Abstract
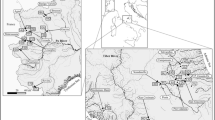
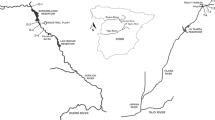
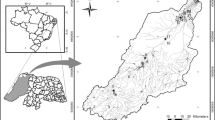
Temporal variation in limnological characteristics favors an increase in aquatic macrophyte diversity in Neotropical riverine environments. We assessed temporal and environmental variability in the aquatic macrophyte community in riverine environments of the Tapajós river basin, southern Amazonia, Brazil. Hydroperiod, type of riverine environment, limnological variables, and surrounding woody vegetation were found to influence aquatic macrophyte richness, cover, and dry and fresh biomass. A total of 98 species from 68 genera and 40 families were recorded. The greatest observed richness in streams was during the dry period. Richness, cover, and biomass were greater in lagoons and rivers during rising water and flood hydroperiods. Amphibious and emergent species had higher biomass in flood and receding water hydroperiods. Higher richness, cover, and fresh biomass were mostly related to electrical conductivity. Suspended and dissolved solids reduced species richness in all environments. Greater tree abundance in the surrounding vegetation was associated with higher macrophyte richness in streams and with macrophyte cover and biomass in rivers. The aquatic macrophyte community in southern Amazonia is subject to variation in riverine ecosystem type, tree composition and structure in surrounding vegetation, hydroperiod (temporal variation), and limnological parameters (environmental/temporal variation).

Source Adapted from Sinop Energia (2013)




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
Alahuhta, J., F. Ecke, L. Johnson, L. Sass & J. Heino, 2017. A comparative analysis reveals little evidence for niche conservatism in aquatic macrophytes among four areas on two continents. Oikos 126: 136–148.
Alba, C., R. Levy & R. Hufft, 2021. Combining botanical collections and ecological data to better describe plant community diversity. PLoS One 16: e0244982.
Ali, M. M., S. A. Hassan & A. M. Shaheen, 2011. Impact of riparian trees shade on aquatic plant abundance in conservation islands. Acta Botanica Croatica 70: 2. https://doi.org/10.2478/v10184-010-0012-7.
American Public Health Association (APHA), 2012. American Public Health Association (APHA) Standard Methods for the Examination of Water and Wastewater (twenty, 2nd ed. American Public Health Association, American Water Works Association, Water Environment Federation, Washington DC, USA:
APG (Angiosperm Phylogeny Group) IV, 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20.
ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS, 1999. ABNT NBR ISO/IEC Guia 43–1: Ensaios de proficiência por comparações interlaboratoriais – Parte 1: Desenvolvimento e operação de programas de ensaios de proficiência. ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS, Rio de Janeiro, 17 pp.
ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS, 2004. ABNT ISO Guia 31: Materiais de referência – Conteúdo de certificados e rótulos. ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS, Rio de Janeiro, 8 pp.
ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS, 2005a. ABNT NBR ISO/IEC 17000: Avaliação da conformidade – Vocabulário e princípios gerais. ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS, Rio de Janeiro, 18 pp.
ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS, 2005b. ABNT NBR ISO/IEC 17025: Requisitos gerais para a competência de laboratórios de ensaio e calibração. ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS, Rio de Janeiro, 31 pp.
ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS, 2008. ABNT NBR ISO 9001: Sistemas de gestão da qualidade – Requisitos, 2 ed. ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS, Rio de Janeiro, 28 pp.
Baart, I., C. Gschöpf, A. P. Blaschke, S. Preiner & T. Hein, 2010. Prediction of potential macrophyte development in response to restoration measures in an urban riverine wetland. Aquatic Botany 93: 153–162. https://doi.org/10.1016/j.aquabot.2010.06.002.
