello, 2010. Behavioral flexibility and species invasions: the adaptive flexibility hypothesis. Ethology Ecology & Evolution 22: 393–404. https://doi.org/10.1080/03949370.2010.505580.
Acknowledgements
Thank you to all the CCCU Natural and Applied Sciences laboratory staff, in particular to Alex Harvey, Charlie Wright, Luis Holdsworth, Csenge Mocsonoky, Naomi Stead, and Dave Belsom, for laboratory assistance. Thank you to Norfolk Wildlife Trust for facilitating fieldwork in the Norfolk Broads, and to Anglian Water for facilitating fieldwork at Pitsford Reservoir. Thanks go to two reviewers for their constructive comments which greatly improved the presentation of the study outcomes.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Conceptualisation: CS & DM. Fieldwork: CS with CDH. Lab work: CS with PB and CDH. Data analysis: CS with KM. Manuscript writing: Led by CS. All authors contributed critically to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling editor: Katya E. Kovalenko
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sanders, C.H., Buckley, P.L., Devereux Hunt, C. et al. Ponto-Caspian amphipod co-location with zebra mussel beds (Dreissena polymorpha) is influenced by substrate size and population source. Hydrobiologia 851, 3507–3523 (2024). https://doi.org/10.1007/s10750-024-05515-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-024-05515-4