Abstract
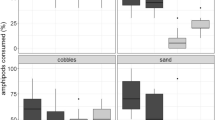
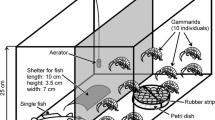
Substrate choice, swimming activity and risk to predation by burbot (Lota lota) of the well established Gammarus roeselii and the invader Dikerogammarus villosus were studied in mixed and single-species aquarium experiments. We used stones, gravel and aquatic weeds (Elodea, Chara) as substrates. We hypothesized that both species have different substrate preferences and that substrate affects the predation risk. We also assumed that presence of D. villosus influences substrate preference and predation risk of G. roeselii since the invader is known to affect the behavior of other gammarids. Adults of D. villosus in single species experiments and juveniles in mixed and single species experiments were evenly distributed over the different substrates but adults in mixed species experiments were more likely to prefer stone substrate. In contrast, adults and juveniles of G. roeselii clearly preferred aquatic weeds independent of the presence/absence of the invader. Both species preferred substrates with fissured surface over substrates with smooth surface. Gammarus roeselii was observed swimming more often than D. villosus in the open water but its swimming activity was lower when its preferred substrate was present compared with its swimming activity if non-preferred substrates were present. Predation rate of burbot on D. villosus was comparatively low and independent of the substrate. Burbot consumed many more G. roeselii than D. villosus, both in mixed and single species experiments. But when the preferred substrate of G. roeselii (weeds) was used in the experiments, predation rate of burbot on G. roeselii was somewhat lower than that when non-preferred substrates were present. The results of the experiments support our hypothesis that the gammarids studied here have different substrate preferences and that presence of the preferred substrate can affect predation risk. However, there is no evidence that presence of D. villosus affected substrate choice or predation risk in G. roeselii. We consider that differences in use of spatial niches permit co-existence of G. roeselii and D. villosus in the wild when substrates are diverse. The fact that G. roeselii than D. villosus is more often observed swimming in the open water may explain its higher risk of being captured by fish.





Similar content being viewed by others
References
Bailey MM (1972) Age, growth, reproduction, and food of the burbot, Lota lota (Linnaeus), in Southwestern Lake Superior. Trans Am Fish Soc 101:667–674. doi:10.1577/1548-8659(1972)101<667:AGRAFO>2.0.CO;2
Baumgärtner D, Jungbluth A-D, Koch U, von Elert E (2002) Effects of infochemicals on microhabitat choice by the freshwater amphipod Gammarus roeseli. Arch Hydrobiol 155:353–367
Baumgärtner D, Koch U, Rothhaupt K-O (2003) Alteration of kairomone-induced response of the freshwater amphipod Gammarus roeseli by sediment type. J Chem Ecol 29:1391–1401. doi:10.1023/A:1024213403537
Bollache L, Kaldonski N, Troussard J-P, Lagrue C, Tierry R (2006) Spines and behaviour as defences against fish predators in an invasive freshwater amphipod. Anim Behav 72:627–633. doi:10.1016/j.anbehav.2005.11.020
Dahl J (1998) Effects of a benthivorous and a drift feeding fish on a benthic stream assemblage. Oecologia 116:426–432. doi:10.1007/s004420050606
Dahl J, Greenberg L (1996) Effects of habitat structure on habitat use by Gammarus pulex in artificial streams. Freshw Biol 36:487–495. doi:10.1046/j.1365-2427.1996.00096.x
