Abstract
Phenotypic plasticity allows organisms to rapidly adjust to environmental changes. Cichlid fish inhabit a wide range of light environments and show a large diversity in visual system properties, which makes them a good model system to address the role of phenotypic plasticity in visual adaptation. Cichlid retinal cone pigments consist of opsin proteins bound to Vitamin A1 or A2-derived chromophores. Plasticity in expression has been shown for cichlid opsin genes, but less is known about the contribution of cyp27c1, the enzyme that converts Vitamin A1 into A2,. Here, we studied both opsin and cyp27c1 expression patterns for three closely related cichlid species from different visual habitats in Lake Victoria, across different light treatments. We found differences in cyp27c1 as well as in opsin expression patterns between the three species. Experimental light treatments affected the developmental trajectory of cyp27c1 expression in one species and opsin expression in all three species. Within each species, we found large individual variation in cyp27c1 expression levels and no consistent association with opsin expression levels. These results indicate that visual system plasticity of even closely related species can be differentially mediated by opsin and cyp27c1 expression, possibly associated with species differences in visual niche.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenotypic plasticity, the ability of a single genotype to express different phenotypes depending on environmental conditions, is a key determinant of organismal performance and greatly influences ecological interactions. Its role in evolution is also increasingly acknowledged (Bradshaw, 1965; Schlichting & Pigliucci, 1998; Agrawal, 2001; Pigliucci, 2001; West-Eberhard, 2003; DeWitt & Scheiner, 2004; Van Snick Gray & Stauffer, 2004; Fusco & Minelli, 2010; Pfennig et al., 2010). Plasticity might weaken selection pressure for genetic change and thus slow down or prevent adaptive evolution (Huey et al., 2003, Price et al., 2003, DeJong, 2005), but it might also promote adaptive evolution by allowing a broad tolerance to environmental conditions (Schlichting, 1986, West-Eberhard, 1989, Scheiner, 1993). Therefore, promotion or inhibition of evolution by plasticity may depend on the trait involved. In the present study, we explore the contribution of phenotypic plasticity to visual system variation in cichlid fish.
Organisms often rely on their visual system to assess and exploit their environments (e.g. foraging and social interaction) (Stevens, 2013) and may differ in visual sensitivity as a result of genetic variation and/or phenotypic plasticity (Collin & Shand, 2003; Smith et al., 2012; Hornsby et al., 2013; Dalton et al., 2015). The visual system is often key in local adaptation, as it is fine-tuned to environmental features. Evidence for visual adaptation to local environments has been documented in numerous taxa, particularly in aquatic species (Cummings & Endler, 2018), probably because aquatic environments constitute highly heterogeneous visual habitats with substantial variation in spectral composition and light intensity (Boughman, 2002; Seehausen et al., 2008; Warrant & Johnsen, 2013). For several fish species, there are indications that divergent visual adaptation to different light environments may contribute to, or even initiate, population divergence (Endler, 1992; Seehausen et al., 1997; Boughman, 2002; Spady et al., 2005; Fuller & Noa, 2010; Maan & Seehausen, 2010). Cichlid fish inhabit a large diversity of visual environments (Schelly et al., 2006) and have highly diverse visual system properties (Carleton & Kocher, 2001; Seehausen et al., 2008; Terai et al., 2017). Moreover, several traits have been shown to be plastic in cichlids (reviewed in Schneider & Meyer, 2017) including the visual system. Here, we address the role of plasticity in cichlid visual system variation by exploring the contributions of both developmental and environmental plasticity to different components of the visual system in three closely related species of Lake Victoria cichlids.
As in other vertebrates, cichlid visual pigments consist of an opsin protein covalently bound to a Vitamin A-based chromophore. Cichlid fish have one rod opsin gene (Rh1), involved in dim light vision, and seven cone opsin genes that are expressed in either one of two morphological cone types (Fernald & Liebman, 1980): single cones: three short-wavelength-sensitive opsins (SWS1, SWS2a, SWS2b) and double cones: three medium-wavelength-sensitive opsins (Rh2b, Rh2aα, Rh2aß) and one long-wavelength-sensitive opsin (LWS) (reviewed in Carleton et al., 2016). Variation in the coding sequence (that may alter the wavelength spectrum absorbed by the pigment) and changes in expression patterns of these opsins, determine differences in visual sensitivity across species. In addition, opsin gene expression has been shown to be plastic (i.e. to be affected by environmental conditions) in cichlids, with varying degrees of plasticity among species (Carleton & Kocher, 2001; Carleton et al., 2005, 2008, 2016; Terai et al., 2006; Hofmann et al., 2010; Nandamuri et al., 2017; Wright et al., 2020). Pigment absorption patterns not only depend on opsins, but also on the type of chromophore. Fish (as well as amphibians and some reptiles) use two types of chromophores, based on either Vitamin A1 (11-cis-retinal) or Vitamin A2 (11-cis 3,4-didehydroretinal). Chromophore-based tuning involves changes in the proportion of these two forms in the retina. Switching from A1- to A2- results in a shift in absorption maxima towards longer wavelengths, with a stronger shift when coupled with longer-wavelength-sensitive opsins (Bridges, 1972; Hárosi, 1994; Parry & Bowmaker, 2000). Chromophore composition varies among species and is associated with variation in light conditions (Bridges & Yoshikami, 1970; Reuter et al., 1971; Provencio et al., 1992; van der Meer & Bowmaker, 1995; Toyama et al., 2008; Morshedian et al., 2017). Underwater light spectra in freshwater habitats are on average more red-shifted than in marine habitats. Consistent with this, freshwater and coastal species tend to use A1/A2 mixtures or only Vitamin-A2-based chromophores, while marine fish use A1-based chromophores (Toyama et al., 2008). In some species, chromophore ratios change during the lifetime of an individual (Munz & McFarland, 1977), often related to seasonal migration (Temple et al., 2006) or developmental alteration in diet (Giovannucci & Stephenson, 1999). In cichlids, microspectrophotometry (MSP) of retinal tissues suggests that chromophore ratios may differ between species: species from clear waters (e.g. Lake Malawi) mainly use A1-derivatives (Carleton et al., 2000; Parry et al., 2005; Sugawara et al., 2005), while cichlids from turbid waters (entailing red-shifted visual conditions; e.g. Lake Victoria, Lake Nicaragua and Lake Managua) tend to have a higher proportion of A2-based pigments (Terai et al., 2006). In zebrafish, conversion of Vitamin A1 to Vitamin A2 is mediated by the enzyme Cyp27c1 (Enright et al., 2015). In line with this, studies in bullfrog and lamprey have documented positive correlations between cyp27c1 expression levels and A2 proportions in retinal pigments (Enright et al., 2015; Morshedian et al., 2017). This suggests that cyp27c1 expression levels can be used as a proxy for A2 proportions. In a previous study, we observed that this gene is expressed in two closely related haplochromine Pundamilia cichlid species from Lake Victoria, with higher cyp27c1 expression in populations from turbid waters than from clear waters in one of the two species (Wilwert et al., 2021). However, cichlid species and populations from such red-shifted environments not only tend to have higher A2 to A1 ratios, but also higher expression of opsin genes that absorb longer wavelengths (i.e. Rh2 and LWS; Hofmann et al., 2009; Carleton et al., 2020). This suggests that opsin expression patterns and chromophore usage can jointly contribute to visual performance.
