Abstract
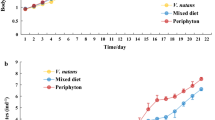
Submerged macrophytes cannot be utilized directly by zooplankton. However, vegetation can serve as an organic carbon resource for heterotrophic bacteria, which are themselves accessible to zooplankton. We therefore hypothesize that submerged macrophytes supply a carbon source to zooplankton by increasing the availability of food such as heterotrophic bacteria. Here, we used stable carbon isotope (13C) labeling to trace the carbon flow from submerged macrophytes to zooplankton with a mesocosm experiment. The carbon stable isotope ratios of zooplankton and their potential food sources were analyzed in mesocosms planted with 13C-labeled Hydrilla verticillata (L. f.) Royle in comparison with the control in which plastic plants were planted. We found that all potential food resources of zooplankton, including phytoplankton, bacterioplankton, macrophyte-associated epiphyton and epibacteria, were significantly enriched with 13C in the presence of 13C-enriched H. verticillata. Zooplankton were significantly more enriched with 13C than phytoplankton, epiphyton and bacterioplankton but significantly less enriched with 13C than epibacteria. Based on a stable isotopic mixing model, we found a macrophyte carbon contribution of 30.5% to epibacteria and 14.7% to zooplankton. Our results indicated that macrophytes might be used by zooplankton as a carbon resource, mainly via a pathway involving epibacteria attached to macrophytes.



Similar content being viewed by others
Availability of data and material
All data are available from the corresponding author upon reasonable request.
References
Agasild, H. & T. Nõges, 2005. Cladoceran and rotifer grazing on bacteria and phytoplankton in two shallow eutrophic lakes: in situ measurement with fluorescent microspheres. Journal of Plankton Research 27: 1155–1174.
Allanson, B. R., 1973. The fine structure of the periphyton of Chara sp. and Potamogeton natans from Wytham Pond, Oxford, and its significance to the macrophyte-periphyton metabolic model of RG Wetzel and HL Allen. Freshwater Biology 3: 535–542.
Allen, H. L., 1971. Primary productivity, chemo-organotrophy, and nutritional interactions of epiphytic algae and bacteria on macrophytes in the littoral of a lake. Ecological Monographs 41: 97–127.
Batt, R. D., S. R. Carpenter, J. J. Cole, M. L. Pace, T. J. Cline, R. A. Johnson & D. A. Seekell, 2012. Resources supporting the food web of a naturally productive lake. Limnology and Oceanography 57: 1443–1452.
Blindow, I., A. Hargeby & G. Andersson, 2002. Seasonal changes of mechanisms maintaining clear water in a shallow lake with abundant Chara vegetation. Aquatic Botany 72: 315–334.
Boschker, H. T. S. & J. J. Middelburg, 2002. Stable isotopes and biomarkers in microbial ecology. FEMS Microbiology Ecology 40: 85–95.
Bowszys, M., B. Jaworska, M. Kruk & A. Goździejewska, 2020. Zooplankton response to organic carbon content in a shallow lake covered by macrophytes. Chemistry and Ecology 36: 309–326.
Bracchini, L., A. Cózar, A. M. Dattilo, S. A. Loiselle, A. Tognazzi, N. Azza & C. Rossi, 2006. The role of wetlands in the chromophoric dissolved organic matter release and its relation to aquatic ecosystems optical properties. A case of study: Katonga and Bunjako Bays (Victoria Lake; Uganda). Chemosphere 63: 1170–1178.
Brendelberger, H., 1991. Filter mesh size of cladocerans predicts retention efficiency for bacteria. Limnology and Oceanography 36: 884–894.
Brett, M. T., M. J. Kainz, S. J. Taipale & H. Seshan, 2009. Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proceedings of the National Academy of Sciences of the United States of America 106: 21197–21201.
Burks, R. L., D. M. Lodge, E. Jeppesen & T. L. Lauridsen, 2002. Diel horizontal migration of zooplankton: costs and benefits of inhabiting the littoral. Freshwater Biology 47: 343–365.