Babur, H., B. Surmen & H. G. Kutbay, 2021. Grime’s csr strategies of aquatic macrophytes in different lagoon lakes in northern Turkey. Turkish Journal of Fisheries and Aquatic Sciences 21: 211–224. https://doi.org/10.4194/1303-2712-v21_05_01.
Bao, F., T. D. Leandro, M. Rocha, V. S. Santos, T. H. Stefanello, R. Arruda, A. Pott & G. A. Damasceno-Júnior, 2017. Plant species diversity in a Neotropical wetland: patterns of similarity, effects of distance, and altitude. Anais Da Academia Brasileira de Ciências 90: 85–97. https://doi.org/10.1590/0001-3765201720150370.
Bates, D. & M. Maechler, 2010. lme4: linear mixed-effects models using S4 classes, 538. R package version 539 0.999375-34. http://CRAN.R-project.org/package=lme4.
Bleich, M., T. M. F. Piedade, P. B. Knopki, N. Góes, D. Castro, S. R. Jati & R. N. Sousa, 2014. Influência das condições do habitat sobre a estrutura de herbáceas aquáticas na região do Lago Catalão, Manaus, AM. Acta Amazonica 44: 481–490.
Bottino, F., M. C. Calijuri & K. J. Murphy, 2013. Temporal and spatial variation of limnological variables and biomass of different macrophyte species in a Neotropical reservoir (São Paulo – Brazil). Acta Limnologica Brasiliensia 25: 387–397. https://doi.org/10.1590/S2179-975X2013000400004.
Braun-Blanquet, J., 1979. Fitosociologia: bases para el estudio de las comunidades vegetales, 3rd ed. Aum. Blume, Madrid:
Bromiley, P. A., N. A. Thacker, & E. Bouhova-Thacker, 2004. Shannon Entropy, Rényi Entropy, and Information. CiteSeerX. 10.1.1.330.9856
Buck, W. R. & B. Goffinet, 2009. Morphology and classification of mosses. In Shaw, A. J. & B. Goffinet (eds), Bryophyte Biology Cambridge University Press, New York: 55–138.
Campos, R., 2021. Evaluating drivers and patterns of aquatic community distribution in Neotropical floodplain systems: an approach based on beta-diversity analyses Evaluating drivers and patterns of aquatic community distribution in Neotropical floodplain systems. http://repositorio.uem.br:8080/jspui/handle/1/6539.
Carranzo, I. V., 2012. Standard Methods for examination of water and wastewater. In Anales De Hidrología Médica, Vol. 5. Universidad Complutense de Madrid, Espanha, 185–187.
Carvalho, G., 2020. Package ‘flora’. https://CRAN.R-project.org/package=flora
Córdova, M. O., J. F. Keffer, D. R. Giacoppini, V. J. Pott, A. Pott, E. G. Moura-Júnior & C. B. R. Munhoz, 2022. Aquatic macrophytes in southern Amazonia, Brazil: richness, endemism, and comparative floristics. Wetlands 42: 1–11. https://doi.org/10.1007/s13157-022-01545-7.
Dapporto, L., M. Ramazzotti, S. Fattorini, G. Talavera & R. H. L. Dennis, 2020. Package ‘recluster’. https://CRAN.R-project.org/package=cluster.
Delignette-Muller, M. L. & C. Dutang, 2015. fitdistrplus: an R package for fitting distributions. Journal of Statistical Software 64: 1–34. https://doi.org/10.18637/jss.
Dubey, D., S. Kumar & V. Dutta, 2022. Impact of nutrient enrichment on habitat heterogeneity and species richness of aquatic macrophytes: evidence from freshwater tropical lakes of Central Ganga Plain, India. International Journal of Environmental Science and Technology 19: 5529–5546. https://doi.org/10.1007/s13762-021-03438-4.
Dufrêne, M. & P. Legendre, 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67: 345–366. https://doi.org/10.2307/2963459.
Esteves, F. A., 1998. Fundamentos de Limnologia. In Intercência. https://doi.org/10.1515/znc-1987-0514.