De Lange HJ, Lüring M, Van Den Borne B, Peeters THM (2005) Attraction of the amphipod Gammarus pulex to water-borne cues of food. Hydrobiologia 544:19–25. doi:10.1007/s10750-004-7896-y
Devin S, Piscart C, Beisel JN, Moreteau JC (2003) Ecological traits of the amphipod invader Dikerogammarus villosus on a mesohabitat scale. Arch Hydrobiol 158:43–56. doi:10.1127/0003-9136/2003/0158-0043
Dick JTA, Platvoet D (2000) Invading predatory crustacean Dikerogammarus villosus eliminates both native and exotic species. P R Soc Lond B Bio 267:977–983. doi:10.1098/rspb.2000.1099
Dick JTA, Platvoet D, Kelly DW (2002) Predatory impact of the freshwater invader Dikerogammarus villosus (Crustacea: Amphipoda). Can J Fish Aquat Sci 59:1078–1084. doi:10.1139/f02-074
Eckmann R, Mörtl M, Baumgärtner D, Berron C, Fischer P, Schleuter D, Weber A (2008) Consumption of amphipods by littoral fish after the replacement of native Gammarus roeseli by invasive Dikerogammarus villosus in Lake Constance. Aquat Invasions 3:184–188
Elliott JM (2005) Day-night changes in the spatial distribution and habitat preferences of freshwater shrimps, Gammarus pulex, in a stony stream. Freshw Biol 50:552–566. doi:10.1111/j.1365-2427.2005.01345.x
Franken RJM, Batten S, Beijer JAJ, Gardeniers JJP, Scheffer M, Peeters ETHM (2006) Effects of interstitial refugia and current velocity on growth of the amphipod Gammarus pulex Linnaeus. J N Am Benthol Soc 25:656–663. doi:10.1899/0887-3593(2006)25[656:EOIRAC]2.0.CO;2
Gergs R, Rothhaupt K-O (2008) Effects of zebra mussels on an native amphipod and the invasive Dikerogammarus villosus: the influence of biodeposition and structural complexity. J N Am Benthol Soc 27:541–548. doi:10.1899/07-151.1
Grabowski M, Jazdzewski K, Konopacka A (2007) Alien crustacea on polish waters–amphipoda. Aquat Invasions 2:25–38. doi:10.3391/ai.2007.2.1.3
Hesselschwerdt J, Necker J, Wantzen KM (2008) Gammarids in Lake Constance: habitat segregation between the invasive Dikerogammarus villosus and the indigenous Gammarus roeselii. Fundam Appl Limnol 173:177–186. doi:10.1127/1863-9135/2008/0173-0177
Hoyle JD, Holomuzki JR (1990) Effect of predatory fish presence on habitat use and diel movement of the stream amphipod Gammarus minus. Freshw Biol 24:509–517. doi:10.1111/j.1365-2427.1990.tb00728.x
Kaldonski N, Perrot-Minnot M-J, Cézilly F (2007) Differential influence of two acanthocephalan parasites on the antipredator behaviour of their common intermediate host. Anim Behav 74:1311–1317. doi:10.1016/j.anbehav.2007.02.027
Kaldonski N, Lagrue C, Motreuil S, Rigaud T, Bollache L (2008) Habitat segregation mediates predation by the benthic fish Cottus gobio on the exotic amphipod species Gammarus roeseli. Naturwissenschaften 95:839–844. doi:10.1007/s00114-008-0392-x
Kinzler W, Maier G (2003) Asymmetry in mutual predation: possible reason for the replacement of native gammarids by invasives. Arch Hydrobiol 157:473–481. doi:10.1127/0003-9136/2003/0157-0473
Kinzler W, Maier G (2006) Selective predation by fish: a further reason for the decline of native gammarids in the presence of invasives? J Limnol 65:27–34
Kley A, Maier G (2003) Life history characteristics of the invasive freshwater gammarids Dikerogammarus villosus and Echinogammarus ischnus in the river Main and the Main–Donau canal. Arch Hydrobiol 156:473–481. doi:10.1127/0003-9136/2003/0156-0457
Kley A, Maier G (2005) An example of niche partitioning between Dikerogammarus villosus and other invasive and native gammarids: a field study. J Limnol 64:85–88
Kley A, Maier G (2006) Reproductive characteristics of invasive gammarids in the Rhine-Main-Danube catchment, South Germany. Limnologica 36:79–90. doi:10.1016/j.limno.2006.01.002