In addition to environmental effects, opsin gene expression is developmentally plastic: individuals from several species shift from high levels of short-wavelength-sensitive opsin expression as larvae and juveniles to high long-wavelength-sensitive opsin expression as adults (Takechi & Kawamura, 2005; Carleton et al., 2008; O’Quin et al., 2011; Dalton et al., 2015; Irazábal-González et al., 2022). These patterns likely reflect differences between life stages in water depth occupation and foraging behaviour (Fryer, 1972; Novales-Flamarique & Hawryshyn, 1994; Jordan et al., 2004). Environmental and developmental plasticity in gene expression is widely documented for opsin genes, but less is known about plasticity at the level of chromophore usage.
In this study we explored (1) patterns of cyp27c1 and cone opsin gene expression across three Lake Victoria haplochromine cichlid species, (2) the developmental trajectory and extent of environmental plasticity of cyp27c1 expression level and (3) the association between cyp27c1 and cone opsin expression patterns.
Methods
Cichlid species
We studied three closely related haplochromine cichlid species, inhabiting different light environments in Lake Victoria (Fig. S1): Pundamilia sp.”pundamilia-like” (P), Pundamilia sp.”nyererei-like” (N) and Paralabidochromis sauvagei (S). Pundamilia pundamilia (Seehausen 1998) and Pundamilia nyererei (Witte-Maas & Witte, 1985) are two closely related cichlids species. Males of the Pundamilia species pair differ in nuptial coloration (blue vs. red) (Seehausen, 1996). Until recently all Pundamilia populations with blue males were classified as P. pundamilia and all populations with red males as P. nyererei. However, population genomic analyses revealed that populations from the southern and western Mwanza Gulf (Kissenda and Python islands) represent a separate speciation event; they are therefore referred to as P. sp.”pundamilia-like” and P. sp.”nyererei-like” (Meier et al., 2017, 2018). For both Pundamilia species, we used first- and second- generation offspring of wild-caught individuals from Python Island in the Mwanza Gulf of Lake Victoria (− 2.6237, 32.8567). At this location, P. sp.”pundamilia-like” occurs in shallow waters (0–2 m) and encounters broad-spectrum light. P. sp.”nyererei-like” extends to greater depths (0–5 m), but is most abundant between 2.5 and 3.5 m (Seehausen et al., 2008). High water turbidity at Python Island results in heterogeneous visual conditions across depth ranges, with a shift of the light spectrum towards longer wavelengths with increasing depth, such that P. sp.”nyererei -like” experiences little short-wavelength light (Seehausen et al., 2008). Pa. sauvagei was collected from Makobe Island (Speke Gulf; − 2.3654, 32.9228), where water transparency is higher than at Python (Seehausen et al., 2006; Castillo Cajas et al., 2012). The species has a relatively broad depth distribution (1.5–14 m; Seehausen, 1996) and therefore encounters a range of light conditions, from broad-spectrum light in shallow waters to red-shifted conditions at the deeper end of its depth distribution (Fig. S1). Families were established opportunistically with 13 dams and 9 sires for P. sp.”pundamilia-like”, 15 dams and 10 sires for P. sp.”nyererei-like” and 15 dams and 8 sires for Pa. sauvagei. In total, eighty-nine crosses resulted in 166 test fish (Table S2). Test fish were randomly selected from these crosses. For individual identification we inserted a microchip in the abdominal cavity (PIT tags, Passive Integrated Transponder, Biomark, ID, Idaho; USA, and Dorset Identification, Aalten, Netherlands) of each test fish. All haplochromines are maternal mouthbrooders; eggs were removed approximately 6 days after fertilization from brooding females and split evenly between two light conditions (see below). Fish were housed at 25 ± 1 °C on a 12L:12D light cycle and fed twice a day with commercial cichlid pellets and frozen raw food (Artemia, spirulina and krill). For gene expression analysis, fish were sacrificed by applying a lethal dose of buffered MS-222 (1 g/L) and subsequent cutting of the vertebral column in the late afternoon (16:00–18:00; synchronized to maximize RNA yield and reduce effects of circadian variation in opsin and cyp27c1 expression; Halstenberg et al., 2005; Yourick et al., 2019). Eyes were extracted, preserved in RNAlater™ (Ambion) and frozen (− 20 °C).
Light conditions
Fish were reared in two different light conditions, mimicking shallow (i.e. broad-spectrum light) and deep (i.e. red-shifted light) water conditions in Lake Victoria (described in Maan et al., 2017; Fig. S1). For both light conditions, we used halogen light bulbs with a green light filter (LEE #243). For the broad-spectrum light condition, we added blue light bulbs (Paulmann, 8809). For the red-shifted light condition we added a yellow filter (LEE #015) to reduce short-wavelength light. Rearing light conditions were designed to mimic the spectral differences between depths and partly recreated intensity differences encountered in the lake (i.e. the red-shifted light environment had a light intensity of ~ 70% of the shallow light condition; see Fig. S1).
Sampling
Fish were sacrificed at several developmental time points, ranging from 10 to 1391 days post fertilization (dpf). Lake Victoria haplochromines reach adulthood at ~ 180 dpf (6 months). Accordingly, fish samples were classified into two categories: juveniles (ranging from 10 to 180 dpf, 5 timepoints: 10; 40; 90; 170; 180 dpf) and adults (older than 190 dpf). We included individuals from at least two families for each timepoint. To obtain sufficient retinal RNA (Carleton et al., 2008), we pooled both eyes from one individual for time points 10–40 dpf and one whole eye from one individual at 90 dpf onwards. From 120 dpf onwards, one retina (instead of the whole eye) per individual was extracted (Table S3).
Measuring expression levels
Real-time quantitative polymerase chain reaction (qRT-PCR) was used to quantify cpy27c1 and opsin gene expression. Total RNA was isolated using Trizol (Ambion) followed by a DNase treatment to remove genomic DNA. 1 µg of RNA was reverse-transcribed into cDNA using Oligo(dT)18 primer (Thermo Fisher Scientific) and RevertAid H Minus (Thermo Fisher Scientific) at 45 °C. cDNA was diluted to a final concentration of 10 ng/µl.
Cyp27c1 expression
As a reference for cyp27c1 expression, we used three housekeeping genes (HKGs): L-lactate dehydrogenase 1 (ldh1), Glyceraldehyde-3-phosphate dehydrogenase 2 (gapdh2) and ß-actin (Jin et al., 2013; Torres-Dowdall et al., 2017). Stability of HKG expression was confirmed using RefFinder (Xie et al., 2012). After a denaturation step (95 °C for 3 min), qRT-PCRs were run for 45 cycles (95 °C for 15 s, 60 °C for 25 s, 72 °C for 30 s). Each reaction mixture (20 µl) contained the gene-specific primer pair (Table S1), diluted cDNA sample and SYBR Green PCR Master Mix (BioRad). Fluorescence was monitored on a StepOnePlus Real-Time PCR System (Applied Biosystems). To test for specificity of amplification, qPCR was followed by a melting curve analysis. We used LinRegPCR (Ruijter & Ramakers, 2003) to determine the initial concentration (N0) of cyp27c1 and the HKGs. Expression levels were based on two technical replicates. We applied the following quality control thresholds: PCR efficiency 1.75–2.25 and standard deviation between duplicates ≤ 0.5. We used the mean of the duplicate No estimates to calculate relative expression levels. The following equation was used to calculate the relative cyp27c1 expression Ecyp for each sample separately:
where N0 cyp is the initial concentration of cyp27c1 cDNA (or mRNA) and N0 Reference is the geometric mean of the starting cDNA (or mRNA) concentration of the three HKGs.