Cattaneo, A. & J. Kalff, 1980. The relative contribution of aquatic macrophytes and their epiphytes to the production of macrophyte beds. Limnology and Oceanography 25: 280–289.
Cazzanelli, M., L. Forsström, M. Rautio, A. Michelsen & K. S. Christoffersen, 2012. Benthic resources are the key to Daphnia middendorffiana survival in a high arctic pond. Freshwater Biology 57: 541–551.
Cole, J. J., M. L. Pace, S. R. Carpenter & J. F. Kitchell, 2000. Persistence of net heterotrophy in lakes during nutrient addition and food web manipulations. Limnology and Oceanography 45: 1718–1730.
Cole, J. J., S. R. Carpenter, J. Kitchell, M. L. Pace, C. T. Solomon & B. Weidel, 2011. Strong evidence for terrestrial support of zooplankton in small lakes based on stable isotopes of carbon, nitrogen, and hydrogen. Proceedings of the National Academy of Sciences of the United States of America 108: 1975–1980.
Connolly, R. M., J. S. Hindell & D. Gorman, 2005. Seagrass and epiphytic algae support nutrition of a fisheries species, Sillago schomburgkii, in adjacent intertidal habitats. Marine Ecology Progress Series 286: 69–79.
Cottenie, K. & L. De Meester, 2004. Metacommunity structure: synergy of biotic interactions as selective agents and dispersal as fuel. Ecology 85: 114–119.
De Kluijver, A., J. Yu, M. Houtekamer, J. J. Middelburg & Z. Liu, 2012. Cyanobacteria as a carbon source for zooplankton in eutrophic Lake Taihu, China, measured by 13C labeling and fatty acid biomarkers. Limnology and Oceanography 57: 1245–1254.
De Kluijver, A., J. Ning, Z. Liu, E. Jeppesen, R. D. Gulati & J. J. Middelburg, 2015. Macrophytes and periphyton carbon subsidies to bacterioplankton and zooplankton in a shallow eutrophic lake in tropical China. Limnology and Oceanography 60: 375–385.
De Melo, M. L., D. N. Kothawala, S. Bertilsson, J. H. Amaral, B. Forsberg & H. Sarmento, 2020. Linking dissolved organic matter composition and bacterioplankton communities in an Amazon floodplain system. Limnology and Oceanography 65: 63–76.
Demarty, M. & Y. T. Prairie, 2009. In situ dissolved organic carbon (DOC) release by submerged macrophyte–epiphyte communities in southern Quebec lakes. Canadian Journal of Fisheries and Aquatic Sciences 66: 1522–1531.
Drechsler, Z. & S. Beer, 1991. Utilization of inorganic carbon by Ulva lactuca. Plant Physiology 97: 1439–1444.
Dunck, B., D. C. Amaral, U. L. Fernandes, N. F. Santana, T. M. Lopes & L. Rodrigues, 2018. Herbivory effects on the periphytic algal functional diversity in lake ecosystems: an experimental approach. Hydrobiologia 816: 231–241.
Faria, D. M., L. S. Cardoso & D. Motta Marques, 2017. Epiphyton dynamics during an induced succession in a large shallow lake: wind disturbance and zooplankton grazing act as main structuring forces. Hydrobiologia 788: 267–280.
Findlay, S., L. Carlough, M. T. Crocker, H. K. Gill, J. L. Meyer & P. J. Smith, 1986. Bacterial growth on macrophyte leachate and fate of bacterial production. Limnology and Oceanography 31: 1335–1341.
Freese, H. M. & D. Martin-Creuzburg, 2013. Food quality of mixed bacteria–algae diets for Daphnia magna. Hydrobiologia 715: 63–76.
Fry, B., 2006. Stable Isotope Ecology. Springer, New York: 1–308.
Gao, J., Z. Liu & E. Jeppesen, 2014. Fish community assemblages changed but biomass remained similar after lake restoration by biomanipulation in a Chinese tropical eutrophic lake. