Abstract
Climatic and hydrological variation is of utmost importance in regions of the globe facing water scarcity and river intermittency (e.g. areas under Mediterranean influence). The main aim of this study was to compare the macroinvertebrate community structure and its bioindicator value (i.e. waterbody ecological status) in streams from three Portuguese regions (Regions C, N and S), representing distinct climatic features and water availability scenarios. Results showed that, overall, sampling sites differed in their climatic, hydromorphological and physical and chemical features, and environmental (abiotic) and ecological (community dissimilarities) gradients among regions were clearly identified. Sites from Regions C (wettest) and S (driest) represented non-overlapping clusters of samples, both in terms of their environmental context and ecological (dis)similarity; sites from Region N occupied an intermediate position, and their macroinvertebrate community was highly variable locally. This coincided with overall higher ecological quality and uniformity in Region C, whereas Regions N and S were more heterogeneous and generally presented lower ecological quality. Our data showed that climate (and associated water scarcity) is coupled with other environmental drivers of the macroinvertebrate community structure, highlighting a shared influence of the three environmental components (climatic, hydromorphological, and physical and chemical) in the modulation of macroinvertebrate communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater importance is undoubtful, given its dual role as a key resource to human populations and habitat to several species (EEA, 2019). As ecosystem holders, freshwaters are estimated to support ca. 40% of global biodiversity and 25% of vertebrate species (Dudgeon et al., 2006). Furthermore, biodiversity is a regulator of ecosystem services, a service itself and a valuable good for humans (Mace et al., 2012). Hence, ensuring biodiverse freshwater ecosystems is crucial to the maintenance of their health and value. In this context, the EU Water Framework Directive (WFD; Directive 2000/06/EC) has assumed a crucial role in Europe since its implementation in 2000. The aim of this piece of legislation and further complementary policies is to follow an integrated ecosystem-based approach in water management, putting the spotlight on ecological status. In fact, the greatest novelty brought by the WFD was the inclusion of an ecological line of evidence in the evaluation of water bodies that formerly was almost exclusively based on chemical monitoring. Two decades after the implementation of the WFD, this ecocentric perspective is now additionally boosted by the European Union (EU) Biodiversity Strategy for 2030 (a part of the European Green Deal) and aligned with the United Nations (UN) Sustainable Development Goals.
One of the biological descriptors within the WFD assessment scheme is the benthic macroinvertebrate community that is amongst the most consolidated biological descriptors or indicators in the WFD bioassessment scheme for riverine ecosystems. The advantages of macroinvertebrates in this context have been highlighted in the literature (e.g. Hellawell, 1986; Metcalfe-Smith, 1994; Chapman & Jackson, 1996; Birk et al., 2012; Pawlowski et al., 2018): (i) the aquatic phase of their life cycle (generally close to or larger than 1 year) is long enough to reflect precedent disturbances in the ecosystem; (ii) the communities usually need long post-disturbance recolonization periods, and therefore, their composition and structure reflects past/historic events; (iii) the high diversity of quantitative metrics available for these specific communities allows the extraction of information about ecological status in different conditions. Therefore, benthic macroinvertebrates are overall well suited to signal disturbances in riverine ecosystems. However, criticisms to the current WFD bioassessment scheme based on macroinvertebrate metrics (e.g. Ramos-Merchante & Prenda, 2017; Carvalho et al., 2019; Santos et al., 2021) have been highlighting the need to fully grasp their sensitivity to disturbances that involve the interplay of anthropogenic and natural contributions, and especially those potentiated by climate change (e.g. hydrological fluctuations, habitat alterations).
According to the SOER 2020 report (EEA, 2019), the main disturbances impacting bioindicators (such as macroinvertebrate communities), and therefore leading to worse ecological statuses of freshwater ecosystems, include hydrological pressures, excessive water abstraction, and the presence of diffuse pollution. Hydrological changes often cause additional indirect effects, such as physical alteration of river channels or the riparian zone, which can jeopardize the ecological evaluation by having a negative impact on aquatic invertebrate communities (EEA, 2019). Hydrological pressures relating to water scarcity generate particular concern in regions under Mediterranean influence, where the occurrence of droughts is not only common but also expected to increase in severity and length under climate change scenarios (IPCC, 2014). These circumstances have been leading to a shift in some rivers from a perennial to an intermittent character. The intermittency of rivers gains particular relevance if extending the context to diffuse pollution threats, as the capacity of diluting contamination decreases through drought periods when flow dramatically decreases (Stubbington et al., 2017) and salinization of freshwaters increases (Cañedo-Argüelles et al., 2013, 2020). Concerning Mediterranean countries, specific macroinvertebrate-based metrics have been developed to accommodate this river type specificities, by incorporating biotic indices that consider sensitive taxa occurring in intermittent or temporary rivers (Munné & Prat, 2009; Feio et al., 2014a, b).
In fact, disturbances to riverine ecosystem health converge to a multiple stressor framework (Ormerod et al., 2010; Carpenter et al., 2011; Reid et al., 2019), where interactions between stressors tend to be very relevant (Côté et al., 2016; Birk et al., 2020). In this multiple stressor context, there is a need to understand biodiversity patterns across environmental gradients and the ability of monitoring tools (such as the WFD bioassessment scheme) to discern between anthropogenic and natural influences, to properly identify threats to freshwater biodiversity and impacts on ecosystem health. In the Mediterranean region, this must be necessarily framed with climate change and its consequences (prolonged droughts, intermittency of rivers), which will directly and indirectly shape regional (hydromorphological and ecological) features of riverine ecosystems, as well as local human pressures. This is the context of the present study, which looked at macroinvertebrate communities across hydrological and climate regimes in a set of Portuguese streams in 2019. This case study is of particular interest, because a water availability gradient can be found along the Iberian Peninsula (Carvalho et al., 2011; Biurrun et al., 2016), associated with typical regional precipitation and temperature regimes. In the last two decades, the frequency of warmer and dryer years in Portugal has been increasing (public data from the Portuguese Institute for Sea and Atmosphere—IPMA), but the 2017–2020 period was highly variable; 2017 was extremely dry and hot, whereas 2018 and 2019 were closer to the norm (1971–2000 period), with drought conditions aggravating again in 2020. This results in heterogeneous pressures and accumulated hydric stress in the most vulnerable regions.
The main aim of this study was to compare the macroinvertebrate community structure and ecological status of streams from three Portuguese regions, representing distinct climatic features and water availability scenarios, along a gradient of water scarcity. To do so, 28 sampling sites were surveyed in a total of 16 streams and six hydrographic basins in the Spring of 2019. Sampling sites were selected to reflect regional and local natural heterogeneity in terms of land use (precluding highly disturbed locations), while reflecting a common set of features (comparable ecological background). It is expected that this study provides important insights on how climatic, hydromorphological, and physical and chemical contexts are reflected in the structure and bioindicator value (ecological status sensu WFD) of the macroinvertebrate communities.
Methodology
Study areas and sampling strategy
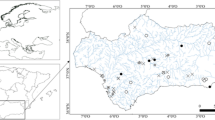
Portugal is part of the Iberian Peninsula, which is dominated by sclerophyllous vegetation adapted to the dry Mediterranean-type climate; in regions where precipitation compensates for the typical dry climate, mixed or deciduous forests can occur (Gavilán et al., 2018). This study contemplated three regions in Portugal along a gradient of Mediterranean features (Carvalho et al., 2011; Biurrun et al., 2016), each one of them presenting different topographic and climate characteristics (Fig. 1). Region C (Littoral Centre) presents Cantabrian mixed forests in northern sampling sites and Southwest Iberian Mediterranean sclerophyllous and mixed forests in southern sampling sites; climate is temperate with rainy winters along with dry and not very hot summers—type Csb, according to Köppen-Geiger classification system (Beck et al., 2018; IPMA, 2021). Among the studied regions, it is the one with milder summers (i.e. less dry), and therefore, less prone to water scarcity. Region N (North-Eastern Portugal) is characterised by the presence of Iberian sclerophyllous and semi-deciduous forests, displaying a temperate climate with rainy winters along with hot and dry summers (type Csa). Most sampling sites of Region N are located on a mountainous part of the country (altitude 250–600 m). Region S (Southern Portugal) belongs to the same climate type (Csa) as Region N, but it is less homogeneous in terms of vegetation, as it includes Iberian sclerophyllous and semi-deciduous forests (in the east) along with Southwest Iberian Mediterranean sclerophyllous and mixed forests (in the West). In contrast to Region N, most sampling sites of Region S are located in lowlands (10–200 m). A quantitative characterization of sampling sites within each region regarding climate variables is available in Supplementary Fig. S1.
Location of sampling sites within the Portuguese continental territory and in Region N (zoomed in the top-left panel), Region C (bottom left panel) and Region S (right-hand panel). Site codes consist of the first three letters of the name of the stream and sequential numbers (1 assigned to the most upstream site)
In each region, sampling sites were selected to reflect regional and local natural heterogeneity of riverine ecosystems (precluding highly disturbed locations, with visible signs of anthropogenic impact), whilst reflecting a common set of features (see below); this required a mix of previous knowledge of the terrain, as well as inspection of satellite information and literature data (sites with existing background information were privileged). A total of 28 sites were selected (Fig. 1): 11 sites (in 7 different streams) in Region C, 7 sites (in 5 different streams) in Region N, 10 sites (in 4 different streams) in Region S (additional information in Supplementary Table S1). We ensured some degree of comparability of site locations to tackle the specific aims of the study. Sampling sites were defined within each area by selecting small to medium size streams, because smaller water courses are more prone to hydrological fluctuations, namely in periods of water scarcity (drought). Streams that had presented dry sections in the past (according to the literature and confirmed by satellite imagery) were selected. Selected sites were shallow (< 1 m deep), narrow (1–12 m wide), and connected to the river continuum in periods of no water scarcity; substrate varied from boulders to gravel, but deposition zones with fine sediments were avoided; each region comprised sampling sites with some degree of vegetation cover (sparse to semi-continuous) and sites where the riparian gallery was absent (see additionally Fig. S2).
Sampling was performed in the Spring of 2019, in May, following the recommendations of the nationally adopted WFD bioassessment protocols (INAG, 2008a).
Climate data
A climatic dataset (see Fig. S1) was assembled with precipitation data from May 2019 (sampling month) and the cumulative precipitation of the previous three and six months (data kindly provided by Portuguese Institute for Sea and Atmosphere—IPMA). The dataset was additionally completed with PDSI (Palmer Drought Severity Index) values for May 2019, as well as three and six months earlier. PDSI considers precipitation, air temperature and availability of ground water, classifying drought periods according to severity and generally ranging within negative values for dry conditions and positive values for wet conditions (Palmer, 1965)—for a better adequacy to the scope of the present study, PDSI values were transformed into a category scale ranging from 1 (no drought) to 5 (severe drought) as detailed in Fig. S1. The records from three and six months before sampling were included in this dataset to provide a more accurate picture of the cumulative climatic and hydrological situation in each region prior to the sampling moment. Given the spatial resolution of the original database, nearby sampling sites share the same climatological information.
Water sampling and analysis
At each sampling site, water temperature (temp, ºC), pH, dissolved oxygen (O2, in mg/L; O2_sat, in % saturation), electrical conductivity (EC, in µS/cm), and total dissolved solids (TDS, in mg/L) were recorded in situ using a multiparameter probe (Aquaread AP-2000). Water samples were collected and transported to the laboratory in refrigerated containers. Turbidity was indirectly assessed by measuring water colour, as the absorption coefficient at 450 nm (Brower et al., 1998). Samples were mineralized with potassium persulphate (Ebina et al., 1983) and then used to measure total phosphorus content (TP, in mg/L) by the tin(II) chloride method (APHA, 2005) and total nitrogen content (TN, in mg/L) by the cadmium reduction method (Lind, 1979). Following vacuum filtration (with glass fibre filters, 1.2 µm pore size) of the samples, the residue was oven-dried at 100 °C for at least two hours and weighed to quantify total suspended solids (TSS, in mg/L) (APHA, 2005), and the filtrate was used to measure coloured dissolved organic carbon (CDOC, in m−1) (Williamson et al., 1999).
Hydromorphological characterization
Each sampling site was characterized concerning hydromorphology and riparian vegetation by measuring channel width, channel depth and flow velocity (flow meter; Global water, FP101), and visually estimating turbidity, shading (% coverage), and continuity of riparian gallery in each bank. These data were categorized in an ordinal scale from 0 to 3 (shading), 0 to 4 (flow velocity and riparian gallery), 1 to 3 (turbidity) or 1 to 4 (channel width and depth), although only a few levels were observed for some variables (see Fig. S2 for scaling details). Additionally, presence/absence (1/0) data were recorded as dummy variables for the types of substrate in the riverbed (blocks, rocks, pebbles, sand), vegetation and logs in the stream channel (and specifically Ranunculus sp. or terrestrial herbs), as well as any existent signals of nearby human influence (structures or constructions, agriculture in the margins, cattle signs). Also, elevation of each sampling site was retrieved from Google Earth for web (https://earth.google.com/web/; assessed on 10/2022).
Macroinvertebrate sampling and analysis
Benthic macroinvertebrates were collected with a standard hand net (0.30 m wide, 500 μm mesh size) as a pooled sample, by kick‐sampling six 1‐m transects respecting the proportion of microhabitats in each sampling site (Hering et al., 2003), following a standardized approach (INAG, 2008a). These samples were stored in plastic containers and preserved with 70–80% v/v ethanol. In the laboratory, samples were washed and organisms were sorted and identified to the lowest practicable taxonomic level, generally the family (and when possible, genus or species) using appropriate taxonomical keys (Hynes, 1993; Wallace et al., 2003; Edington & Hildrew, 2005; Sundermann et al., 2007; Elliott & Humpesch, 2010; Tachet et al., 2010). The abundance of each macroinvertebrate taxon in each sampling site was recorded (see Table S2).
Determination of ecological status
Ecological status was evaluated using multi-metric indices derived from the macroinvertebrate community. Briefly, this is done by estimating the deviation from pristine reference conditions, which constitute the benchmark for ecological assessment. The approach involves the calculation of ecological quality ratios (EQR), which are then translated to ecological status classes (High, Good, Moderate, Poor, Bad) according to river typology. The process was intercalibrated by EU Member States in the scope of the WFD implementation, and reference values for the various macroinvertebrate metrics and ecological quality boundaries were set-up by each member state (van de Bund, 2009; Feio et al., 2014b; Santos et al., 2021). As recommended by the national authorities (see Table S1), we used the biological quality indices IPtIN and IPtIS (respectively, North and South Invertebrate Portuguese Index; INAG, 2009; van de Bund, 2009; APA, 2016), which assess general degradation impacts on invertebrate fauna and were previously intercalibrated—they correspond to ICM-7 and ICM-10 (or IMMi-T), respectively (Munné & Prat, 2009; van de Bund, 2009; Feio et al., 2014b). Their calculation is as follows:
where S is the number of families (richness); EPT is the number of families from the orders Ephemeroptera, Plecoptera and Trichoptera (sensitive taxa); J’ is Pielou’s equitability index (evenness); IASPT is the average sensitivity score per taxon—these scores are derived from the Iberian adaptation of the BMWP index, IBMWP, which essentially sums up scores defining tolerance to pollution of the taxa present in each sample (Alba-Tercedor & Sánchez-Ortega, 1988); and log (sel. ETD + 1) and log (sel. EPTCD + 1) stand for the logarithm of the sum of the abundances of selected sensitive taxa. Before the calculation of the multi-metric indices, each macroinvertebrate metric was normalized to the corresponding reference values (type-specific benchmarks for the pristine reference condition; see Table S1 for details), which were obtained from official guidance documents (INAG, 2009; APA, 2016). A second normalization step was carried out by dividing the multi-metric index value by its type-specific reference value, thus, obtaining the EQR and corresponding ecological status.