Opsin gene expression
Opsin gene expression was quantified only for adults. Expression levels of the opsin genes (SWS2b, SWS2a, Rh2, LWS) were determined in relation to total opsin gene expression, using previously described methods (Wright et al., 2019). Expression was quantified using gene-specific Taqman primers and probes (Table S1). Rh2aα and Rh2Aβ were combined as Rh2A, as they are more than 99% similar in sequence and function (Parry et al., 2005). Opsin gene expression (SWS2a, SWS2b, Rh2 and LWS) was monitored on StepOnePlus Real-Time PCR System (Applied Biosystems TMStepOnePlusTMReal-Time PCR System) for (95 °C for 2 min, 95 °C for 50 s, 60 °C for 1 min). Using LinRegPCR (Ruijter & Ramakers, 2003), we determined the initial concentrations (N0) and the critical threshold cycle numbers (Ct) of all four opsin genes. This approach determines the upper and lower limits of a “window-of linearity” by examining the log-linear of the PCR curve of each sample (Ruijter & Ramakers, 2003). On each plate, we included a serial dilution of a construct composed of one fragment of each of the four opsin genes ligated together. We used the mean of the technical replicates No estimates to calculate relative expression levels. Individual opsin gene expression was quantified relative to total opsin expression, using the following equation:
where N0/N0,all is the expression of a given opsin gene relative to the total expression of all measured opsin genes, Ct is the critical threshold cycle number of the sample, b the intercept and m the slope value derived from the construct linear regression (Gallup, 2011).
Data analysis
All analyses were performed in R (v4.1.2; R Development Core Team, 2021). Prior to statistical analysis, relative gene expression data were tested for outliers (1.5 * the interquartile range), separately for each species, light treatment and age class (juveniles and adults). Outlier removal resulted in 149 samples for cyp27c1 expression (15 removed) and 83 samples for opsin gene expression (7 removed). To analyse the effects (and interaction) of species differences and light treatments on cyp27c1 expression across life stages, we modelled expression as: relative gene expression ~ species * treatment * age + (1|mother ID) + (1|father ID). To analyse the relationship between opsin and cyp27c1 expression in adult fish, we included opsin expression data of an additional 38 Pundamilia adults, reported in Wright et al., 2019. These data were obtained using the same protocol as applied in our study. In addition, to describe changes at the cone type level, we calculated ratios of SWS2a/SWS2b (single cones) and LWS/Rh2 (double cones). We used linear mixed effects modelling (lmer, R package lme4) for each gene and ratio separately. To determine minimum adequate models, we used stepwise backward selection based on statistical significance. We used Anova (car package; Fox et al., 2017) of the minimum adequate model to estimate the parameter effects, degrees of freedom, and P values of the significant factors with Kenward–Roger degrees of freedom (Kenward & Roger, 1997; Halekoh & Højsgaard, 2014). To estimate parameter effects, P values and degrees of freedom, we performed ´KRmodcomp´ (pbkrtest package). In case of more than two categories, we used Tukey tests (glht – multcomp package, Hothorn et al., 2008) to estimate parameter effects and P values. Finally, principal component analysis (PCA) was used to obtain a visual representation of species- and treatment-specific expression profiles in terms of both opsin gene expression levels and cyp27c1 expression levels.
Results
Species differences in cyp27c1 and opsin gene expression
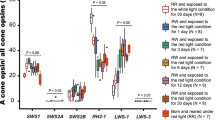
Cyp27c1 expression levels differed significantly between species (juveniles and adults combined; F2,12.1 = 29.18, P < 0.001; n = 146): expression was highest in Pa. sauvagei (compared to P. sp.“pundamilia-like”: z = 4.133, P < 0.001; compared to P. sp.“nyererei-like”: z = 3.72, P = 0.001), while the two Pundamilia species showed similar levels of cyp27c1 (z = − 0.37, P = 0.983). For opsin expression patterns (adults only, n = 90), we found that proportions of LWS (70.9% ± 1.3) and Rh2 (16.6% ± 1.2) were high across species, together making up 87.5% ± 2.5 of total opsin expression, while the expression levels of SWS2a (11.1% ± 0.5) and SWS2b (1.4% ± 0.03) were low. Independent of light treatments, we found species differences in opsin gene expression (Rh2: F2,11.5 = 45.40, P < 0.001; LWS: F2,7.04 = 47.38, P < 0.001). Consistent with earlier work (Wright et al., 2019), P. sp.”pundamilia-like” expressed a higher proportion of LWS (z = 3.08, P = 0.011) and lower Rh2 (z = 5.17, P < 0.001) compared to P. sp.”nyererei-like” (Fig. 2a). Pa. sauvagei had lower LWS expression than both Pundamilia species (compared to P. sp.”pundamilia-like”: z = − 10.09, P < 0.001; compared to P. sp.”nyererei-like”: z = 5.39, P < 0.001), and higher Rh2 expression (compared to P. sp.”pundamilia-like”: z = 12.16, P < 0.001; compared to P. sp.”nyererei-like”: z = − 5.31, P < 0.001). Proportions of SWS2a and SWS2b did not differ between species (SWS2a: F2,7.516 = 2.81, P = 0.122; SWS2b: F2,18.9 = 0.83, P = 0.451; Fig. 2a).
Developmental pattern and effect of light conditions on cyp27c1 expression
Cyp27c1 expression levels changed with age (F1,114.4 = 106.75, P < 0.001; n = 146), but the developmental pattern of cyp27c1 expression differed between species (i.e. significant species by age interaction; F3,120 = 4.88, P = 0.003). In the Pundamilia species cyp27c1 expression slightly decreased with age (P. sp. “pundamilia-like”: F1,18.3 = 15.88, P = 0.001; P. sp. “nyererei-like”: F1,24.8 = 25.53, P = 0.001) and did not differ between rearing light conditions (P. sp. “pundamilia-like”: F1,41.56 = 1, P = 0.408; P. sp. “nyererei-like”: F1,44.23 = 1, P = 0.455; Fig. 1a). In Pa. sauvagei, however, the developmental pattern of cyp27c1 expression was significantly influenced by the light conditions (F1,41.1 = 12.14, P = 0.001): in both light conditions, cyp27c1 was expressed at low levels in early life stages (10–90 dpf), but in the broad-spectrum light condition it increased with age while it remained low in the red-shifted light condition (Fig. 1). This was also reflected by a significant species by light treatment interaction (F2,127.9 = 2.88, P = 0.038), indicating that species responded differently to the different light treatments. Pa. sauvagei individuals reared in broad-spectrum light tended to express higher cyp27c1 compared to their siblings reared in red-shifted light (z = 2.36, P = 0.072), while in the Pundamilia species, expression levels did not differ between light conditions (P. sp. “pundamilia-like”: z = 0.63, P = 0.950; P. sp.”nyererei-like”: z = 0.54, P = 0.972) (Note that we did not document the developmental patterns in opsin expression in Pa.sauvagei; for the Pundamilia species the developmental is reported in Irazabal et al., 2021).
Cyp27c1 expression. (a) Over development and (b) in adulthood (> 190 dpf). Cyp27c1 expression decreased with age in the Pundamilia species, but increased in Pa. sauvagei individuals reared in broad-spectrum light. Irrespective of the light conditions, cyp27c1 expression was higher in Pa. sauvagei than in the Pundamilia species. Each symbol represents an individual (for 10 dpf two individuals were pooled). The grey line indicates the transition from juvenile state to adulthood. Shaded areas indicate ± 95% confidence intervals. Boxes represent 25–75th percentiles intercepted by the median. Error bars indicate 95% confidence intervals. Colours indicate light treatments (blue = broad-spectrum light; yellow = red-shifted light)
Effects of light conditions on cyp27c1 and opsin gene expression in adult stage
To explore the effects of light conditions on cyp27c1 expression at the adult stage, we used the same dataset as above, but now only included adults (n = 93). We found a similar pattern as described in the previous section: the different rearing light conditions did not induce a plastic response in cyp27c1 expression in the Pundamilia species (P. sp. “pundamilia-like”: z = 1.41, P = 0.407; P. sp.”nyererei-like”: z = 1.18, P = 0.559), while Pa. sauvagei reared in broad-spectrum light expressed higher cyp27c1 compared to siblings reared in red-shifted light (z = 3.03, P = 0.007; Fig. 1b). For opsins (n = 89),we found a significant effect of rearing light on the relative expression levels of SWS2b (F1,22.1 = 22.11, P < 0.001) and a tendency for an effect on SWS2a (F1,70.8 = 2.83, P = 0.097) and LWS (F1,73.9 = 3.51, P = 0.065) in adult fish. No effect on Rh2 expression was observed (F1,73.2 = 1, P = 0.349) (Fig. 2c). We found that P. sp.”nyererei-like” individuals reared in the red-shifted environment expressed lower proportions of SWS2b (z = -3.08, P = 0.008) and SWS2a (z = -3.32, P = 0.003) and higher proportions of LWS (z = -2.87, P = 0.016), compared to their siblings in the broad-spectrum light condition. Similar to P. sp.”nyererei-like”, Pa. sauvagei reared in the red-shifted light condition expressed lower SWS2b proportions compared to their siblings reared in the broad-spectrum light condition (z = -5.89, P < 0.001). P. sp.”pundamilia-like” individuals showed no difference in opsin expression profiles between rearing light conditions (SWS2b: z = 0.84, P = 0.864; SWS2a: z = -0.05, P = 0.999; Rh2: z = 0.25, P = 0.998; LWS: z = -0.53, P = 0.971).
Relationship between opsin gene and cyp27c1 expression. (a) Opsin expression in adult P. sp. “pundamilia-like”, P. sp. “nyererei-like” and Pa. sauvagei, (b–c) Relationship between opsin gene expression (SWS2a/SWS2b and LWS/RH2 ratio) and cyp27c1 expression across species. (d) Light-induced changes in opsin expression, (e–f) Relationship between light-induced changes in opsin and cyp27c1 expression across species. Boxes represent 25–75th percentiles, intercepted by the median and black symbols are outliers. Shapes in (e–f) indicate species (Pa. sauvagei (■), P. sp. ”nyererei-like”(▲) and P. sp. ”pundamilia-like”(●). Sample sizes are indicated above each boxplot and error bars represent ± 95% confidence interval. *** indicates P < 0.001, **P < 0.01 and *P < 0.05
No clear association between cyp27c1 and opsin gene expression in adult fish
To visualize the relationship between species differences in opsin expression and species differences in cyp27c1 expression, we calculated the ratios of opsin expression within single cones (SWS2a/SWS2b) and double cones (LWS/Rh2) and plotted these against mean cyp27c1 expression levels, for each species (Fig. 2b, 2c). This shows that Pa. sauvagei combines low SWS2a/SWS2b and LWS/Rh2 ratios with a high cyp27c1 expression level, while P. sp. “pundamilia-like” combines high SWS2a/SWS2b and LWS/Rh2 ratios with a low cyp27c1 expression level. In P. sp. “nyererei-like”, SWS2a/SWS2b ratio, LWS/Rh2 ratio and cyp27c1 expression were low. Thus, Pa. sauvagei and P. sp. “pundamilia-like” differ in both cyp27c1 expression and opsin ratios, while P. sp. “nyererei-like” differs from Pa. sauvagei mostly in cyp27c1 expression level. In line with this, PCA indicated that the cluster dominated by Pa. sauvagei had positive PC1 values (indicating high cyp27c1 and Rh2, but low LWS), while the cluster dominated by P. sp. “pundamilia-like” had negative PC1 values (indicating low cyp27c1 and Rh2, but high LWS; Fig. 3a). The cluster dominated by P. sp. “nyererei-like” shows that P. sp. “nyererei-like” overlaps primarily with P. sp. “pundamilia-like”, but also with Pa. sauvagei.
Expression profiles of cyp27c1 and opsins (a) form two clusters: the cluster dominated by Pa. sauvagei (▲) was characterized by positive PC1 scores, indicating high expression levels of cyp27c1 and Rh2, but low expression levels of LWS. The cluster dominated by P. sp. ”pundamilia-like” was characterized by negative PC1 scores, indicating low expression levels of cyp27c1 and Rh2, but high expression levels of LWS, while the cluster dominated by P. sp. ”nyererei-like” overlaps with Pa. sauvagei and P. sp. ”pundamilia-like”. Each symbol represents an individual. (b) Plastic responses in cyp27c1 and opsin expression were more pronounced in the two species experiencing a broader visual niche (i.e. P. sp. ”nyererei-like” and Pa. sauvagei). Colours indicate species: P. sp. ”pundamilia-like”(blue) P. sp. ”nyererei-like” (red) and Pa. sauvagei (orange). Linetypes indicate light treatment: broad-spectrum light (solid) and red-shifted light (dashed)
Using the same cone-class-specific opsin expression ratios, we visualized the light-induced changes in opsin and cyp27c1 expression for each species (Fig. 2d–f). We observed that in response to the different light conditions, P. sp.”pundamilia-like” did not change opsin or cyp27c1 expression, while P. sp. ”nyererei-like” changed SWS2a/SWS2b ratios, but not LWS/Rh2 ratios or cyp27c1 expression levels. Pa. sauvagei in red-shifted light conditions did not change LWS/Rh2, but increased SWS2a/SWS2b ratio and decreased cyp27c1 expression. In line with this, PCA indicated more pronounced plastic responses in the cluster dominated by Pa. sauvagei and P. sp. ”nyererei-like” than P. sp.”pundamilia-like” (Fig. 3b).
At the individual level, we found no consistent relationship between cyp27c1 and opsin gene expression (Fig. 4; Fig. S2). There was substantial individual variation, but the nature and extent of variation differed between species. For instance, we observed large individual variation in cyp27c1 expression in Pa. sauvagei, particularly in individuals reared in broad-spectrum light conditions, but not in the Pundamilia species.
Discussion
Phenotypic plasticity allows organisms to rapidly adjust to environmental changes, but its potential role in evolutionary change is unclear. Here, we explored the extent of phenotypic plasticity in multiple components of the visual system in three closely related haplochromine cichlid species from distinct photic environments in Lake Victoria, to start to understand the role of plasticity in cichlid visual system variation. We explored variation across species, environments and life stages by measuring the expression levels of opsin genes and, as a proxy for chromophore usage, the cyp27c1 gene (Enright et al., 2015). We have previously shown that cyp27c1 is expressed in two of these species (P. sp. “pundamilia-like” and P. sp. “nyererei-like”), although at very low levels, with variation in expression levels across species and populations (Wilwert et al., 2021). In the present study, we added a third, more distantly related haplochromine species (Pa. sauvagei) and explored environmental and developmental plasticity in cyp27c1 expression of all three species. We also explored the relationship between cyp27c1 expression and opsin gene expression profiles.