Kobak J, Zytkowicz (2007) Preferences of invasive Ponto-Caspian and native gammarids for zebra mussel (Dreissena polymorpha, Bivalvia) shell habitat. Hydrobiologia 589:43–54. doi:10.1007/s10750-007-0716-4
Krisp H (2004) Substratpräferenz, Aktivität, Prädationsneigung und Wachstum von neozoischen und heimischen Gammaridenarten. Diploma Thesis in Biology, University of Ulm,. 67 pp
Lods-Crozet B, Reymond O (2006) Bathymetric expansion of an invasive gammarid (Dikerogammarus villosus, crustacea, amphipoda) in Lake Leman. J Limnol 65:141–144
Mac Neil C, Elwood RW, Dick JTA (1999) Predator-prey interactions between brown trout Salmo trutta and native and introduced amphipods; their implications for fish diets. Ecography 22:686–696. doi:10.1111/j.1600-0587.1999.tb00518.x
MacNeil C, Dick JTA, Elwood RW (1997) The trophic ecology of freshwater Gammarus (crustacea: amphipoda); problems and perspectives concerning the functional feeding group concept. Biol Rev Camb Philos Soc 72:349–364. doi:10.1017/S0006323196005038
MacNeil C, Elwood RW, Dick JTA (2000) Factors influencing the importance of Gammarus spp. (Crustacea: Amphipoda) in riverine salmonid diets. Arch Hydrobiol 149:87–107
MacNeil C, Platvoet D, Dick JTA (2008) Potential roles for differential body size and microhabitat complexity in mediating biotic interactions within invasive freshwater amphipod assemblages. Fundam Appl Limnol 172:175–182. doi:10.1127/1863-9135/2008/0172-0175
Mathis A, Hoback W (1997) The influence of chemical stimuli from predators on precopulatory pairing by the amphipod, Gammarus pseudolimnaeus. Ethology 103:33–40
Mayer G, Maier G, Maas A, Waloszek D (2008) Mouthparts of the Ponto-Caspian invader Dikerogammarus villosus (amphipoda: pontogammaridae). J Crustac Biol 28:1–15. doi:10.1651/07-2867R.1
Mayer G, Maier G, Maas A, Waloszek D (2009) Mouthpart morphology of Gammarus roeselii compared to a successful invader, Dikerogammarus villosus (Amphipoda). J Crustac Biol (in press)
Mazzi D, Bakker TCM (2003) A predator’s dilemma: prey choice and parasite susceptibility in three-spined sticklebacks. Parasitology 126:339–347. doi:10.1017/S0031182003003019
McGrath KE, Peeters ETHM, Beijer JAJ, Scheffer M (2007) Habitat-mediated cannibalism and microhabitat restriction in the stream invertebrate Gammarus pulex. Hydrobiologia 589:155–164. doi:10.1007/s10750-007-0731-5
Mörtl M, Mürle U, Ortlepp J, Rey P, Schleifhacken N, Werner S (2004) Dikerogammarus villosus (crustacea: amphipoda) und Corbicula fluminea (Bivalvia: Veneroidea) im Bodensee. In: Wirbellose Neozoen im Bodensee. LfU Baden-Württemberg, Institut für Seenforschung. City Satz GmbH, Herxheim, pp 15–30
Mürle U, Becker A, Rey P (2004) Dikerogammarus villosus (amphipoda), new in Lake Constance. Lauterbornia 49:77–79
Newman RM, Waters TH (1984) Size-selective predation on Gammarus pseudolimnaeus by trout and sculpins. Ecology 65:1535–1545. doi:10.2307/1939133
Palmer ME, Ricciardi A (2004) Physical factors affecting the relative abundance of native and invasive amphipods in the St Lawrence River. Can J Zool 82:1886–1893. doi:10.1139/z04-186
Pennuto C, Keppler D (2008) Short-term predator avoidance behaviours by invasive and native amphipods in the Great Lakes. Aquat Ecol 42:629–641. doi:10.1007/s10452-007-9139-6
Piscart C, Manach A, Copp GH, Marmonier P (2007) Distribution and microhabitats of native and non-native gammarids (amphipoda, crustacea) in Brittany, with particular reference to the endangered endemic sub-species Gammarus duebeni celticus. J Biogeogr 34:524–533. doi:10.1111/j.1365-2699.2006.01609.x