Data analysis
The three environmental datasets (climate, hydromorphology, and physical and chemical) were separately analysed using Principal Components Analysis (PCA). Prior to analysis, variables were centred and standardized to accommodate for differential scales. Variables coding for the presence of rocks and pebbles (hydromorphological dataset) were not considered, as these substrates were common to almost all sites; analogously, TN and water colour (physical and chemical dataset) were also discarded because they were uninformative. This initial analysis allowed inspecting the main environmental gradients and the main sources of variation in the data (regional vs. local).
The macroinvertebrate community dataset was analysed using Principal Coordinates Analysis (unconstrained PCoA), computed on a distance matrix based on the square root of Bray–Curtis dissimilarity. This is a popular distance in ecological research, as it circumvents the typical non-linearity of ecological gradients and the double-zero problem—“shared absence” of species across samples (Legendre & Gallagher, 2001; Borcard et al., 2011). Prior to the analysis, rare macroinvertebrate taxa and dubious taxonomical entities (e.g. partially unidentified specimens) were excluded from the dataset; despite reservations on the removal of rare taxa (for considerations on the removal of rare taxa, see Vidal et al., 2014), we excluded all taxa under (i) 5% occurrence or (ii) under 10% occurrence and low total abundance (< 10 individuals). Abundance data were transformed (square root transformation followed by Wisconsin double standardization) to explore extended dissimilarities (metaMDS option in PCoA). The PCoA allowed assessing the main sources of variation (regional vs. local) in the macroinvertebrate community, and the taxa associated with the main ecological gradients. To explore the association between community structure and ecological status, PCoA samples (i.e. site) scores (from the first and second axis) were correlated with the corresponding site EQR (using Pearson correlation index, r).
To assess the contribution of each explanatory environmental dataset on the macroinvertebrate community, models were built using distance-based Redundancy Analysis (db-RDA) based on Bray–Curtis dissimilarity, through the use of a constrained form of PCoA (Legendre & Anderson, 1999; Borcard et al., 2011). Similarly to the unconstrained ordination procedure (see above), abundance data were transformed prior to analysis (square root followed by Wisconsin double standardization) and the square root of Bray–Curtis dissimilarities was used. Each environmental dataset was used in its standardized form (as for the PCA, to minimize scale effects) and a priori selection of variables for each dataset was performed. A stepwise selection procedure of explanatory variables was coupled with the inspection of variance inflation factors (collinearity) in the final model (Borcard et al., 2011); when in presence of two (or more) correlated variables with similar weights in the model, we selected the most simple and informative combination (e.g. variables “sand” and “rocks” constituted opposite vectors that were highly correlated with “flow”; therefore, only “flow” was included in the final model). The purpose of this selection step was to avoid the noise provoked by redundant or less informative variables (Borcard et al., 2011) and to obtain parsimonious models, i.e. models with a good level of explanation using as few predictor variables as possible (Gauch Jr., 1993). This combination of mathematical and logical criteria led to the inclusion of the following explanatory variables in the final models: (i) temperature, dissolved oxygen (in mg/L) and conductivity (physical and chemical); (ii) presence of Ranunculus sp., elevation and flow velocity (hydromorphology); and (iii) cumulative precipitation in the previous 6 months and PDSI in December 2018 (climate).
The use of db-RDA allowed calculating the amount of variation (in terms of ecological distance based on the macroinvertebrate community) explained by each set of explanatory variables, as well as by the overall environmental dataset (global model). The significance of each model was tested using a permutation test, and the adjusted R2 was computed (it provides the proportion of variation in the ecological distance that is explained by the environmental variables). Additionally, a variation partitioning technique (Borcard et al., 1992; Peres-Neto et al., 2006) was applied to quantify the variation explained by the various environmental datasets whilst controlling for (partialling out) the effect of others. In this way, it was possible to discern the unique contribution of each explanatory dataset (e.g. “pure” hydromorphological variation) as well as the joint (i.e. overlapping) contributions of climate, hydromorphology and physical and chemical variables. A Venn diagram was built using Microsoft® Office tools to show the individual and joint contribution of the environmental subsets.
Multivariate analyses and graphics were elaborated using with R software (R Core Team, 2022), using the IDE interface RStudio (RStudio Team, 2020). Specific R packages were required, namely “ggord” (Beck, 2021) and “ggplot2” (Wickham, 2016) for plotting, as well as “vegan” (Oksanen et al., 2020) for ordination. Preliminary steps to support model choice additionally required “vegan” functions rankindex()—to evaluate the association between ecological distance and environmental gradients in various combinations of dissimilarity measures and macroinvertebrate data transformations—and ordiR2step()—to assist on environmental variable selection based on the adjusted R2 and permutational P value of the models.
Results
Environmental context of sampling sites
Overall, samples differed in their climatic, hydromorphological and physical and chemical features (Fig. 2; see Figs. S1-S3 for full details). In most cases, regions (N, C and S) were distinguishable according to their abiotic context, although local variations also occurred. The heterogeneity among regions was more evident concerning climatic variables (Fig. 2, left), with higher precipitation occurring in Region C when compared to Regions N and S (which have overall similar precipitation regimes); however, Region S differs from N by having less water availability (expressed by higher PDSI levels in S). The three regions were also distinct in their hydromorphological features (Fig. 2, centre), since sites from Region C (right side of PCA biplot) were associated with higher flow velocity, higher riparian cover and associated shadow, whilst sites of Regions S and N (with some exceptions) were associated with macrophyte presence (especially Ranunculus sp.) and sandy substrate. Northern (N) sites displayed narrower river channels and the presence of terrestrial herbs (second axis of the PCA biplot). Despite these general regional patterns, local variations occur, as seen by the spread of the data points. Variation in physical and chemical variables (Fig. 2, right) was large for Regions N and S, but Region C was very homogeneous. The extreme variation in Region N was mostly due to site Xed (bottom left data point), where very low oxygen levels were recorded. A clear mineralization and temperature gradient was visible in the PCA biplot (mostly related to the first axis), with sampling sites from Region S displaying higher conductivity and temperature, dissolved solids and organic carbon than sites from Regions C and N, as well as higher phosphorus concentrations (in some sites).
PCA biplots for climatic (left), hydromorphological (middle) and water physical and chemical (right) data. Symbols represent sampling sites according to geographical region (squares—Region N; circles—Region C; triangles—Region S); arrows represent measured environmental variables (corresponding labels shown close to arrow tip). Ellipses (95% confidence) for each region are shown in grey; for simplification, labels of individual sampling sites are not shown. Percent variation explained by each PC is given within brackets
Variation in macroinvertebrate communities
The macroinvertebrate community structure and composition often reflected local variation, although some general inter-regional differences were found (Fig. 3). Unconstrained analysis (PCoA) showed an overall separation of Regions C (right) and S (left) along the first ordination axis, indicating relevant differences in community composition between these regions (except for a few sites). Whereas sites from Region C were more homogeneous, sites from Region S were scattered throughout the second axis (high local variation). Surprisingly, sites from the north (N) were more heterogeneously distributed throughout the ordination plot, despite the fact they all belong to the same hydrographic basin and river typology (Table S1) with the exception of Moi.
PCoA ordination of macroinvertebrate abundance data based on Bray–Curtis dissimilarity. Species (left) and site (right) scores are shown separately to improve visualization. Crosses (×) depict species scores, whereas other symbols represent sampling sites according to geographical region (squares—Region N; circles—Region C; triangles—Region S); ellipses (95% confidence) for each region are shown in grey. Taxa abbreviations are decoded in Table S2 (Supplementary Information). Percent variation explained by each PC is given within brackets
Important taxa associated with the first PCoA gradient were Leuctridae (Leuct), Atherix sp. (Ather), Rhyacophila sp. (Rhya), Gomphidae (Gomph), Serratella sp. (Serrat) and Polycentropodidae (Polyce), which were more frequent and abundant in sites from Region C (see right side of the first axis in Fig. 3). Some of these taxa were shared with sites Cap2 (Region S), Vil2 and Moi (Region N), thus explaining the association of these sites with those from Region C. A Siphonoperla morphospecies (Siphon) also contributed to the separation of sites from Region C, as it was found exclusively in this region. A total of 12 taxa (including the Siphonoperla morphospecies) occurred exclusively in this region (Table S3). On the opposite side of this gradient, taxa such as Oulimnius sp. larvae (Oullar), Physidae (Physi) and Hydrobius sp. larvae (Hydblar) were distinctly more abundant in Region S and in some sites in Region N, segregating sites on the left side of the diagram (Fig. 3).