Species differences in cyp27c1 and opsin gene expression
We observed species differences in cyp27c1 expression levels, with Pa. sauvagei expressing higher levels than the two Pundamilia species. Possibly, this relatively low expression in Pundamilia species indicates that it is not relevant for visual adaptation in these species. For opsin gene expression profiles, we found that P. sp.”pundamilia-like” expressed higher LWS and lower Rh2 than P. sp.”nyererei-like”, in line with previous work in wild-caught and laboratory-reared Pundamilia (Wright et al., 2019, 2020). In Pa. sauvagei, we found substantially lower LWS and higher Rh2 proportions compared to the two Pundamilia species. Together, these findings show that, even when reared under the same light conditions, Lake Victoria cichlids show species-specific expression levels of opsin and cyp27c1, supporting a genetic basis of species differences in visual system gene expression profiles. Thus, Pa. sauvagei has a markedly different expression profile for both opsins and cyp27c1. There are at least two not mutually exclusive explanations for these observations. First, the three species inhabit different visual niches. This implies that species occupying similar visual niches (Pundamilia) show similar visual system properties, compared to a species with a more diverse visual niche (Pa. sauvagei). Second, species differences might be related to their phylogenetic history: The two Pundamilia species are very closely related and even hybridize incidentally, while Pa. sauvagei is less closely related to both Pundamilia species. Thus, visual system properties in Pundamilia may resemble each other more not because of current adaptative significance, but due to shared evolutionary history. To evaluate the contributions of each of these, assessing expression patterns in additional species is needed.
Effects of environmental light on cyp27c1 and opsin gene expression
Adaptive evolution and diversification of the visual system may be facilitated by phenotypic plasticity. Given the red-shifted sensitivity associated with increased A2, one would expect higher cyp27c1 expression levels in fish reared under red-shifted light conditions. We found no such effect. In Pundamilia, expression levels were low and did not change in response to light manipulation. This is in line with our previous study (Wilwert et al., 2021), where we also observed very low cyp27c1 expression in both Pundamilia species, and hypothesised that cyp27c1 may not contribute significantly to visual functioning in these species. The absence of phenotypically plastic responses to light manipulation may indicate that cyp27c1 also contributes little to individual adjustment to prevailing light conditions in these species. In contrast, in Pa. sauvagei, the different light treatments induced a strong plastic response in cyp27c1 expression. However, against the expectation, fish reared in broad-spectrum light expressed more cyp27c1 than fish reared in red-shifted light. Because Cyp27c1 is not a structural component of the pigments, it could be that in broad-spectrum light, the expression of cyp27c1 ensures a buffer of the enzyme to be employed when the light suddenly changes towards longer wavelengths. This could yield a swifter response than altering opsin expression levels (Munz & McFarland, 1977; Collin & Shand, 2003). In red-shifted light, the opsin expression profile may ensure sufficient visual flexibility, precluding the need for high expression levels of cyp27c1. It is important to note that the plastic response observed in Pa. sauvagei does not fully account for the observed species difference (i.e. Pundamilia vs. Pa. sauvagei) in cyp27c1 expression. Even when comparing within the same light condition, Pa. sauvagei expressed higher levels of cyp27c1 than Pundamilia, suggesting a genetic difference in the regulation of cyp27c1 expression between the species. Together with its stronger response to the light treatments, this suggests that chromophore-level variation may be more important in visual system functioning in Pa. sauvagei than in Pundamilia, or that Pundamilia species have a different pathway to modulate chromophore ratios. Further studies are needed to investigate the effects of cyp27c1 and opsin expression levels on actual visual functioning in cichlids.
In line with previous work (Wright et al., 2019), light conditions significantly influenced opsin expression patterns (i.e. SWS2b, SWS2a and LWS) in P. sp. ”nyererei-like”, but not in P. sp. ”pundamilia-like”, however it should be noted that part of the opsin expression data reported in this study (22 out of 64 of Pundamilia individuals) came from Wright et al., 2019. In Pa. sauvagei, we observed that these different light conditions induced a change in SWS2b proportions. Apparently, P.”nyererei-like” respond to different light conditions by changes in opsin expression levels, while Pa. sauvagei respond by changes in cyp27c1 and SWS2b expression levels. We discuss this further below.
Cyp27c1 expression across life stages
Over the course of an individual’s lifetime, opsin gene expression profiles may change to match developmental changes in water depth occupancy and feeding behaviour (Carleton et al., 2008; Dalton et al., 2015; Härer et al., 2018). Haplochromine cichlid larvae and juveniles typically feed on zooplankton (Fryer, 1972), naturally occurring in shallow waters where the light spectrum is still relatively rich in short-wavelength light (Goldschmidt et al., 1990). Over development fish change their foraging strategies (Fryer, 1972) and explore greater depths encountering different photic conditions. Prior work has shown that the Pundamilia species pair shifts from shorter-wavelength sensitive to longer-wavelength sensitive opsins with increasing age (Irazábal-González et al., 2022). Here, we find that cyp27c1 expression slightly decreases with age in Pundamilia. If this decrease is functionally relevant, it would be consistent with the expected decrease in long-wavelength sensitivity with age, adding to the pattern observed for opsins. In Pa. sauvagei, cyp27c1 expression levels were also low at the start of development, but increased significantly with age in the broad-spectrum light condition, while remaining low in the red-shifted light condition. Establishing whether or not these species differences in developmental and environmental plasticity reflect different ecologies requires detailed observation of juvenile behaviour in (semi-) natural conditions. In addition, the developmental pattern of opsin expression in Pa. sauvagei must be determined, to assess whether this also differs from that of Pundamilia species and whether it covaries with light conditions.
Possibly, the observed differences are related to the different visual niches experienced by the three species. P. sp. “pundamilia like” inhabits a narrow visual niche (0–2 m) occurring exclusively in rock crevices at shallow depths (Seehausen et al., 1998). The two other species, P. sp. “nyererei like” and Pa. sauvagei, experience broader visual niches and are also more responsive to the different light manipulations (Fig. S1): P. sp. “nyererei like” inhabits shallow and deep waters between 0 and 5 m, while Pa. sauvagei extends to even greater depths (1.4–15 m) (Seehausen et al., 1998).
Based on our findings, we can formulate three possible ways in which opsin and cyp27c1 expression together may contribute to visual performance. First, opsin expression and chromophore usage may act in an additive fashion, where red-shifted light conditions select for increased expression of both LWS opsin and cyp27c1. This has been observed in Neotropical Midas cichlids, where populations from turbid waters showed increased LWS and cyp27c1 expression compared to populations from clear waters (Torres-Dowdall et al., 2017; Härer et al., 2018). A second scenario could involve a compensatory mechanism, where reduced long-wavelength sensitivity (i.e. lower LWS proportions) is counteracted by higher Vitamin A2 usage (i.e. higher cyp27c1 expression) and vice versa, as was observed in populations of A. cf. citrinellus from turbid waters (Härer et al., 2018). Third, there might be no functional relationship at all between opsin genes and cyp27c1 expression. Our observations could be explained by two of these scenarios: first, the high expression of cyp27c1, but low LWS in Pa. sauvagei and the low cyp27c1, yet high LWS proportions in P. sp. “pundamilia-like” suggests a compensatory effect. Second, the absence of a clear relationship between expression levels of opsin genes and cyp27c1 at individual level, despite substantial inter-individual variation, is consistent with the third scenario in which there is no relationship between the two, suggesting that other factors are more important in regulating visual system properties.
