Abstract
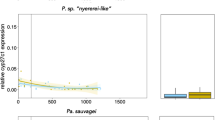
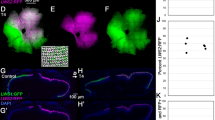
Cichlid fishes show remarkable variation in visual sensitivities through differential expression of seven cone opsin genes. Many species undergo spectral sensitivity shifts from shorter to longer wavelengths as they develop from larvae to adults. However, while some species retain larval-like short wavelength sensitivities, others show adult-like longer wavelength sensitivities throughout life. The riverine cichlid, Astatotilapia burtoni, shows a single cone progression from ultraviolet to violet to blue sensitivity, while their long wavelength double cones maintain green and red sensitivities throughout life. To identify mechanisms that regulate these sensitivities, we asked whether thyroid hormone (TH) or light environment can drive shifts. We find that developmental treatment with TH can speed shifts to longer wavelength sensitivity, but only in single cones. TH inhibition can short wavelength shift adult opsin expression. Exposure to light regimes containing UV wavelengths induce short wavelength shifts in single cones early in development. None of the treatments produces double cone shifts or significant expression of the shortest wavelength double cone opsin, rh2b, although we detect no cis-regulatory variation. This suggests that while single cones show both TH and light plasticity, A. burtoni double cones have lost this plasticity, perhaps through changes in trans-acting opsin regulation.





Similar content being viewed by others
Data availability
Quantitative PCR data are given in Supplementary Tables S2–S5.
References
Allison, W. T., S. G. Dann, K. M. Veldhoen & C. W. Hawryshyn, 2006. Degeneration and regeneration of ultraviolet cone photoreceptors during development in rainbow trout. Journal of Comparative Neurology 499(5): 702–715.
Applebury, M. L., F. Farhangfar, M. Glosmann, K. Hashimoto, K. Kage, J. T. Robbins, N. Shibusawa, F. E. Wondisford & H. Zhang, 2007. Transient expression of thyroid hormone nuclear receptor TRbeta2 sets S opsin patterning during cone photoreceptor genesis. Development Dynamics 236(5): 1203–1212.
Beaudet, L., I. Novales Flamarique & C. W. Hawryshyn, 1997. Cone photoreceptor topography in the retina of sexually mature Pacific salmonid fishes. Journal of Comparative Neurology 383(1): 49–59.
Bedolla, D. E. & V. Torre, 2011. A component of retinal light adaptation mediated by the thyroid hormone cascade. PLoS ONE 6(10): e26334.
Bowmaker, J. K., 1995. The visual pigments of fish. Progress in Retinal and Eye Research 15(1): 1–31.
Carleton, K. L., 2011. Quantification of transcript levels with quantitative RT-PCR. Methods in Molecular Biology 772: 279–95.
Carleton, K. L., T. C. Spady, J. T. Streelman, M. R. Kidd, W. N. McFarland & E. R. Loew, 2008. Visual sensitivities tuned by heterochronic shifts in opsin gene expression. BMC Biology 6(1): 22.
Carleton, K. L., B. E. Dalton, D. Escobar-Camacho & S. P. Nandamuri, 2016. Proximate and ultimate causes of variable visual sensitivities: Insights from cichlid fish radiations. Genesis 54(6): 299–325.
Carleton, K. L., M. A. Conte, M. Malinsky, S. P. Nandamuri, B. A. Sandkam, J. I. Meier, S. Mwaiko, O. Seehausen & T. D. Kocher, 2020a. Movement of transposable elements contributes to cichlid diversity. Molecular Ecology 29(24): 4956–4969.
Carleton, K. L., D. Escobar-Camacho, S. M. Stieb, F. Cortesi & J. Marshall, 2020. Seeing the rainbow: mechanisms underlying spectral sensitivity in teleost fishes. Journal of Experimental Biology. https://doi.org/10.1242/jeb.193334.
Castro-Mondragon, J. A., R. Riudavets-Puig, I. Rauluseviciute, R. Berhanu Lemma, L. Turchi, R. Blanc-Mathieu, J. Lucas, P. Boddie, A. Khan, N. Manosalva Perez, O. Fornes, T. Y. Leung, A. Aguirre, F. Hammal, D. Schmelter, D. Baranasic, B. Ballester, A. Sandelin, B. Lenhard, K. Vandepoele, W. W. Wasserman, F. Parcy & A. Mathelier, 2022. JASPAR 2022: the 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Research 50(D1): D165–D173.
Cheng, C. L., K. J. Gan & I. N. Flamarique, 2009. Thyroid hormone induces a time-dependent opsin switch in the retina of salmonid fishes. Investigative Ophthalmology & Vision Science 50(6): 3024–3032.