Fearnside, P. M., 2019. Hidrelétricas na Amazônia, Vol. 3. http://inct-servamb.inpa.gov.br/publ_livres/2019/Hidro-v3/Livro_Hidrelétricas_Vol_3.pdf#page=7.
Ferreira, F. A., R. P. Mormul, S. M. Thomaz, A. Pott & V. J. Pott, 2011. Macrophytes in the upper Paraná river floodplain: checklist and comparison with other large South American wetlands. Revista De Biología Tropical 59: 541–556.
Fidelis, E. G., M. A. S. Reis, M. Negrini & D. N. M. Ferreira, 2019. Life table parameters of the red palm mite Raoiella indica (Acari: Tenuipalpidae) at various temperatures and for sexual and asexual reproduction. Experimental and Applied Acarology 78: 535–546.
Flora e Funga do Brasil, 2020. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/.
Fortney, R. H., M. Benedict, J. F. Gottgens, T. L. Walters, B. S. Leady & J. Rentch, 2004. Aquatic plant community composition and distribution along an inundation gradient at two ecologically-distinct sites in the Pantanal region of Brazil. Wetlands Ecology and Management 12: 575–585. https://doi.org/10.1007/s11273-005-1763-0.
Galvanese, E. F., A. P. Lula-Costa, E. S. Araújo, B. C. Falkievicz, G. G. V. Melo, J. R. S. Vitule & A. A. Padial, 2022. Community stability and seasonal biotic homogenisation emphasize the effect of the invasive tropical tanner grass on macrophytes from a highly dynamic neotropical tidal river. Aquatic Sciences 84: 30. https://doi.org/10.1007/s00027-022-00858-3.
Grimaldo, J. T., L. M. Bini, V. L. Landeiro, M. T. O’Hare, J. Caffrey, A. Spink, S. V. Martins, M. P. Kennedy & K. J. Murphy, 2016. Spatial and environmental drivers of macrophyte diversity and community composition in temperate and tropical calcareous rivers. Aquatic Botany 132: 49–61. https://doi.org/10.1016/J.AQUABOT.2016.04.006.
Hachoł, J., E. Bondar-Nowakowska & E. Nowakowska, 2019. Factors influencing macrophyte species richness in unmodified and altered watercourses. Polish Journal of Environmental Studies 28: 609–622. https://doi.org/10.15244/pjoes/85220.
Holtmann, L., K. Kerler, L. Wolfgart, C. Schmidt & T. Fartmann, 2019. Habitat heterogeneity determines plant species richness in urban stormwater ponds. Ecological Engineering 138: 434–443. https://doi.org/10.1016/j.ecoleng.2019.07.035.
Hubbell, S. P., F. He, R. Condit, L. Borda-de-Água, J. Kellner & H. T. Steege, 2009. How many tree species are there in the Amazon and how many of them will go extinct? PNAS 105: 11498–11504. https://doi.org/10.1073/pnas.0801915105.
Irgang, B. E. & C. V. S. Gastal Jr., 1996. Plantas aquáticas da planície costeira do Rio Grande do Sul, UFRGS, Porto Alegre:
Janauer, G. A., N. Exler, G. Anačkov, V. Barta, Á. Berczik, P. Boža, M. Dinka, V. Georgiev, M. Germ, M. Holcar, R. Hrivnák, R. Igić, S. Ozimec, A. Sârbu, B. Schmidt, U. Schmidt-Mumm, W. Schütz, K. Sipos, E. Szalma, J. Topić, S. Tsoneva, M. Valachovič, V. Valchev, D. Vukov, I. Zelnik & A. Gaberščik, 2021. Distribution of the macrophyte communities in the danube reflects river serial discontinuity. Water 13: 918. https://doi.org/10.3390/w13070918.
Kaijser, W., S. Birk & D. Hering, 2022. Environmental ranges discriminating between macrophytes groups in European rivers. PLoS ONE 17: e0269744. https://doi.org/10.1371/journal.pone.0269744.
Khedr, A. H. A. & M. A. El-Demerdash, 1997. Distribution of aquatic plants in relation to environmental factors in the Nile Delta. Aquatic Botany 56: 75–86. https://doi.org/10.1016/S0304-3770(96)01090-X.
Kindt, R. & R. Coe, 2005. Tree Diversity Analysis. A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies. World Agroforestry Centre (ICRAF), Nairobi.
Legendre, P., M. J. Fortin & D. Borcard, 2015. Should the Mantel test be used in spatial analysis? Methods in Ecology and Evolution 6: 1239–1247. https://doi.org/10.1111/2041-210X.12425.
Li, S., T. Sun, W. Yang, B. Cui, X. Yin & C. Tao Sun, 2020. A biodiversity evaluation framework for restoration of aquatic macrophyte communities in shallow lakes driven by hydrological process management. Hydrological Processes. 35: e13983. https://doi.org/10.1002/hyp.13983.
Lind, L., R. L. Eckstein & R. A. Relyea, 2022. Direct and indirect effects of climate change on distribution and community composition of macrophytes in lentic systems. Biological Reviews 97: 1677–1690. https://doi.org/10.1111/brv.12858.
Lindholm, M., J. Alahuhta, J. Heino & H. Toivonen, 2021. Temporal beta diversity of lake plants is determined by concomitant changes in environmental factors across decades. Journal of Ecology 109: 819–832. https://doi.org/10.1111/1365-2745.13508.
Liu, H., G. Liu & W. Xing, 2021. Functional traits of submerged macrophytes in eutrophic shallow lakes affect their ecological functions. Science of the Total Environment 760: 143332. https://doi.org/10.1016/j.scitotenv.2020.143332.
Lobato-de Magalhães, T., K. Murphy, A. Efremov, V. Chepinoga, T. A. Davidson & E. Molina-Navarro, 2021. Ploidy state of aquatic macrophytes: global distribution and drivers. Aquatic Botany 173: 103417. https://doi.org/10.1016/J.AQUABOT.2021.103417.
Machado, N. G., L. Sanches, L. B. Silva, J. W. Z. Novais, A. M. Aquino, M. S. Biudes, O. B. Pinto-Junior & J. S. Nogueira, 2015. Soil nutrients and vegetation structure in a neotropical seasonal wetland. Applied Ecology and Environmental Research 13: 289–305. https://doi.org/10.15666/aeer/1302_289305.
Maltchik, L., A. S. Rolon & P. Schott, 2007. Effects of hydrological variation on the aquatic plant community in a floodplain palustrine wetland of southern Brazil. Limnology 8: 23–28. https://doi.org/10.1007/s10201-006-0192-y.
Maracahipes Santos, L., E. Lenza, J. O. dos Santos, B. S. Marimon, P. V. Eisenlohr, B. H. Marimon Junior & T. R. Feldpausch, 2015. Diversity, floristic composition, and structure of the woody vegetation of the Cerrado in the Cerrado–Amazon transition zone in Mato Grosso, Brazil. Brazilian Journal of Botany 38: 877–887. https://doi.org/10.1007/s40415-015-0186-2.
Marchetti, Z. Y. & P. A. Scarabotti, 2016. Macrophyte assemblages in relation to environmental, temporal and spatial variations in lakes of a subtropical floodplain-river system, Argentina. Flora - Morphology, Distribution, Functional Ecology of Plants 225: 82–91. https://doi.org/10.1016/J.FLORA.2016.10.004.
Marimon, B. S., E. S. Lima, T. G. Duarte, L. C. Chieregatto & J. A. Ratter, 2006. Observations on the vegetation of northeastern Mato Grosso, Brazil. Iv. An analysis of the cerrado–amazonian forest ecotone. Edinburgh Journal of Botany 63: 323–341. https://doi.org/10.1017/S0960428606000576.