An implicit assumption of this study is that cyp27c1 expression levels represent A1/A2 ratios. Previous studies in zebrafish and bullfrog have shown that the expression of cyp27c1 is correlated with the presence of Vitamin A2. In cichlids, however, only a few data points are available to substantiate this pattern (Torres-Dowdall et al., 2017). The fact that our findings are different from previous findings (Härer et al., 2018), shows that we do not have a full understanding of the mechanism by which cyp27c1 may or may not mediate chromophore composition and, how its expression interacts with other genes involved in visual system functioning. In zebrafish and bullfrogs cyp27c1 is expressed in the retinal pigment epithelium (Enright et al., 2015). However, in cichlids, Vitamin A1/A2 conversion might be regulated differently and does not necessarily have to occur in the retina. Hence, to interpret cyp27c1 expression levels, direct measurements of Vitamin A1/A2 ratios directly in the visual pigments of Lake Victoria cichlids are required.
Our current picture of cichlid visual adaptation and its role in species divergence is primarily based on opsin level variation. This study illustrates that visual adaptation is more complex, as we not only observe developmental and environmental plasticity of cyp27c1 expression, but also find that even closely related haplochromine cichlids may use different visual strategies. Ultimately, a better understanding of the molecular basis of the fish visual system will enhance its value as a model system for evolutionary research. This will allow us to exploit it for understanding how organisms adapt, how fast they can respond to environmental changes and how the components of the visual system and phenotypic plasticity contribute to local adaptation.
Data availability
Once the manuscript is accepted, we will submit our data and R-scripts to Dryad.
References
Agrawal, A. A., 2001. Phenotypic plasticity in the interactions and evolution of species. Science 294(5541): 321–326.
Bradshaw, A. D., 1965. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics 13: 115–155.
Bridges, C. D. B., 1972. The rhodopsin-porphyropsin visual system. Photochemistry of Vision: 417–480.
Bridges, C. D. B. & S. Yoshikami, 1970. The rhodopsin-porphyropsin system in freshwater fishes-1. Effects of age and photic environment. Vision Research, 10: 1315-IN4.
Boughman, J. W., 2002. How sensory drive can promote speciation. Trends in Ecology and Evolution 17: 571–577.
Carleton, K. L., F. I. Hárosi & T. D. Kocher, 2000. Visual pigments of African cichlid fishes: evidence for ultraviolet vision from microspectrophotometry and DNA sequences. Vision Research 40: 879–890.
Carleton, K. L., & T.D. Kocher. 2001. Cone opsin genes of African cichlid fishes: Tuning spectral sensitivity by differential gene expression. Molecular Biology and Evolution 18(8): 1540–1550.
Carleton, K. L., J. W. Parry, J. K. Bowmaker, D. M. Hunt & O. Seehausen, 2005. Colour vision and speciation in Lake Victoria cichlids of the genus Pundamilia. Molecular Ecology 14: 4341–4353.
Carleton, K. L., T. C. Spady, J. T. Streelman, M. R. Kidd, W. N. McFarland & E. R. Loew, 2008. Visual sensitivities tuned by heterochronic shifts in opsin gene expression. BMC Biology 6: 1–14.
Carleton, K. L., B. E. Dalton, D. Escobar-Camacho & S. P. Nandamuri, 2016. Proximate and ultimate causes of variable visual sensitivities: insights from cichlid fish radiations. Genesis 54: 299–325.
Carleton, K. L., D. Escobar-Camacho, S. M. Stieb, F. Cortesi & N. J. Marshall, 2020. Seeing the rainbow: mechanisms underlying spectral sensitivity in teleost fishes. Journal of Experimental Biology 223: jeb193334.
Castillo Cajas, R. F., O. M. Selz, E. A. Ripmeester, O. Seehausen, & M. E. Maan, 2012. Species-specific relationships between water transparency and male coloration within and between two closely related Lake Victoria cichlid species. International Journal of Evolutionary Biology, 2012
Collin, S. P. & J. Shand, 2003. Retinal Sampling and the Visual Field in Fishes. Sensory Processing in Aquatic Environments, Springer, New York:, 139–169.
Cummings, M. E. & J. A. Endler, 2018. 25 Years of sensory drive: the evidence and its watery bias. Current Zoology 64: 471–484.
Dalton, B. E., J. Lu, J. Leips, T. W. Cronin & K. L. Carleton, 2015. Variable light environments induce plastic spectral tuning by regional opsin coexpression in the African cichlid fish, Metriaclima Zebra. Molecular Ecology 24: 4193–4204.
DeJong, G., 2005. Evolution of phenotypic plasticity: patterns of plasticity and the emergence of ecotypes. New Phytologist 166(1): 101–118.
DeWitt, T. J. & S. M. Scheiner, 2004. Phenotypic Plasticity: Functional and Conceptual Approaches, Oxford University Press, Oxford:
Endler, J. A., 1992. Signals, signal conditions, and the direction of evolution. American Naturalist 139: S125–S153.
Enright, J. M., M. B. Toomey, S. Y. Sato, S. E. Temple, J. R. Allen, R. Fujiwara, V. M. Kramlinger, L. D. Nagy, K. M. Johnson, Y. Xiao, M. J. How, S. L. Johnson, N. W. Roberts, V. J. Kefalov, F. P. Guengerich & J. C. Corbo, 2015. Cyp27c1 red-shifts the spectral sensitivity of photoreceptors by converting Vitamin A1 into A2. Current Biology 25: 3048–3057.
Fernald, R. D., & P.A. Liebman. 1980. Visual receptor pigments in the African cichlid fish, Haplochromis burtoni. Vision Research 20(10): 857–864.
Fryer, G., 1972. The Cichlid Fishes of the Great Lakes of Africa. Their Biology and Evolution: 641.
Fox, J., D. Adler, D. Bates, G. Baud-Bovy, S. Ellison, D. Firth, M. Friendly, G. Gorjanc & S. Graves, 2017. Package ‘car‘.
Fuller, R. C. & L. A. Noa, 2010. Female mating preferences, lighting environment, and a test of the sensory bias hypothesis in the bluefin killifish. Animal Behaviour 80: 23–35.
Fusco, G. & A. Minelli, 2010. Phenotypic plasticity in development and evolution: facts and concepts. Introduction. Philosophical Transactions of the Royal Society b: Biological Sciences 365: 547–556.
Gallup, J. M., 2011. qPCR inhibition and amplification of difficult templates. PCR troubleshooting and optimization: the essential guide, Caister Academic Press, Norfolk:
Giovannucci, D. R. & R. S. Stephenson, 1999. Identification and distribution of dietary precursors of the Drosophila visual pigment chromophore: analysis of carotenoids in wild type and ninaD mutants by HPLC. Vision Research 39(2): 219–229.
Goldschmidt, T., F. Witte & J. De Visser, 1990. Ecological segregation in zooplanktivorous haplochromine species (Pisces: Cichlidae) from Lake Victoria. Oikos 58: 343–355.
Halekoh, U. & S. Højsgaard, 2014. A kenward-roger approximation and parametric bootstrap methods for tests in linear mixed models–the R package pbkrtest. Journal of Statistical Software 59(9): 1–30.