Variation in the second PCoA axis was most pronounced for Regions S and N (Region C was more homogeneous). Important taxa include Atyaephyra sp. (Atyae), Caenis sp. (Caen) and some Chironomidae groups (Chironomini—Chini; Tanypodinae—Tanyp; and Tanytarsini—Tanyt), which were predominantly found in Oei2, Oei3, Oei4 and Cai1 (Region S), as well as Vil1 (Region N)—bottom left in the diagram (Fig. 3). Curiously, Oei1 was distinct from the remaining Oeiras sites, as it did not have such high abundances of these taxa. On a distinct note, Notonecta sp. (Noto), Dytiscidae larvae (Dytlar) and Sympecma sp. (Sympec) occurred predominantly in sites from Regions N (Cal1, Cal2, Sou1, Xed) and S (top left in the diagram; Fig. 3), being almost absent from Region C. Only 7 and 3 taxa were exclusively found in Regions N and S, respectively (Table S3).
Overall, there was some correspondence between community composition and ecological quality (Fig. 4), which partly relates to regional and local differences. Indeed, EQR values were significantly correlated with the first PCoA axis (r = 0.65, P < 0.001), but were not associated with the second PCoA axis (r = 0.04, P = 0.85). All sites from Region C (all located on the right side of the diagram) were of High or Good ecological status. Sampling sites from Regions S and N were more heterogenous in terms of ecological quality, ranging from Poor to Good ecological status, apart the few exceptions of sites Cai1 and Cai2 in Region S, as well as Moi in Region N, which were found to be of High ecological status. Considering IBMWP as an indicator of species sensitivity, it becomes clear that sites from Region C had a high proportion of exclusive sensitive taxa (67% of macroinvertebrate taxa with an IBMWP score had a score ≥ 7), whereas northern and southern sites were dominated by tolerant taxa—in both, 100% of the exclusive taxa had a score < 7 (Table S3).
Relationship between ecological status and PCoA ordination of macroinvertebrate abundance data based on Bray–Curtis dissimilarity. On the left, PCoA ordination of site scores is shown with data labels identifying corresponding ecological status (Poor to High); on the right, a positive association is shown between ecological quality ratios and PCoA site scores from the first axis. Symbols represent sampling sites according to geographical region (squares—Region N; circles—Region C; triangles—Region S); data point labels show ecological status (left) or site identification (right)
Constrained analysis and variation partitioning
The physical and chemical, hydrological and climatic variables explained a significant and comparable portion (7.7–10%, based on the adjusted R2) of the ecological distance among sites (Table 1). A global environmental model pooling these three subsets explained 17% of the macroinvertebrate community gradients. The unique contribution of each environmental subset (partial db-RDA) dropped to 2–5%, indicating that physical and chemical features, hydromorphology and climate influences are inter-correlated (i.e. they jointly explain variation in the macroinvertebrate data). Indeed, the shared proportion of variance explained was substantial (Fig. 5).
The global model (db-RDA with all environmental variables; Fig. 6) showed a separation of Region C from Regions N and S along the first axis, analogously to unconstrained PCoA (see above). This separation was associated with higher flow velocity, dissolved oxygen and precipitation in Region C, along with lower water temperature and PDSI values; Regions N and S (with the exception of Moi, Vil2 and Cap2 as previously noticed) were on opposite gradients to Region C (low flow, low precipitation, higher temperature and PDSI) and were more prone to the presence of Ranunculus sp. and other macrophytes in the channel. Taxa strongly associated to the first axis of the model (Fig. 6) were Leuctridae (Leuct) and Atherix sp. (Ather), among others, which were more frequent and abundant in the sites from Region C, Vil2 and Moi (Region N) and Cap2 (Region S). These sites scored higher in terms of EQR, thus linking increasing gradients of flow velocity, dissolved oxygen and precipitation to better ecological status. A gradient of elevation was reflected on the second axis, a secondary gradient that explains some degree of intra-regional differences, especially associated with sites at higher altitudes (> 500 m), such as Mau1, Cer, Can, and Cat—Fig. 6. The position of Oei2, Oei3, Oei4 (Region S) in the model and the presence of Atyaephyra sp. (Atyae) and Caenis sp. (Caen) (as well as some dipterans) were associated with increased conductivity and PDSI (drought) level. Overall, the environmental gradients associated with the ecological distance between samples (i.e. dissimilarities in macroinvertebrate community composition) show an interplay between climatic, hydromorphological and physical and chemical variables (note for example the association between precipitation, flow velocity and dissolved oxygen; Fig. 6), confirming that it is difficult to ascertain their isolated influence (i.e. the unique contribution of each environmental subset), as shown in Fig. 5.
Distance-based redundancy analysis (db-RDA) ordination of macroinvertebrate abundance data based on Bray–Curtis dissimilarity, representing the relationship between sampling sites (different symbols according to region) and species scores (small circles or crosses) with the selected physical and chemical, hydromorphological and climatic variables (arrows); ellipses (95% confidence) for each region are shown in grey. Percent variation explained by each axis is given within brackets. Environmental explanatory variables include: (i) water temperature (temp), dissolved oxygen in mg/L (O2) and electrical conductivity (EC); (ii) presence of Ranunculus sp. (ranunc), elevation of the sampling site (elevation) and flow velocity (flow); and (iii) cumulative precipitation in the previous 6 months (prec_6m) and PDSI (drought index) in December 2018 (pdsi_dec). Species (top right) and site (bottom right) scores are shown separately to improve visualization; taxa abbreviations are decoded in Table S2 (Supplementary Information)
Discussion
Riverine ecosystems face a multitude of environmental stressors as well as their interactions, and the biological communities therein respond accordingly. A deep understanding of this natural picture, as well as of deviations from common scenarios, is critical to promote informed management decisions (Birk et al., 2020). To properly identify threats to freshwater biodiversity and impacts on ecosystem health, there is a need to understand how community composition and structure change along environmental gradients, and whether this is reflected in riverine ecological status as defined by standard assessment metrics. Here, we sampled and analysed macroinvertebrate communities across hydrological and climate regimes in a set of Portuguese streams in 2019, distributed along a gradient defined by regional precipitation and temperature regimes. Climatic and hydrological variation is of utmost importance in regions of the globe facing water scarcity and river intermittency (e.g. areas under Mediterranean influence), shaping regional features of riverine ecosystems and putatively affecting ecosystem health.
The last two decades have been generally warmer than the norm (1971–2000) in Portugal, but the period that preceded our sampling campaign was heterogeneous. Specifically, 2017 was an extremely dry and hot year (IPMA, 2017), with intense drought (extending through Autumn), substantially affecting aquatic ecosystems. On the contrary, 2018 presented normal levels of precipitation (IPMA, 2018), which may have allowed some degree of recovery of the ecosystems. However, this was largely due to a very rainy Spring, which was followed by a very hot and dry Summer (IPMA, 2018) that may have contributed to the return of stressful conditions, especially in the most drought-prone regions. In turn, the sampling year (2019) was also closer to the norm, although high PDSI levels were still recorded, especially in the South (IPMA, 2019). In 2018, eight sites out of 28 were dry in October/November (Table S4); small streams from Region N were particularly prone to drought. So, despite this paper provides a picture of the status of macroinvertebrate communities and the riverine environment at a specific moment (Spring 2019), it is reasonable to claim that our samples captured the cumulative effects of the combination of the harsh climatic conditions in 2017 and the hydrological fluctuations in subsequent years.