Cronin, T. W., S. Johnsen, N. J. Marshall & E. J. Warrant, 2014. Visual Ecology, Princeton University Press, Princeton, N.J.
Dalton, B. E., E. R. Loew, T. W. Cronin & K. L. Carleton, 2014. Spectral tuning by opsin coexpression in retinal regions that view different parts of the visual field. Proceedings: Biological Science 281(1797): 20141980.
Dalton, B. E., J. Lu, J. Leips, T. W. Cronin & K. L. Carleton, 2015. Variable light environments induce plastic spectral tuning by regional opsin coexpression in the African cichlid fish Metriaclima Zebra. Molecular Ecology 24(16): 4193–4204.
Dalton, B. E., F. de Busserolles, N. J. Marshall & K. L. Carleton, 2017. Retinal specialization through spatially varying cell densities and opsin coexpression in cichlid fish. Journal of Experimental Biology 220(Pt 2): 266–277.
Deal, C. K. & H. Volkoff, 2020. The role of the thyroid axis in fish. Frontiers Endocrinology (Lausanne) 11: 596585.
Ebrey, T. & Y. Koutalos, 2001. Vertebrate photoreceptors. Progress in Retinal and Eye Research 20(1): 49–94.
Eldred, K. C., S. E. Hadyniak, K. A. Hussey, B. Brenerman, P. W. Zhang, X. Chamling, V. M. Sluch, D. S. Welsbie, S. Hattar, J. Taylor, K. Wahlin, D. J. Zack & R. J. Johnston Jr., 2018. Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science. https://doi.org/10.1126/science.aau6348.
Fernald, R. D., 1984. Vision and behavior in an African cichlid fish. American Scientist 72: 58–65.
Fernald, R. D. & P. A. Liebman, 1980. Visual receptor pigments in the African cichlid fish, Haplochromis burtoni. Vision Research 20(10): 857–864.
Forrest, D. & A. Swaroop, 2012. Minireview: the role of nuclear receptors in photoreceptor differentiation and disease. Molecular Endocrinology 26(6): 905–915.
Forsell, J., P. Ekstrom, I. N. Flamarique & B. Holmqvist, 2001. Expression of pineal ultraviolet- and green-like opsins in the pineal organ and retina of teleosts. Journal of Expermental Biology 204(Pt 14): 2517–2525.
Fujimura, K. & N. Okada, 2007. Development of the embryo, larva and early juvenile of Nile tilapia Oreochromis niloticus (Pisces: Cichlidae) Developmental Staging System. Development, Growth & Differentiation 49(4): 301–324.
Gan, K. J. & I. Novales Flamarique, 2010. Thyroid hormone accelerates opsin expression during early photoreceptor differentiation and induces opsin switching in differentiated TRα-expressing cones of the salmonid retina. Developmental Dynamics 239(10): 2700–2713.
Halstenberg, S., K. M. Lindgren, S. P. Samagh, M. Nadal-Vicens, S. Balt & R. D. Fernald, 2005. Diurnal rhythm of cone opsin expression in the teleost fish Haplochromis burtoni. Visual Neuroscience 22(2): 135–141.
Harer, A., J. Torres-Dowdall & A. Meyer, 2017. Rapid adaptation to a novel light environment: the importance of ontogeny and phenotypic plasticity in shaping the visual system of Nicaraguan Midas cichlid fish (Amphilophus citrinellus spp.). Molecular Ecology 26(20): 5582–5593.
Harer, A., A. Meyer & J. Torres-Dowdall, 2018. Convergent phenotypic evolution of the visual system via different molecular routes: How Neotropical cichlid fishes adapt to novel light environments. Evolution Letters 2(4): 341–354.
Harer, A., N. Karagic, A. Meyer & J. Torres-Dowdall, 2019. Reverting ontogeny: rapid phenotypic plasticity of colour vision in cichlid fish. Royal Society Open Science 6(7): 190841.
Harpavat, S. & C. L. Cepko, 2003. Thyroid hormone and retinal development: an emerging field. Thyroid 13(11): 1013–1019.
Hofmann, C. M., K. E. O’Quin, N. J. Marshall, T. C. Cronin, O. Seehausen & K. L. Carleton, 2009. The eyes have it: Regulatory and structural changes both underlie cichlid visual pigment diversity. PLoS Biology 7(12): e1000266.
Hofmann, C. M., K. E. O’Quin, A. R. Smith & K. L. Carleton, 2010. Plasticity of opsin gene expression in cichlids from Lake Malawi. Molecular Ecology 19(10): 2064–2074.
Karagic, N., A. Harer, A. Meyer & J. Torres-Dowdall, 2018. Heterochronic opsin expression due to early light deprivation results in drastically shifted visual sensitivity in a cichlid fish: possible role of thyroid hormone signaling. Journal of Experimental Zoology Part B 330(4): 202–214.
Karagic, N., A. Harer, A. Meyer & J. Torres-Dowdall, 2022. Thyroid hormone tinkering elicits integrated phenotypic changes potentially explaining rapid adaptation of color vision in cichlid fish. Evolution. https://doi.org/10.1111/evo.14455.
Kroger, R. H. & R. D. Fernald, 1994. Regulation of eye growth in the African cichlid fish Haplochromis burtoni. Vision Research 34(14): 1807–1814.
Lukats, A., A. Szabo, P. Rohlich, B. Vigh & A. Szel, 2005. Photopigment coexpression in mammals: comparative and developmental aspects. Histology and Histopathology 20(2): 551–574.
Mackin, R. D., R. A. Frey, C. Gutierrez, A. A. Farre, S. Kawamura, D. M. Mitchell & D. L. Stenkamp, 2019. Endocrine regulation of multichromatic color vision. Proceedings of the National Academy of Science USA 116(34): 16882–16891.
Maruska, K. P. & J. M. Butler, 2021. Reproductive- and social-state plasticity of multiple sensory systems in a cichlid fish. Integrative and Comparative Biology 61(1): 249–268.
Maruska, K. P., C. M. Anselmo, T. King, R. B. Mobley, E. J. Ray & R. Wayne, 2022. Endocrine and neuroendocrine regulation of social status in cichlid fishes. Hormones and Behaviour 139: 105110.
Nandamuri, S. P., M. R. Yourick & K. L. Carleton, 2017. Adult plasticity in African cichlids: rapid changes in opsin expression in response to environmental light differences. Molecular Ecology 26(21): 6036–6052.
Ng, L., J. B. Hurley, B. Dierks, M. Srinivas, C. Salto, B. Vennstrom, T. A. Reh & D. Forrest, 2001. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nature Genetics 27(1): 94–98.
O’Quin, K. E., C. M. Hofmann, H. A. Hofmann & K. L. Carleton, 2010. Parallel evolution of opsin gene expression in African cichlid fishes. Molecular Biology and Evolution 27(12): 2839–2854.
O’Quin, K. E., A. R. Smith, A. Sharma & K. L. Carleton, 2011. New evidence for the role of heterochrony in the repeated evolution of cichlid opsin expression. Evolution & Development 13(2): 193–203.
Okano, T., D. Kojima, Y. Fukada, Y. Shichida & T. Yoshizawa, 1992. Primary structures of chicken cone visual pigments: vertebrate rhodopsins have evolved out of cone visual pigments. Proceedings of the National Academy of Science U S A 89(13): 5932–5936.
Paris, M. & V. Laudet, 2008. The history of a developmental stage: metamorphosis in chordates. Genesis 46(11): 657–672.
Prazdnikov, D. V. & F. N. Shkil, 2019. Experimental evidence of the role of heterochrony in evolution of the Mesoamerican cichlids pigment patterns. Evolution & Development 21(1): 3–15.
Raine, J. C. & C. W. Hawryshyn, 2009. Changes in thyroid hormone reception precede SWS1 opsin downregulation in trout retina. Journal of Experimental Biology 212(17): 2781–2788.
Reddy, P. K., C. L. Brown, J. F. Leatherland & T. J. Lam, 1992. Role of thyroid hormones in tilapia larvae (Oreochromis mossambicus): II. Changes in the hormones and 5'-monodeiodinase activity during development. Fish Physiology and Biochemistry 9(5-6): 487–496.
Roberts, M. R., M. Srinivas, D. Forrest, G. Morreale de Escobar & T. A. Reh, 2006. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proceedings of the National Academy of Science USA 103(16): 6218–6223.
Sawant, O. B., A. M. Horton, O. F. Zucaro, R. Chan, V. L. Bonilha, I. S. Samuels & S. Rao, 2017. The circadian clock gene bmal1 controls thyroid hormone-mediated spectral identity and cone photoreceptor function. Cell Rep 21(3): 692–706.
Schulte, J. E., C. S. O’Brien, M. A. Conte, K. E. O’Quin & K. L. Carleton, 2014. Interspecific variation in Rx1 expression controls opsin expression and causes visual system diversity in African Cichlid fishes. Molecular Biology & Evolution 31(9): 2297–2308.
Schwartz, S., Z. Zhang, K. A. Frazer, A. Smit, C. Riemer, J. Bouck, R. Gibbs, R. Hardison & W. Miller, 2000. PipMaker: a web server for aligning two genomic DNA sequences. Genome Research 10(4): 577–586.
Spady, T. C., J. W. Parry, P. R. Robinson, D. M. Hunt, J. K. Bowmaker & K. L. Carleton, 2006. Evolution of the cichlid visual palette through ontogenetic subfunctionalization of the opsin gene arrays. Molecular Biology and Evolution 23(8): 1538–1547.