Marques, E. Q., B. H. Marimon-Junior, B. S. Marimon, E. A. T. Matricardi, H. A. Mews & G. R. Colli, 2020. Redefining the Cerrado–Amazonia transition: implications for conservation. Biodiversity and Conservation 29: 1501–1517. https://doi.org/10.1007/s10531-019-01720-z.
Mitchell, A., 2005. The ESRI Guide to GIS Analysis, Vol. 2. ESRI Press, Redlands:
Moi, D. A. & F. Teixeira-de-Mello, 2022. Cascading impacts of urbanization on multitrophic richness and biomass stock in neotropical streams. Science of the Total Environment 806: 151398. https://doi.org/10.1016/j.scitotenv.2021.151398.
Morais, M., M. S. A. Abdo, C. Santos, N. L. Sander, J. R. Silva Nunes, W. L. Lázaro & C. J. Silva, 2022. Long-term analysis of aquatic macrophyte diversity and structure in the Paraguay river ecological corridor. Brazilian Pantanal Wetland. Aquatic Botany 178: 103500. https://doi.org/10.1016/j.aquabot.2022.103500.
Mori, G. B., M. T. F. Piedade, A. Lopes, S. F. B. Ferraz, L. F. Cancian & A. F. M. Camargo, 2021. Different scales determine the occurrence of aquatic macrophyte species in a tropical stream. Acta Botanica Brasilica 35: 37–45. https://doi.org/10.1590/0102-33062020ABB0362.
Moura-Júnior, E. G. & V. M. Cotarelli, 2019. An update on the knowledge of aquatic macrophytes in Northeast Brazil. Abstract We updated the first checklist of aquatic macrophytes from Northeast Brazil. Rodriguésia. https://doi.org/10.1590/2175-7860201970076.
Moura-Júnior, E. G., R. M. S. Paiva, A. C. Ferreira, L. D. Pacopahyba, A. S. Tavares, F. A. Ferreira & A. Pott, 2015. Updated checklist of aquatic macrophytes from Northern Brazil. Acta Amazonica 45: 111–132.
Moura-Júnior, E. G., A. Pott, W. Severi & C. S. Zickel, 2017. Water level rise induced limnological changes indirectly influencing the structure of aquatic macrophyte communities in a tropical reservoir. Journal of Plant Sciences 4: 195. https://doi.org/10.11648/j.jps.20160406.18.
Murphy, K. J., G. Dickinson, S. M. Thomaz, L. M. Bini, K. Dick, K. Greaves, M. P. Kennedy, S. Livingstone, H. McFerran, J. M. Milne, J. Oldroyd & R. A. Wingfield, 2003. Aquatic plant communities and predictors of diversity in a sub-tropical river floodplain: the upper Rio Paraná, Brazil. Aquatic Botany 77: 257–276. https://doi.org/10.1016/S0304-3770(03)00108-6.
Murphy, K. J., A. Efremov, T. A. Davidson, E. Molina-Navarro, K. C. Fidanza, P. Chambers, J. Tapia-Grimaldo, S. Varandas-Martins, I. Springuel, M. Kennedy, R. P. Mormul, E. Dibble, D. Hofstra, B. A. Lukács, D. Gebler, L. Baastrup-Spohr & J. Urrutia-Estrada, 2019. World distribution, diversity and endemism of aquatic macrophytes. Aquatic Botany 158: 103127. https://doi.org/10.1016/J.AQUABOT.2019.06.006.
Naimi, B., N. A. Hamm, T. A. Groen, A. K. Skidmore & A. G. Toxopeus, 2014. Where is positional uncertainty a problem for species distribution modelling. Ecography 37: 191–203. https://doi.org/10.1111/j.1600-0587.2013.00205.x.
Nascimento, C. P., J. C. Alves, J. D. Latini & L. C. Gomes, 2022. Anthropogenic Activities and Habitat Complexity Influence Fish Functional Diversity in a Neotropical Reservoir. 84: 35. https://doi.org/10.1007/s00027-022-00865-4.
Oksanen, J., G. L. Simpson, & F. G. Blanchet, 2022. Vegan: Community Ecology Package. R package version 2.5-7. https://cran.r-project.org/web/packages/vegan/vegan.pdf.
Oliveira, L. S., B. O. Andrade, I. I. Boldrini & M. C. C. Moço, 2019. Aquatic vascular plants of south Brazil: Checklist and a comparative floristic approach. Acta Botanica Brasilica 33: 709–715. https://doi.org/10.1590/0102-33062019abb0194.
Paradis, E., 2022. Analyses of Phylogenetics and Evolution. http://ape-package.ird.fr/.
Pereira, S. A., C. R. T. Trindade, E. F. Albertoni & C. Palma-Silva, 2012. Aquatic macrophytes as indicators of water quality in subtropical shallow lakes, Southern Brazil. Acta Limnologica Brasiliensia 24: 52–63. https://doi.org/10.1590/S2179-975X2012005000026.
Pereira, K. M., S. M. Hefler, G. Trentin & A. S. Rolon, 2021. Influences of landscape and climatic factors on aquatic macrophyte richness and composition in ponds. Flora 279: 151811. https://doi.org/10.1016/j.flora.2021.151811.
Pereto, S. C. A. S. & A. A. Padial, 2021. Macrophyte functional composition is stable across a strong environmental gradient of a Neotropical floodplain. Acta Botanica Brasilica 35: 62–69. https://doi.org/10.1590/0102-33062020abb0348.
Petsch, D. K., K. A. Cottenie, A. Padial, J. D. Dias, C. C. Bonecker, S. M. Thomaz & A. S. Melo, 2021. Floods homogenize aquatic communities across time but not across space in a Neotropical floodplain. Aquatic Sciences 83: 19. https://doi.org/10.1007/s00027-020-00774-4.
Piedade, M. T. F., A. Lopes, L. O. Demarchi, W. Junk, F. Wittmann, J. Schöngart & J. Cruz, 2018. Guia de campo de herbáceas aquáticas: várzea Amazônica, Editora INPA, Manaus:
Pivari, M. O. D., P. H. A. Melo, F. S. Souza, J. R. Stehmann, E. G. Moura-Júnior, S. N. Moreira, V. J. Pott, A. Pott, A. Lopes, M. C. C. Moço, L. S. Oliveira, A. L. A. Lins, R. Arruda, I. L. Morais, G. S. Silva & R. M. Ferreira, 2019. New initiatives for Brazilian aquatic plant data management. Acta Botanica Brasilica 33: 78–87. https://doi.org/10.1590/0102-33062018abb0280.
Pott, V. J. & A. Pott, 2000. Plantas aquáticas do Pantanal, Embrapa Pantanal (Corumbá, MS), Brasília:
Pott, A., V. J. Pott, S. N. Moreira & F. A. Ferreira, 2012. Macrófitas aquáticas do Pantanal e de outras áreas úmidas em Mato Grosso do Sul. Heringeriana 6: 72–75.
Pozzobom, U. M., V. L. Landeiro, M. T. S. Brito, J. Alahuhta & J. Heino, 2021. Multiple facets of macrophyte beta diversity are shaped by environmental factors, directional spatial processes, and connectivity across tropical floodplain lakes in the dry season. Hydrobiologia 848: 3587–3602. https://doi.org/10.1007/s10750-021-04613-x.
PPG I (Pteridophyte Phylogeny Group), 2016. A community-derived classification for extant lycophytes and ferns. Journal of Systematics and Evolution 54(6): 563–603. https://doi.org/10.1111/jse.12229.