Other studies have provided evidence of long time lags between climatic variation and its effects, such as on the productivity of vegetation from the riparian gallery (Pace et al., 2021), which in turn can be reflected on plant-associated insect communities (Schweiger et al., 2012). The intermittency of flow for unexpectedly long periods of time often translates into negative repercussions for aquatic and terrestrial biota (Datry et al., 2017; Pace et al., 2021). On the other hand, isolated pools can work as reservoirs of biodiversity of groups of limnophilic macroinvertebrates (Bonada et al., 2006). These pools contribute to the overall maintenance of biodiversity throughout cycles of drought and flow even though communities structure and functioning might change with the progressive disappearance of more drought-sensitive species (Bonada et al., 2006; Banegas-Medina et al., 2021). Also, the increasing variation in the outbreak and duration of drought periods can compromise the robustness of the macroinvertebrate communities (Cid et al., 2017). Flow interruptions undeniably play an important role in disturbing biodiversity even when these alterations are expected to happen (Datry et al., 2014; Leigh & Datry, 2017), by progressively eliminating taxa with less effective resistance and/or resilience mechanisms (Stubbington et al., 2017). Changes in flow are even more worrying in Mediterranean streams, which are more prone to aridity, as they may alter responses of their macroinvertebrate communities to drought phenomena in an abrupt way (Bonada et al., 2006). This scenario of low flow, intermittency or drought was confirmed in our sampling sites during some periods of time, based on our own observations in the 2018–2019 period (Table S4) and on historical/literature data.
Our sampling strategy was successful in capturing regional environmental gradients (climatic and water availability features), whilst reflecting local natural heterogeneity. This was visible in the clear distinction of the three regions according to precipitation (higher in C when compared to N and S) and proneness to drought (higher in S, where low precipitation was coupled with higher evapotranspiration). A gradient related with water availability was, therefore, observed from Region C (wettest) to S (driest), with Region N occupying an intermediate position (although sites from Region N were particularly prone to intermittency; Table S4). Hydromorphological and physical and chemical features of sampling sites also revealed regional differences, but the degree of overlap between regions was higher because of the considerable scatter of samples. The dispersion of data points confirms that we were still able to encompass local natural variation whilst complying with a common set of local features during site selection (ensuring a comparable background across samples). Other studies (e.g. Datry et al., 2014; Leigh et al., 2016; Pařil et al., 2019) used similar approaches to explore macroinvertebrate biodiversity across spatial scales, by establishing specific criteria for site admissibility whilst covering the natural array of environmental variation.
Overall, the analysis of the macroinvertebrate communities demonstrated that climatic influences were closely linked to hydromorphology and, to a lesser extent, to the physical and chemical context; unique contributions of climatic, hydromorphological and physical and chemical explanatory variables were difficult to distinguish, and the macroinvertebrate communities were found to be modulated by their joint (i.e. shared) influence. We argue that this joint influence reflects direct and indirect effects of climate. Indeed, flow and macrophyte cover were dependent on precipitation patterns, whilst dissolved oxygen and flow velocity were negatively correlated with temperature. Drier sites (mostly samples from Region S) displayed a less extensive cover (and shading) of riparian gallery, as well as a more pronounced occurrence of in-bed macrophytes associated with lower flow velocity. In fact, riparian forests (represented by Alnus glutinosa (L.) Gaertn., Fraxinus angustifolia Vahl, Populus spp., Salix spp., etc.) are considered Eurosiberian islands in Mediterranean landscapes (Gavilán et al., 2018), as this type of vegetation is supported by the local availability of water in an otherwise unfavourable environment. Especially in the South (Region S), droughts, water scarcity (both surface and groundwater) and river intermittency may represent a challenge to this type of vegetation, thus explaining the overlap between climatic (lower precipitation, higher PDSI) and hydromorphological (lower riparian cover) features. This interconnection between climate and hydromorphology is present in the importance of accumulated rainfall in the seasonality and productivity of riparian vegetation (Pace et al., 2021), as well as in the adaptations of algae and vascular plants to harsh conditions to overcome periods of water intermittency in stream beds (Sabater et al., 2017). In contrast, Region C recorded higher precipitation and lower PDSI, translating into higher flow and riparian cover. Region N occupied an intermediate position between Regions C and S; despite the tendency for Summer or post-Summer droughts (Table S4), water availability in Region N does not seem as problematic as in Region S. Indeed, higher temperatures were associated with proneness to drought, and corresponding decrease of dissolved oxygen and flow velocity, especially due to Region S. Conductivity was also higher in this region (mostly due to geological differences; Alves et al., 2004; INAG, 2008b; Feio et al., 2009). The close interconnection between climate, hydromorphology and physical and chemical features contributes to the idea that the ecological dissimilarities found reflect both a direct climatic gradient and an indirect climatic gradient identified through critical hydromorphological (mostly) and physical and chemical variables.
Both unconstrained and constrained analyses of the macroinvertebrate communities resulted in a clear distinction between regions, especially between C and S. Additionally, sampling sites from Region S were more heterogenous (large scatter in multivariate plots) than Region C (homogeneous cluster of samples). Although sampling sites from Region C belong to distinct hydrographic basins, the sampled streams are geographically close enough to allow the establishment of gene flow between streams by the flying life phases of many macroinvertebrates (Banegas-Medina et al., 2021). This may concur to explain the observed homogeneity of communities within this region, along with the fairly homogeneous physical and chemical features of the sites in this region (unlike S and N). On the contrary, the heterogeneity of macroinvertebrate communities in Region N is surprising, taking into account the narrow geographic range of N sites and similar climatic and hydromorphological features. Although the existence of unmeasured constraints cannot be ruled out considering the amount of variance explained by the models, differences in water availability (and susceptibility to drought) of the sites of this region can help explaining the heterogeneity of its biological communities despite the geographical proximity of streams. In fact, Region N presented more locations without water in the preceding Autumn (2018) than any other region (Table S4), which is likely to be reflected in the health of the biological communities in the following Spring. Differential drying of the sites in this region could have compromised emergence and oviposition patterns in some sites, causing this unexpected within-region heterogeneity. Hydromorphological characteristics and/or climate can cause different stages of drought in close locations. For example, the condition of riparian galleries and consequently exposure to sunlight can provoke differential drying among sites, as well as slope (Cartwright et al., 2020). In fact, the sites from Region N were indeed more prone to drying (Table S4).
The differences across regions (ecological distance) were partially translated into differences in ecological status (bioindicator value of the macroinvertebrate community). The uniqueness of Region C concerning climatic, hydromorphological and physical and chemical characteristics was reflected in overall high EQRs and High (~ 80% of sites) or Good ecological status, which did not happen in the other regions. Many of the taxa exclusive to Region C (Table S3) are sensitive to disturbance, which is certainly contributing to the overall high ecological status observed. The consequent separation of Region C from the other regions in the analyses (mostly along the first ordination axes) justifies the positive correlation found between ecological distance and EQRs. On the contrary, Regions N and S presented overall lower EQRs, yet meeting a broader range of ecological statuses (Poor to High). In Region N, Moi and Vil2 were the only sites with High and Good ecological status (respectively), which is likely due to higher flow and water oxygenation that favour healthier (e.g. richer, more diverse) biological communities. The remaining sites of this region scored low EQRs, which is consistent with lower water availability conditions across the region in the past (see above), reflecting cumulative effects of drought. Concerning Region S, lower EQRs (Poor or Moderate ecological status) belong to Oeiras (Oei2-Oei4) and Grândola (Gra2) streams, where hydromorphological conditions are challenging, accumulated with the climatic stress imposed to the region as a whole. The other sites scored higher EQRs (Good and High ecological status). These differences illustrate variation in biological patterns in this region due to the vastness of the geographical distribution of its sampling sites and different intensity of climatic stress.
On a finer scale, the analysis of the macroinvertebrates associated with each region allowed the identification of some patterns. Sensitive organisms such as Leuctridae (Plecoptera) and Athericidae (Diptera), which are not very tolerant to drought periods and hydromorphological alterations, were mostly present in sites with high flow. Streams with permanent flow and riffles had a marked presence of Leuctridae (Plecoptera), Siphlonurus sp. and Serratella sp. (Ephemeroptera), as well as Rhyacophila sp. (Trichoptera) in Region C (high flow velocity and water oxygenation), confirming the preference for this type of habitats by EPT taxa (Bonada et al., 2006). Mediterranean streams usually present macroinvertebrate communities with organisms that are adapted (to a certain degree) to the typical harsh conditions of this climate type (Bonada et al., 2007; Cid et al., 2017). In our case, this trend was observed within Regions N and S (e.g. in Oei2-Oei4, Sou1, Xed, Cal1 and Cal2, which all have a history of dry periods), with the presence of Notonecta sp. and Dytiscidae larvae and adults, which tend to be frequent in pools formed during the drying process (Bonada et al., 2006). Elmidae larvae and adults, although characteristic of riffles (White, 2009), were also present, as previously happened in streams subjected to drying in Mediterranean climate streams (Bonada et al., 2006). Also, thermo and limnophilic ephemeropterans (e.g. Caenis sp.; Péran et al., 1999) were also present in these sites, which concurs with the Mediterranean characteristics of Regions N and S.
Overall, our study shows that regions with distinct climate and water availability backgrounds were distinguishable according to their environmental features, but also—partially—in terms of the composition and bioindicator value of the macroinvertebrate community. Additionally, climate (and associated water scarcity) was coupled with other environmental drivers of the macroinvertebrate community, namely hydromorphology (riparian cover and flow velocity). Lower ecological status seemed to be associated with accumulated hydric stress in more vulnerable regions (namely S and N). It is unclear whether these ecological differences across regions will be potentiated by climate change and extreme events, via differential pressures (Region C vs. Regions N and S). In such scenarios, it is important to understand whether the background conditions influence the resilience of macroinvertebrate communities to the impacts of drought or low flow. In our case, this could imply that macroinvertebrate communities that are less used to water scarcity and droughts (i.e. Region C) may respond more dramatically to droughts, being most affected; alternatively, it could be the case that communities that are already displaying signs of disturbance (Regions N and S) will be most affected, as a consequence of cumulative pressures. Future work from our team is ongoing to compare macroinvertebrate community patterns across Regions N, C and S in hydrologically distinct years. This is a much-needed venture to understanding resilience patterns.
Data availability
Data are accessible from supplementary material and raw datasets are available from the authors upon reasonable request.
References
Alba-Tercedor, J. & A. Sánchez-Ortega, 1988. Un método rápido y simple para evaluar la calidad biológica de las águas corrientes basado en el de Hellawell (1978). Limnética 4: 51–56.
Alves, M. H., J. M. Bernardo, H. Figueiredo, J. Pádua, P. Pinto, & M. T. Rafael, 2004. Aplicação do Sistema B da Directiva-Quadro da Água na identificação de tipos de rios em Portugal Continental. In: 7° Congresso da Água: 14.
APA, 2016. Plano de Gestão de Bacia Hidrográfica, Parte 2 - Caracterização e Diagnóstico.
APHA, 2005. Standard methods for the examination of water and wastewater. American Public Health Association (APHA), American Water Works Association (AWWA) and the Water Environment Federation (WEF), Washington DC.
Banegas-Medina, A., I.-Y. Montes, O. Tzoraki, L. Brendonck, T. Pinceel, G. Diaz, P. Arriagada, J.-L. Arumi, P. Pedreros & R. Figueroa, 2021. Hydrological, environmental and taxonomical heterogeneity during the transition from drying to flowing conditions in a mediterranean intermittent river. Biology 10: 316.
Beck, M. W., 2021. ggord: ordination Plots with ggplot2.
Beck, H. E., N. E. Zimmermann, T. R. McVicar, N. Vergopolan, A. Berg & E. F. Wood, 2018. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Scientific Data 5: 180214.
Birk, S., W. Bonne, A. Borja, S. Brucet, A. Courrat, S. Poikane, A. Solimini, W. van de Bund, N. Zampoukas & D. Hering, 2012. Three hundred ways to assess Europe’s surface waters: an almost complete overview of biological methods to implement the Water Framework Directive. Ecological Indicators 18: 31–41.
Birk, S., D. Chapman, L. Carvalho, B. M. Spears, H. E. Andersen, C. Argillier, S. Auer, A. Baattrup-Pedersen, L. Banin, M. Beklioğlu, E. Bondar-Kunze, A. Borja, P. Branco, T. Bucak, A. D. Buijse, A. C. Cardoso, R.-M. Couture, F. Cremona, D. de Zwart, C. K. Feld, M. T. Ferreira, H. Feuchtmayr, M. O. Gessner, A. Gieswein, L. Globevnik, D. Graeber, W. Graf, C. Gutiérrez-Cánovas, J. Hanganu, U. Işkın, M. Järvinen, E. Jeppesen, N. Kotamäki, M. Kuijper, J. U. Lemm, S. Lu, A. L. Solheim, U. Mischke, S. J. Moe, P. Nõges, T. Nõges, S. J. Ormerod, Y. Panagopoulos, G. Phillips, L. Posthuma, S. Pouso, C. Prudhomme, K. Rankinen, J. J. Rasmussen, J. Richardson, A. Sagouis, J. M. Santos, R. B. Schäfer, R. Schinegger, S. Schmutz, S. C. Schneider, L. Schülting, P. Segurado, K. Stefanidis, B. Sures, S. J. Thackeray, J. Turunen, M. C. Uyarra, M. Venohr, P. C. von der Ohe, N. Willby & D. Hering, 2020. Impacts of multiple stressors on freshwater biota across spatial scales and ecosystems. Nature Ecology and Evolution 4: 1060–1068.
Biurrun, I., J. A. Campos, I. García-Mijangos, M. Herrera & J. Loidi, 2016. Floodplain forests of the Iberian Peninsula: vegetation classification and climatic features. Applied Vegetation Science 19: 336–354.
Bonada, N., M. Rieradevall, N. Prat & V. H. Resh, 2006. Benthic macroinvertebrate assemblages and macrohabitat connectivity in Mediterranean-climate streams of northern California. Journal of the North American Benthological Society 25: 32–43.
Bonada, N., S. Dolédec & B. Statzner, 2007. Taxonomic and biological trait differences of stream macroinvertebrate communities between Mediterranean and temperate regions: implications for future climatic scenarios. Global Change Biology 13: 1658–1671.
Borcard, D., P. Legendre & P. Drapeau, 1992. Partialling out the spatial component of ecological variation. Ecology 73: 1045–1055.
Borcard, D., F. Gillet & P. Legendre, 2011. Numerical Ecology with R, Springer, New York.
Brower, J. E., J. H. Zar & C. N. von Ende, 1998. Field and Laboratory Methods of General Ecology, McGraw-Hill.
Cañedo-Argüelles, M., C. Gutiérrez-Cánovas, R. Acosta, D. Castro-López, N. Cid, P. Fortuño, A. Munné, C. Múrria, A. R. Pimentão, R. Sarremejane, M. Soria, P. Tarrats, I. Verkaik, N. Prat & N. Bonada, 2020. As time goes by: 20 years of changes in the aquatic macroinvertebrate metacommunity of Mediterranean river networks. Journal of Biogeography 47: 1861–1874.
Cañedo-Argüelles, M., B. J. Kefford, C. Piscart, N. Prat, R. B. Schäfer & C.-J. Schulz, 2013. Salinisation of rivers: an urgent ecological issue. Environmental Pollution 173: 157–167.
Carpenter, S. R., E. H. Stanley & M. J. Vander Zanden, 2011. State of the world’s freshwater ecosystems: physical, chemical, and biological changes. Annual Review of Environment and Resources 36: 75–99.
Cartwright, J. M., C. E. Littlefield, J. L. Michalak, J. J. Lawler & S. Z. Dobrowski, 2020. Topographic, soil, and climate drivers of drought sensitivity in forests and shrublands of the Pacific Northwest, USA. Scientific Reports 10: 18486.
Carvalho, J. C., P. Cardoso, L. C. Crespo, S. Henriques, R. Carvalho & P. Gomes, 2011. Determinants of beta diversity of spiders in coastal dunes along a gradient of mediterraneity. Diversity and Distributions 17: 225–234.
Carvalho, L., E. B. Mackay, A. C. Cardoso, A. Baattrup-Pedersen, S. Birk, K. L. Blackstock, G. Borics, A. Borja, C. K. Feld, M. T. Ferreira, L. Globevnik, B. Grizzetti, S. Hendry, D. Hering, M. Kelly, S. Langaas, K. Meissner, Y. Panagopoulos, E. Penning, J. Rouillard, S. Sabater, U. Schmedtje, B. M. Spears, M. Venohr, W. van de Bund & A. L. Solheim, 2019. Protecting and restoring Europe’s waters: an analysis of the future development needs of the Water Framework Directive. Science of the Total Environment 658: 1228–1238.
Chapman, D., & J. Jackson, 1996. Biological Monitoring. In: Bartram, J., & R. Ballance (eds), Water Quality Monitoring - A Practical Guide to the Design and Implementation of Freshwater Quality Studies and Monitoring Programmes. United Nations Environment Programme and the World Health Organization, pp. 263–302.
Cid, N., N. Bonada, S. M. Carlson, T. E. Grantham, A. Gasith & V. H. Resh, 2017. High variability is a defining component of Mediterranean-climate rivers and their biota. Water 9: 52.
Côté, I. M., E. S. Darling & C. J. Brown, 2016. Interactions among ecosystem stressors and their importance in conservation. Proceedings of the Royal Society B: Biological Sciences 283: 1–9.
Datry, T., S. T. Larned, K. M. Fritz, M. T. Bogan, P. J. Wood, E. I. Meyer & A. N. Santos, 2014. Broad-scale patterns of invertebrate richness and community composition in temporary rivers: effects of flow intermittence. Ecography 37: 94–104.
Datry, T., N. Bonada, & A. J. Boulton, 2017. General introduction. In: Intermittent Rivers and Ephemeral Streams: Ecology and Management. Elsevier Inc.: 1–20.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z.-I. Kawabata, D. J. Knowler, C. Lévêque, R. J. Naiman, A.-H. Prieur-Richard, D. Soto, M. L. J. Stiassny & C. A. Sullivan, 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81: 163–182.
Ebina, J., T. Tsutsui & T. Shirai, 1983. Simultaneous determination of total nitrogen and total phosphorus in water using peroxodisulfate oxidation. Water Research 17: 1721–1726.
Edington, J. M., & A. G. Hildrew, 2005. A Revised Key to the Caseless Caddis Larvae of the British Isles. Freshwater Biological Association, UK.
EEA, 2019. The European environment — state and outlook 2020 (SOER 2020). .
Elliott, J. M., & U. H. Humpesch, 2010. Mayfly Larvae (Ephemeroptera) of Britain and Ireland: Keys and a Review of their Ecology. Freshwater Biological Association, UK.
Feio, M. J., R. H. Norris, M. A. S. Graça & S. Nichols, 2009. Water quality assessment of Portuguese streams: regional or national predictive models? Ecological Indicators 9: 791–806.
Feio, M. J., F. C. Aguiar, S. F. P. Almeida, J. Ferreira, M. T. Ferreira, C. Elias, S. R. Q. Serra, A. Buffagni, J. Cambra, C. Chauvin, F. Delmas, G. Dörflinger, S. Erba, N. Flor, M. Ferréol, M. Germ, L. Mancini, P. Manolaki, S. Marcheggiani, M. R. Minciardi, A. Munné, E. Papastergiadou, N. Prat, C. Puccinelli, J. Rosebery, S. Sabater, S. Ciadamidaro, E. Tornés, I. Tziortzis, G. Urbanič & C. Vieira, 2014a. Least disturbed condition for European Mediterranean rivers. Science of the Total Environment 476–477: 745–756.
Feio, M. J., J. Ferreira, A. Buffagni, S. Erba, G. Dörflinger, M. Ferréol, A. Munné, N. Prat, I. Tziortzis & G. Urbanič, 2014b. Comparability of ecological quality boundaries in the Mediterranean basin using freshwater benthic invertebrates. Statistical options and implications. Science of the Total Environment 476–477: 777–784.
Gauch, H. G., Jr., 1993. Prediction, parsimony and noise. American Scientist 81: 468–478.
Gavilán, R. G., B. Vilches, A. Gutiérrez-Girón, J. M. Blanquer, & A. Escudero, 2018. Sclerophyllous Versus Deciduous Forests in the Iberian Peninsula: A Standard Case of Mediterranean Climatic Vegetation Distribution. In: Greller, A., K. Fujiwara, & F. Pedrotti (eds), Geographical Changes in Vegetation and Plant Functional Types. Springer, Cham: 101–106.
Hellawell, J. M., 1986. Biological indicators of freshwater pollution and environmental management. Elsevier Applied Science Publishers.
Hering, D., A. Buffagni, O. Moog, L. Sandin, M. Sommerhäuser, I. Stubauer, C. Feld, R. Johnson, P. Pinto, N. Skoulikidis, P. Verdonschot & S. Zahrádková, 2003. The development of a system to assess the ecological quality of streams based on macroinvertebrates – design of the sampling programme within the AQEM project. International Review of Hydrobiology 88: 345–361.
Hynes, H. B. N., 1993. A Key to the Adults and Nymphs of the British Stoneflies (Plecoptera): With Notes on Their Ecology and Distribution. Freshwater Biological Association, UK.
INAG, 2008a. Manual para a avaliação biológica da qualidade da água em sistemas fluviais segundo a Directiva Quadro da Água - Protocolo de amostragem e análise para os macroinvertebrados bentónicos. Ministério do Ambiente, Ordenamento do Território e do Desenvolvimento Regional, Portugal.
INAG, 2008b. Tipologia de rios em Portugal Continental no âmbito da implementação da Directiva Quadro da Água, I - Caracterização abiótica. Ministério do Ambiente, Ordenamento do Território e do Desenvolvimento Regional, Portugal.
INAG, 2009. Critérios para a Classificação do Estado das Massas de Água Superficiais - Rios e Albufeiras. Ministério do Ambiente, Ordenamento do Território e do Desenvolvimento Regional, Portugal.
IPCC, 2014. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge.
IPMA, 2017. Boletim Climático Anual, Portugal Continental - 2017. Portugal.
IPMA, 2018. Boletim Climático Anual, Portugal Continental - 2018. Portugal.
IPMA, 2019. Boletim Climático Anual, Portugal Continental - 2019. Portugal.
IPMA, 2021. Normais climatológicas, https://www.ipma.pt/pt/oclima/normais.clima/.
Legendre, P. & M. J. Anderson, 1999. Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs 69: 1–24.
Legendre, P. & E. D. Gallagher, 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129: 271–280.
Leigh, C. & T. Datry, 2017. Drying as a primary hydrological determinant of biodiversity in river systems: a broad-scale analysis. Ecography 40: 487–499.
Leigh, C., N. Bonada, A. J. Boulton, B. Hugueny, S. T. Larned, R. Vander Vorste & T. Datry, 2016. Invertebrate assemblage responses and the dual roles of resistance and resilience to drying in intermittent rivers. Aquatic Sciences 78: 291–301.
Lind, O. T., 1979. Handbook of common methods in Limnology. Kendall Hunt Publishing.
Mace, G. M., K. Norris & A. H. Fitter, 2012. Biodiversity and ecosystem services: a multilayered relationship. Trends in Ecology and Evolution 27: 19–26.
Metcalfe-Smith, J. L., 1994. Biological Water-Quality Assessment of Rivers: Use of Macroinvertebrate Communities. In: Calow, P., & G. E. Petts (eds), The Rivers Handbook: Hydrological and Ecological Principles, Volume Two. Blackwell Science Ltd, Oxford, UK.