Halstenberg, S., K. M. Lindgren, S. P. Samagh, M. Nadal-Vicens, S. Balt & R. D. Fernald, 2005. Diurnal rhythm of cone opsin expression in the teleost fish Haplochromis burtoni. Visual Neuroscience 22: 135.
Härer, A., J. Torres-Dowdall & A. Meyer, 2018. Rapid adaptation to a novel light environment: the importance of ontogeny and phenotypic plasticity in shaping the visual system of Nicaraguan Midas cichlid fish (Amphilophus citrinellus spp.). Molecular Ecology 26: 5582–5593.
Hárosi, F. I., 1994. An analysis of two spectral properties of vertebrate visual pigments. Vision Research 34: 1359–1367.
Hofmann, C. M., K. E. O’Quin, N. J. Marshall, T. W. Cronin, O. Seehausen & K. L. Carleton, 2009. The eyes have it: regulatory and structural changes both underlie cichlid visual pigment diversity. PLoS Biology 7: e1000266.
Hofmann, C. M., K. E. O’Quin, A. R. Smith & K. L. Carleton, 2010. Plasticity of opsin gene expression in cichlids from Lake Malawi. Molecular Ecology 19: 2064–2074.
Hornsby, M. A., S. Sabbah, R. M. Robertson & C. W. Hawryshyn, 2013. Modulation of environmental light alters reception and production of visual signals in Nile tilapia. Journal of Experimental Biology 216: 3110–3122.
Hothorn, T., F. Bretz & P. Westfall, 2008. Simultaneous inference in general parametric models. Biometrical Journal 50: 346–363.
Huey, R. B., P. E. Hertz & B. Sinervo, 2003. Behavioral drive versus behavioral inertia in evolution: a null model approach. The American Naturalist 161(3): 357–366.
Irazábal-González L., D. S. Wright & M. E. Maan, 2022. Developmental and environmental plasticity in opsin gene expression in Lake Victoria cichlid fish. BioRxiv 2021.09.01.458542.
Jin, Y. H., J. W. Park, J. H. Kim & J. Y. Kwon, 2013. The expression pattern of melatonin receptor 1a gene during early life stages in the Nile tilapia (Oreochromis niloticus). Development and Reproduction 17: 45.
Jordan, R., D. Howe, F. Juanes, J. Stauffer Jr. & E. Loew, 2004. Ultraviolet radiation enhances zooplanktivory rate in ultraviolet sensitive cichlids. African Journal of Ecology 42: 228–231.
Kenward, M. G. & J. H. Roger, 1997. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53: 983–997.
Maan, M. E. & O. Seehausen, 2010. Mechanisms of species divergence through visual adaptation and sexual selection: perspectives from a cichlid model system. Current Zoology 56: 285–299.
Maan, M. E., O. Seehausen & T. G. Groothuis, 2017. Differential survival between visual environments supports a role of divergent sensory drive in cichlid fish speciation. The American Naturalist 189(1): 78–85.
Meier, J. I., V. C. Sousa, D. A. Marques, M. Selz, C. E. Wagner, L. Excoffier & O. Seehausen, 2017. Demographic modelling with whole-genome data reveals parallel origin of similar Pundamilia cichlid species after hybridization. Molecular Ecology 26: 123–141.
Meier, J. I., D. A. Marques, C. E. Wagner, L. Excoffier & O. Seehausen, 2018. Genomics of parallel ecological speciation in Lake Victoria cichlids. Molecular Biology and Evolution 35(6): 1489–1506.
Morshedian, A., M. B. Toomey, G. E. Pollock, R. Frederiksen, J. M. Enright, S. D. McCormick, M. C. Cornwall, G. L. Fain & J. C. Corbo, 2017. Cambrian origin of the CYP27C1-mediated vitamin A1-to-A2 switch, a key mechanism of vertebrate sensory plasticity. Royal Society Open Science 4: 170362.
Munz, F. W. & W. N. McFarland, 1977. Evolutionary Adaptations of fishes to the photic environment, The Visual System in Vertebrates Springer, Berlin: 193–274.
Nandamuri, S. P., M. R. Yourick & K. L. Carleton, 2017. Adult plasticity in African cichlids: rapid changes in opsin expression in response to environmental light differences. Molecular Ecology 26: 6036–6052.
Novales-Flamarique, H. & C. Hawryshyn, 1994. Ultraviolet photoreception contributes to prey search behaviour in two species of zooplanktivorous fishes. Journal of Experimental Biology 186: 187–198.
O’Quin, K. E., A. R. Smith, A. Sharma & K. L. Carleton, 2011. New evidence for the role of heterochrony in the repeated evolution of cichlid opsin expression. Evolution and Development 13: 193–203.
Parry, J. W. L. & J. K. Bowmaker, 2000. Visual pigment reconstitution in intact goldfish retina using Synthetic retinaldehyde isomers. Vision Research 40: 2241–2247.
Parry, J. W., K. L. Carleton, T. Spady, A. Carboo, D. M. Hunt & J. K. Bowmaker, 2005. Mix and match color vision: tuning spectral sensitivity by differential opsin gene expression in Lake Malawi cichlids. Current Biology 15: 1734–1739.
Pfennig, D. W., M. A. Wund, E. C. Snell-Rood, T. Cruickshank, C. D. Schlichting & A. P. Moczek, 2010. Phenotypic plasticity’s impacts on diversification and speciation. Trends in Ecology & Evolution 25: 459–467.
Pigliucci, M., 2001. Phenotypic Plasticity: Beyond Nature and Nurture, JHU Press, Baltimore:
Price, T. D., A. Qvarnström & D. E. Irwin, 2003. The role of phenotypic plasticity in driving genetic evolution. Proceedings of the Royal Society of London. Series B: Biological Sciences 270: 1433–1440.
Provencio, I., E. R. Loew & R. G. Foster, 1992. Vitamin A2-based visual pigments in fully terrestrial vertebrates. Vision Research 32: 2201–2208.
R Development Core Team, 2018. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna:
Reuter, T. E., R. H. White & G. Wald, 1971. Rhodopsin and Porphyropsin fields in the adult bullfrog retina. Journal of General Physiology 58(4): 351–371.
Ruijter, J. M. and C. Ramakers, 2003. LinRegPCR: analysis of real-time quantitative PCR data. User Manual provided with software.
Scheiner, S. M., 1993. Genetics and evolution of phenotypic plasticity. Annual Review of Ecology and Systematics 24: 35–68.
Schelly, R., W. Salzburger, S. Koblmüller, N. Duftner & C. Sturmbauer, 2006. Phylogenetic relationships of the lamprologine cichlid genus Lepidiolamprologus (Teleostei: Perciformes) based on mitochondrial and nuclear sequences, suggesting introgressive hybridization. Molecular Phylogenetics and Evolution 38: 426–438.
Schlichting, C. D., 1986. The evolution of phenotypic plasticity in plants. Annual Review of Ecology and Systematics 17: 667–693.
Schlichting, C. D. & M. Pigliucci, 1998. Phenotypic Evolution: A Reaction Norm Perspective, Sinauer Associates Incorporated, Sunderland:
Schneider, R. F. & A. Meyer, 2017. How plasticity, genetic assimilation and cryptic genetic variation may contribute to adaptive radiations. Molecular Ecology 26: 330–350.
Seehausen, O., 1996. Lake Victoria Rock Cichlids: Taxonomy, Ecology, and Distribution, Verduyn Cichlids, Herten:
Seehausen, O., J. J. Van Alphen & F. Witte, 1997. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277: 1808–1811.
Seehausen, O., E. Lippitsch, N. Bouton & H. Zwennes, 1998. Mbipi, the rock-dwelling cichlids of Lake Victoria: description of three new genera and fifteen new species (Teleostei). Ichthyological Exploration of Freshwaters 9(2): 129–228.
Seehausen, O., Y. Terai, I. S. Magalhaes, K. L. Carleton, H. D. Mrosso, R. Miyagi, I. Van Der Sluijs, M. V. Schneider, M. E. Maan, H. Tachida & H. Imai, 2008. Speciation through sensory drive in cichlid fish. Nature 455: 620–626.
Smith, A. R., M. J. van Staaden & K. L. Carleton, 2012. An evaluation of the role of sensory drive in the evolution of lake Malawi cichlid fishes. International Journal of Evolutionary Biology, 12.
Spady, T. C., O. Seehausen, E. R. Loew, R. C. Jordan, T. D. Kocher & K. L. Carleton, 2005. Adaptive molecular evolution in the opsin genes of rapidly speciating cichlid species. Molecular Biology and Evolution 22: 1412–1422.
Stevens, M., 2013. Sensory Ecology, Behaviour, and Evolution, Oxford University Press, Oxford:
Sugawara, T., Y. Terai, H. Imai, G. F. Turner, S. Koblmüller, C. Sturmbauer, Y. Shichida & N. Okada, 2005. Parallelism of amino acid changes at the RH1 affecting spectral sensitivity among deep-water cichlids from Lakes Tanganyika and Malawi. Proceedings of the National Academy of Sciences 102: 5448–5453.
Takechi, M. & S. Kawamura, 2005. Temporal and spatial changes in the expression pattern of multiple red and green subtype opsin genes during zebrafish development. Journal of Experimental Biology 208: 1337–1345.
Temple, S. E., E. M. Plate, S. Ramsden, T. J. Haimberger, W. M. Roth & C. W. Hawryshyn, 2006. Seasonal cycle in vitamin A 1/A 2-based visual pigment composition during the life history of coho salmon (Oncorhynchus kisutch). Journal of Comparative Physiology A 192: 301–313.
Terai, Y., O. Seehausen, T. Sasaki, K. Takahashi & S. Mizoiri, 2006. Divergent Selection on Opsins Drives Incipient Speciation in Lake Victoria Cichlids. PLoS Biology 4: 433.
Terai, Y., R. Miyagi, M. Aibara, S. Mizoiri, H. Imai, T. Okitsu, A. Wada, S. Takahashi-Kariyazono, A. Sato, H. Tichy & H. D. Mrosso, 2017. Visual adaptation in Lake Victoria cichlid fishes: depth-related variation of color and scotopic opsins in species from sand/mud bottoms. BMC Evolutionary Biology 17: 200.
Torres-Dowdall, J., M. E. Pierotti, A. Härer, N. Karagic, J. M. Woltering, F. Henning, K. R. Elmer & A. Meyer, 2017. Rapid and parallel adaptive evolution of the visual system of Neotropical Midas cichlid fishes. Molecular Biology and Evolution 34: 2469–2485.
Toyama, M., M. Hironaka, Y. Yamahama, H. Horiguchi, O. Tsukada, N. Uto, Y. Ueno, F. Tokunaga, K. Seno & T. Hariyama, 2008. Presence of rhodopsin and porphyropsin in the eyes of 164 fishes, representing marine, diadromous, coastal and freshwater species—a qualitative and comparative study. Photochemistry and Photobiology 84: 996–1002.
Van der Meer, H. J. & J. K. Bowmaker, 1995. Interspecific Variation of photoreceptors in four co-existing Haplochromine cichlid fishes. Brain, Behavior and Evolution 45: 232–240.
Van Snick Gray, E. & J. Stauffer, 2004. Phenotypic plasticity: its role in trophic radiation and explosive speciation in cichlids (Teleostei: Cichlidae). Animal Biology 54: 137–158.
Warrant, E. J. & S. Johnsen, 2013. Vision and the light environment. Current Biology 23: R990–R994.
West-Eberhard, M. J., 1989. Phenotypic plasticity and the origins of diversity. Annual Review of Ecology and Systematics 20: 49–278.
West-Eberhard, M. J., 2003. Developmental Plasticity and Evolution, Oxford University Press, Oxford:
Wilwert, E., R. S. Etienne, L. van de Zande & M. E. Maan, 2021. Contribution of opsins and chromophores to cone pigment variation across populations of Lake Victoria cichlids. Journal of Fish Biology
Witte-Maas, E. L. M. and F. Witte, 1985. Haplochromis nyererei, a new cichlid fish from Lake Victoria named in honour of Mwalimu Nyerere President of Tanzania: 1–13—Private publication. Witte & Van Ojen: Lake Victoria haplochromine trophic groups, 47.
Wright, D. S., R. Meijer, R. van Eijk, W. Vos, O. Seehausen & M. E. Maan, 2019. Geographic variation in opsin expression does not align with opsin genotype in Lake Victoria cichlid populations. Ecology and Evolution 9: 8676–8689.
Wright, D. S., R. van Eijk, L. Schuart, O. Seehausen, T. G. Groothuis & M. E. Maan, 2020. Testing sensory drive speciation in cichlid fish: Linking light conditions to opsin expression, opsin genotype and female mate preference. Journal of Evolutionary Biology 33: 422–434.
Xie, F., P. Xiao, D. Chen, L. Xu & Zhang, 2012. miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs. Plant Molecular Biology 80: 75–84.
Yourick, M. R., B. A. Sandkam, W. J. Gammerdinger, D. Escobar-Camacho, S. P. Nandamuri, F. E. Clark, B. Joyce, M. A. Conte, T. D. Kocher & K. L. Carleton, 2019. Diurnal variation in opsin expression and common housekeeping genes necessitates comprehensive normalization methods for quantitative real-time PCR analyses. Molecular Ecology Resources 19: 447–1460.
Acknowledgements
The authors thank Roel van Eijk and Jolien Gay for helping to design the qPCR protocol. We thank Sjoerd Veenstra, Brendan Verbeek, Lucia Irazábal-González and Willem Diderich for taking care of the fish in the laboratory.
Funding
This study was supported by the Ecology Fund of the Royal Netherlands Academy of Arts and Sciences and an Adaptive Life Programme grant made possible by the Board of the University of Groningen, the Faculty of Science and Engineering and the Groningen Institute for Evolutionary Life Sciences (GELIFES).
Author information
Authors and Affiliations
Contributions
MEM designed the study, together with RSE, LVDZ and EW. EW designed the qPCR protocol for cyp27c1 and completed the laboratory work. EW performed the analysis, with assistance from MEM, RSE and LVDZ. EW wrote the manuscript with contributions of MEM, RSE and LVDZ. All authors approved the contents of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted under the approval of the Institutional Animal Care and Use Committee of the University of Groningen.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: S. Koblmüller, R. C. Albertson, M. J. Genner, K. M. Sefc & T. Takahashi / Advances in Cichlid Research V: Behavior, Ecology and Evolutionary Biology
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wilwert, E., Etienne, R.S., van de Zande, L. et al. Visual system plasticity is differently mediated by cone opsin expression and chromophore composition in closely related cichlid species. Hydrobiologia 850, 2299–2314 (2023). https://doi.org/10.1007/s10750-022-04932-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04932-7