Quinn, G. P. & M. J. Keough, 2002. Experimental Design and Data Analysis for Biologists, Cambridge University Press, Cambridge:
R Development Core Team, 2019. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna
Reid, M. A. & G. P. Quinn, 2004. Hydrologic regime and macrophyte assemblages in temporary floodplain wetlands: Implications for detecting responses to environmental water allocations. Wetlands 24: 586–599. https://doi.org/10.1672/0277-5212(2004)024[0586:HRAMAI]2.0.CO;2.
Ripley, B., B. Venables, D. M. Bates & D. Firth, 2022. Modern Applied Statistics with S, 4th ed. Springer, New York. https://www.stats.ox.ac.uk/pub/MASS4/.
Rolon, A. S., H. F. Homem & L. Maltchik, 2010. Aquatic macrophytes in natural and managed wetlands of Rio Grande do Sul State, Southern Brazil. Acta Limnologica Brasiliensia 22: 133–146. https://doi.org/10.4322/actalb.02202003.
Sârbu, A., G. Janauer, U. Schmidt-Mumm, P. Filzmoser, D. Smarandache & G. Pascale, 2021. Characterisation of the potamal Danube River and the Delta: connectivity determines indicative macrophyte assemblages. Hydrobiologia 671: 75–93. https://doi.org/10.1007/s10750-011-0705-5.
Schneider, B., E. R. Cunha, L. A. Espínola, M. Marchese & S. M. Thomaz, 2019. The importance of local environmental, hydrogeomorphological and spatial variables for beta diversity of macrophyte assemblages in a Neotropical floodplain. Journal of Vegetation Science 30: 269–280. https://doi.org/10.1111/jvs.12707.
Silva, S. C. A., A. C. Cervi, C. Bona & A. A. Padial, 2014. Aquatic macrophyte community varies in urban reservoirs with different degrees of eutrophication. Acta Limnologica Brasiliensia 26: 129–142. https://doi.org/10.1590/S2179-975X2014000200004.
Simão, C. H., F. M. Alves, A. Barros, P. M. Simão, A. Pott & C. Aoki, 2021. Reproductive phenology of aquatic macrophytes in the Cerrado-Pantanal ecotone. Acta Botanica Brasilica 35: 92–103. https://doi.org/10.1590/0102-33062020abb0364.
Singh, K. K., K. K. Singh, K. Usha, S. Das & S. S. Singh, 2022. Evaluation of seasonal dynamics of the surface water hydrochemistry using multivariate statistical techniques and aquatic macrophyte productivity in a mountainous lake, Northeast India. Environmental Science and Pollution Research 29: 69048–69067. https://doi.org/10.1007/s11356-022-20631-1.
Souza, A. F. & F. R. Martins, 2005. Spatial variation and dynamics of flooding, canopy openness, and structure in a Neotropical swamp forest. Plant Ecology 180: 161–173. https://doi.org/10.1007/s11258-004-7811-7.
Sousa, W. T. Z., S. M. Thomaz & K. J. Murphy, 2011. Drivers of aquatic macrophyte community structure in a Neotropical riverine lake. Acta Oecologica 37: 462–475. https://doi.org/10.1016/j.actao.2011.05.015.
Souza, S. N., M. T. F. Piedade, L. O. Demarchi & A. Lopes, 2021. Implications of global climate change for the development and ecological interactions between two key Amazonian aquatic macrophytes. Acta Botanica Brasilica 35: 111–121. https://doi.org/10.1590/0102-33062020abb0138.
Teixeira, A. D. P., M. A. Assis, F. R. Siqueira & J. C. Casagrande, 2008. Tree species composition and environmental relationships in a Neotropical swamp forest in Southeastern Brazil. Wetlands Ecology and Management 16: 451–461. https://doi.org/10.1007/s11273-008-9082-x.
Thomaz, S. M. & E. R. Cunha, 2010. The role of macrophytes in habitat structuring in aquatic ecosystems: methods of measurement, causes and consequences on animal assemblages’ composition and biodiversity. Acta Limnologica Brasiliensia 22: 218–236.