Munné, A. & N. Prat, 2009. Use of macroinvertebrate-based multimetric indices for water quality evaluation in Spanish Mediterranean rivers: an intercalibration approach with the IBMWP index. Hydrobiologia 628: 203–225.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs, & H. Wagner, 2020. vegan: Community Ecology Package. https://cran.r-project.org/package=vegan.
Ormerod, S. J., M. Dobson, A. G. Hildrew & C. R. Townsend, 2010. Multiple stressors in freshwater ecosystems. Freshwater Biology 55: 1–4.
Pace, G., C. Gutiérrez-Cánovas, R. Henriques, F. Boeing, F. Cássio & C. Pascoal, 2021. Remote sensing depicts riparian vegetation responses to water stress in a humid Atlantic region. Science of the Total Environment 772: 145526.
Palmer, W. C., 1965. Meteorological drought. Research Paper No. 45. US Weather Bureau.
Pařil, P., M. Polášek, B. Loskotová, M. Straka, J. Crabot & T. Datry, 2019. An unexpected source of invertebrate community recovery in intermittent streams from a humid continental climate. Freshwater Biology 64: 1971–1983.
Pawlowski, J., M. Kelly-Quinn, F. Altermatt, L. Apothéloz-Perret-Gentil, P. Beja, A. Boggero, A. Borja, A. Bouchez, T. Cordier, I. Domaizon, M. J. Feio, A. F. Filipe, R. Fornaroli, W. Graf, J. Herder, B. van der Hoorn, J. I. Jones, M. Sagova-Mareckova, C. Moritz, J. Barquín, J. J. Piggott, M. Pinna, F. Rimet, B. Rinkevich, C. Sousa-Santos, V. Specchia, R. Trobajo, V. Vasselon, S. Vitecek, J. Zimmerman, A. Weigand, F. Leese & M. Kahlert, 2018. The future of biotic indices in the ecogenomic era: integrating (e)DNA metabarcoding in biological assessment of aquatic ecosystems. Science of the Total Environment 637–638: 1295–1310.
Péran, A., J. Velasco & A. Millán, 1999. Life cycle and secondary production of Caenis luctuosa (Ephemeroptera) in a semiarid stream (Southeast Spain). Hydrobiologia 400: 187–194.
Peres-Neto, P. R., P. Legendre, S. Dray & D. Borcard, 2006. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87: 2614–2625.
R Core Team, 2022. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Ramos-Merchante, A. & J. Prenda, 2017. Macroinvertebrate taxa richness uncertainty and kick sampling in the establishment of Mediterranean rivers ecological status. Ecological Indicators 72: 1–12.
Reid, A. J., A. K. Carlson, I. F. Creed, E. J. Eliason, P. A. Gell, P. T. J. Johnson, K. A. Kidd, T. J. MacCormack, J. D. Olden, S. J. Ormerod, J. P. Smol, W. W. Taylor, K. Tockner, J. C. Vermaire, D. Dudgeon & S. J. Cooke, 2019. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews 94: 849–873.
RStudio Team, 2020. RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA, http://www.rstudio.com/.
Sabater, S., X. Timoner, G. Bornette, M. De Wilde, J. C. Stromberg, & J. C. Stella, 2017. The Biota of Intermittent Rivers and Ephemeral Streams: Algae and Vascular Plants. In: Intermittent Rivers and Ephemeral Streams: Ecology and Management. Elsevier Inc.: 189–216.
Santos, J. I., T. Vidal, F. J. M. Gonçalves, B. B. Castro & J. L. Pereira, 2021. Challenges to water quality assessment in Europe – is there scope for improvement of the current Water Framework Directive bioassessment scheme in rivers? Ecological Indicators 121: 107030.
Schweiger, O., R. K. Heikkinen, A. Harpke, T. Hickler, S. Klotz, O. Kudrna, I. Kühn, J. Pöyry & J. Settele, 2012. Increasing range mismatching of interacting species under global change is related to their ecological characteristics. Global Ecology and Biogeography 21: 88–99.
Stubbington, R., M. T. Bogan, N. Bonada, A. J. Boulton, T. Datry, C. Leigh & R. Vander Vorste, 2017. The Biota of Intermittent Rivers and Ephemeral Streams: Aquatic Invertebrates. In: Intermittent Rivers and Ephemeral Streams: Ecology and Management. Elsevier Inc.: 217–243.
Sundermann, A., S. Lohse, L. A. Beck & P. Haase, 2007. Key to the larval stages of aquatic true flies (Diptera), based on the operational taxa list for running waters in Germany. Annales De Limnologie - International Journal of Limnology 43: 61–74.
Tachet, H., P. Richoux, M. Bournaud, & P. Usseglio-Polatera, 2010. Invertébrés d’eau douce: systématique, biologie, écologie. CNRS Editions.
van de Bund, W., 2009. Water Framework Directive intercalibration technical report, Part 1: Rivers.
Vidal, T., J. I. Santos, C. R. Marques, J. L. Pereira, M. T. Claro, R. Pereira, B. B. Castro, A. Soares & F. Gonçalves, 2014. Resilience of the macroinvertebrate community of a small mountain river (Mau River, Portugal) subject to multiple stresses. Marine and Freshwater Research 65: 633.
Wallace, I. D., B. Wallace, & G. N. Philipson, 2003. Keys to the Case-bearing Caddis Larvae of Britain and Ireland. Freshwater Biological Association, UK.
White, D. S., 2009. Coleoptera (Beetles) in Aquatic Ecosystems. In: Likens, G. E. (ed), Encyclopedia of Inland Waters. Academic Press, Murray, KY. pp. 144–156.
Wickham, H., 2016. ggplot2: Elegant Graphics for Data Analysis, Springer, New York.
Williamson, C. E., D. P. Morris, M. L. Pace & O. G. Olson, 1999. Dissolved organic carbon and nutrients as regulators of lake ecosystems: resurrection of a more integrated paradigm. Limnology and Oceanography 44: 795–803.
Acknowledgements
This work was supported by national funds (through FCT/MCTES) and by the European Regional Development Fund (through COMPETE2020 and PT2020) by means of the institutional programmes UIDP/50017/2020, UIDB/50017/2020 and LA/P/0094/2020 (CESAM), as well as UID/BIA/04050/2019 and UIDB/04050/2020 (CBMA). Joana Isabel Santos and Carlos Silva were supported by individual doctoral grants (SFRH/BD/121341/2016 and SFRH/BD/138389/2018) by FCT. Funding institutions played no role in study design, data collection and interpretation, nor in manuscript preparation and submission. The authors acknowledge the assistance of Sandra Nogueira, who produced Fig. 1 using GIS software (location of sites and regions).
Funding
Open access funding provided by FCT|FCCN (b-on). This work was supported by national funds (through FCT/MCTES) and by the European Regional Development Fund (through COMPETE2020 and PT2020) by means of the institutional programmes UIDP/50017/2020, UIDB/50017/2020 and LA/P/0094/2020 (CESAM), as well as UID/BIA/04050/2019 and UIDB/04050/2020 (CBMA). Joana Isabel Santos and Carlos Silva were supported by individual doctoral grants (SFRH/BD/121341/2016 and SFRH/BD/138389/2018) by FCT.
Author information
Authors and Affiliations
Contributions
JIS: Conceptualization, Investigation, Formal analysis, Writing—original draft, Writing—review and editing. CS: Investigation, Writing—review and editing. FJMG: Conceptualization, Supervision, Writing—review and editing. JLP: Conceptualization, Supervision, Formal analysis, Writing—original draft, Writing—review and editing. BBC: Conceptualization, Supervision, Formal analysis, Writing—original draft, Writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Handling editor: María del Mar Sánchez-Montoya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santos, J.I., Silva, C., Gonçalves, F.J.M. et al. Macroinvertebrate community structure and ecological status in Portuguese streams across climatic and water scarcity gradients. Hydrobiologia 850, 967–984 (2023). https://doi.org/10.1007/s10750-023-05137-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05137-2