Suliman, T. & I. Novales Flamarique, 2014. Visual pigments and opsin expression in the juveniles of three species of fish (rainbow trout, zebrafish, and killifish) following prolonged exposure to thyroid hormone or retinoic acid. Journal of Comparative Neurology 522(1): 98–117.
Swaroop, A., D. Kim & D. Forrest, 2010. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nature Reviews Neuroscience 11(8): 563–576.
Temple, S. E., E. M. Plate, S. Ramsden, T. J. Haimberger, W. M. Roth & C. W. Hawryshyn, 2006. Seasonal cycle in vitamin A1/A2-based visual pigment composition during the life history of coho salmon (Oncorhynchus kisutch). Journal of Comparative Physiology A 192(3): 301–313.
Temple, S. E., K. M. Veldhoen, J. T. Phelan, N. J. Veldhoen & C. W. Hawryshyn, 2008. Ontogenetic changes in photoreceptor opsin gene expression in coho salmon (Oncorhynchus kisutch, Walbaum). Journal of Experimental Biology 211(Pt 24): 3879–3888.
Torres-Dowdall, J., M. E. R. Pierotti, A. Harer, N. Karagic, J. M. Woltering, F. Henning, K. R. Elmer & A. Meyer, 2017. Rapid and parallel adaptive evolution of the visual system of neotropical midas cichlid fishes. Molecular Biology and Evolution 34(10): 2469–2485.
Veldhoen, K., W. T. Allison, N. Veldhoen, B. R. Anholt, C. C. Helbing & C. W. Hawryshyn, 2006. Spatio-temporal characterization of retinal opsin gene expression during thyroid hormone-induced and natural development of rainbow trout. Visual Neuroscience 23(2): 169–179.
Volkov, L. I., J. S. Kim-Han, L. M. Saunders, D. Poria, A. E. O. Hughes, V. J. Kefalov, D. M. Parichy & J. C. Corbo, 2020. Thyroid hormone receptors mediate two distinct mechanisms of long-wavelength vision. Proceedings of the National Academy of Science USA 117(26): 15262–15269.
Woltering, J. M., M. Holzem, R. F. Schneider, V. Nanos & A. Meyer, 2018. The skeletal ontogeny of Astatotilapia burtoni – a direct-developing model system for the evolution and development of the teleost body plan. BMC Developmental Biology 18(1): 8.
Xiang, M., 2013. Intrinsic control of mammalian retinogenesis. Cellular and Molecular Life Sciences 70(14): 2519–2532.
Yang, F., H. Ma & X. Q. Ding, 2018. Thyroid hormone signaling in retinal development, survival, and disease. Vitamins and Hormones 106: 333–349.
Yokoyama, S., 2008. Evolution of dim-light and color vision pigments. Annual Review of Genomics and Human Genetics 9: 259–282.
Yourick, M. R., 2021. Internal and External Environmental Regulation of Opsin Expression in African Cichlids. University of Maryland.
Yourick, M. R., B. A. Sandkam, W. J. Gammerdinger, D. Escobar-Camacho, S. P. Nandamuri, F. E. Clark, B. Joyce, M. A. Conte, T. D. Kocher & K. L. Carleton, 2019. Diurnal variation in opsin expression and common housekeeping genes necessitates comprehensive normalization methods for quantitative real-time PCR analyses. Molecular Ecology Resources 19(6): 1447–1460.
Acknowledgements
We would like to thank the Juntti and Kocher labs for help with fish care and breeding. We also thank Douglas Forrest for encouraging our studies of thyroid hormone responses. Thanks also to two anonymous reviewers for their suggestions and improvements.
Funding
This work was supported by the National Eye Institute at the National Institutes of Health (NIH R01EY024639B (KC and SJ) and the National Science Foundation (IOS-1825723 (SJ) and IOS-0841270 (KC) and Human Frontiers in Science Program (RGY0079; SJ).
Author information
Authors and Affiliations
Contributions
This study was conceived by all authors. MS and MY performed data collection and analysis. SJ and KC contributed to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests in the performance of this research.
Ethical approval
This work has been completed under the approval of the University of Maryland’s Institutional Animal Care and Use Committee (R-AUG-18-41; R-FEB-21-06).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest Editors: S. Koblmüller, R. C. Albertson, M. J. Genner, K. M. Sefc & T. Takahashi / Advances in Cichlid Research V: Behavior, Ecology and Evolutionary Biology
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schreiner, M.M., Yourick, M.R., Juntti, S.A. et al. Environmental plasticity in opsin expression due to light and thyroid hormone in adult and developing Astatotilapia burtoni. Hydrobiologia 850, 2315–2329 (2023). https://doi.org/10.1007/s10750-022-04957-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04957-y