Trindade, C. R. T., V. L. Landeiro & F. Schneck, 2018. Macrophyte functional groups elucidate the relative role of environmental and spatial factors on species richness and assemblage structure. Hydrobiologia 823: 217–230. https://doi.org/10.1007/s10750-018-3709-6.
Tundisi, J. G. & T. M. Tundisi, 2008. Limnologia I. Tundisi, Takako Matsumura.
U.S. EPA., 1991. Amendments to the water quality standardsregulation; compliance with CWA Section 303(c) (2) (B);proposed rule. Fed Reg 56: 58420–58437.
Umetsu, C. A., R. K. Umetsu, K. C. Aparecida, H. José, D. E. Alex & V. Krusche, 2007. Aspectos Físico-Químicos De Dois Rios Da Bacia Do Alto Tapajós – Teles Pires e Cristalino – MT, Durante Período De Estiagem E Cheia. Revista De Ciências Agro-Ambientais 5(1): 59–70.
Wang, J., W. Wang, J. Xiong, L. Li, B. Zhao, I. Sohail & Z. He, 2021. A constructed wetland system with aquatic macrophytes for cleaning contaminated runoff/storm water from urban area in Florida. Journal of Environmental Management 280: 111794. https://doi.org/10.1016/J.JENVMAN.2020.111794.
Wenzel, D. A., E. M. Uliana, F. T. Almeida, A. P. Souza, M. A. S. Mendes & L. G. S. Souza, 2017. Características fisiográficas de sub-bacias do Médio e Alto Rio Teles Pires, Mato Grosso. Revista De Ciências Agroambientais 15: 123–131. https://doi.org/10.5327/z1677-606220172193.
Wittmann, F., 2011. Tree species composition and diversity in Brazilian freshwater floodplains. In Mycorrhiza: Occurrence in Natural and Restored Environments. Nova Science Publishers, Hauppauge.
Yang, W., J. Yan, Y. Wang, B. T. Zhang & H. Wang, 2020. Seasonal variation of aquatic macrophytes and its relationship with environmental factors in Baiyangdian Lake, China. Science of the Total Environment 708: 135112. https://doi.org/10.1016/j.scitotenv.2019.135112.
Acknowledgements
We thank the CNMT Herbarium for providing logistical support for this study. Our thanks to Decanato de Pós-Graduação, Universidade de Brasília for finance support and to the Brazilian governmental agency CAPES (Coordenação de Aperfeiçoamento de Pessoal e Nível Superior or “Coordination of Personnel Improvement and Higher Education”) for a grant (M. O. Cordova and J. F. Keffer, Finance Code 001). We thank Dr. Arnildo Pott and Dr. Vali Pott for contribution with taxonomic identification. To the professors of the Universidade Federal de Mato Grosso – Sinop, Brazil, Dr. Domingos Rodrigues, Dr. Gustavo Canale, and Dr. Rafael Arruda project coordinators in partnership with Energia Sinop S.A.
Funding
This article was financially supported by the Sinop Energia S.A. (Aquatic Macrophyte Monitoring Subprogram (Water Quality Program) and Decanato de Pós-Graduação, Universidade de Brasília, by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001) for the doctoral thesis grant awarded to the first author.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Milton Omar Cordova, Josiane Fernandes Keffer, Dienefe Rafaela Giacoppini, and Cássia Beatriz Rodrigues Munhoz. The first draft of the manuscript was written by Milton Omar Cordova, and all authors commented on previous versions of the manuscript. Later versions were corrected and commented on by Cássia Beatriz Rodrigues Munhoz. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Written—informed consent for publication was obtained from all participants.
Additional information
Handling editor: Katya E. Kovalenko
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Córdova, M.O., Keffer, J.F., Giacoppini, D.R. et al. Environmental and temporal variability of the aquatic macrophyte community in riverine environments in the southern Amazonia. Hydrobiologia 851, 1415–1433 (2024). https://doi.org/10.1007/s10750-023-05385-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05385-2