Platvoet D, Dick JTA, Konijnendijk N, van der Velde G (2006) Feeding of micro-algae in the invasive Ponto-Caspian amphipod Dikerogammarus villosus (Sowinsky, 1894). Aquat Ecol 40:237–245. doi:10.1007/s10452-005-9028-9
Pöckl M (1995) Laboratory studies on growth, feeding, moulting and mortality in the freshwater amphipods Gammarus fossarum and G. roeseli. Arch Hydrobiol 134:223–253
Ponyi E (1956) Ökologische, ernährungsbiologische und systematische Untersuchungen an verschiedenen Gammarus-Arten. Annu Rev Ecol Syst 20:297–330
Ponyi E (1961) Über Ernährung einiger Amphipoden (Crustacea) in Ungarn. Ann Inst Biol Tihany 28:117–123
Ryder RA, Pesendorfer J (1992) Food, growth, habitat, and community interactions of young-of-the year burbot, Lota lota L., in a precambrian Shield lake. Hydrobiologia 243–244:211–227
Sudo H, Azeta M (2001) The microhabitat and size of gammarid species selectively predated by young red sea bream Pagrus major. Fish Sci 67:389–400. doi:10.1046/j.1444-2906.2001.00274.x
Van Dolah RF (1978) Factors regulating the distribution and population dynamics of the amphipod Gammarus palustris in an intertidal salt mars community. Ecol Monogr 48:191–217. doi:10.2307/2937299
Van Overdijk CDA, Grigorovich IA, Mabee T, Ray WJ, Ciborowski JJH, MacIsaac HJ (2003) Microhabitat selection by the invasive amphipod Echinogammarus ischnus and native Gammarus fasciatus in laboratory experiments and in Lake Erie. Freshw Biol 48:567–578. doi:10.1046/j.1365-2427.2003.01041.x
Van Riel M, van der Velde G, Rajagopal S, Marguillier S, Dehairs F, bij de Vaate A (2006) Trophic relationships in the Rhine food web during invasion and after establishment of the Ponto-Caspian invader Dikerogammarus villosus. Hydrobiologia 565:39–58. doi:10.1007/s10750-005-1904-8
Van Riel M, Healy EP, van der Velde G, bij de Vaate A (2007) Interference competition among native and invader amphipods. Acta Oecol 31:282–289. doi:10.1016/j.actao.2006.12.006
Wijnhoven S, van Riel MC, van der Velde G (2003) Exotic and indigenous freshwater gammarid species: physiological tolerance to water temperature in relation to ionic content of the water. Aquat Ecol 37:151–158. doi:10.1023/A:1023982200529
Williams DD, Moore (1982) The effect of environmental factors on the activity of Gammarus pseudolimnaeus (amphipoda). Hydrobiologia 96:137–147. doi:10.1007/BF02185429
Wooster DE (1998) Amphipod (Gammarus minus) reseponses to predators and predator impact on amphipod density. Oecologia 115:253–259. doi:10.1007/s004420050514
Wudkevich K, Wisenden BD, Chivers DP, Smith RJF (1997) Reactions of Gammarus lacustris to chemical stimuli from natural predators and injured conspecifics. J Chem Ecol 23:1163–1173. doi:10.1023/B:JOEC.0000006393.92013.36
Acknowledgments
This study is part of the project “ANEBO” (Aquatische Neozoen im Bodensee und seinen Zuflüssen); it is financially supported by the European Union within the scope of the Interreg III A programme. We are grateful for this support. We thank anonymous reviewers and R. Gulati for valuable critical comments on an earlier version of this manuscript. We also thank members of the staff of Reiner Eckmann (Konstanz) who provided burbots used in our experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kley, A., Kinzler, W., Schank, Y. et al. Influence of substrate preference and complexity on co-existence of two non-native gammarideans (Crustacea: Amphipoda). Aquat Ecol 43, 1047–1059 (2009). https://doi.org/10.1007/s10452-009-9242-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-009-9242-y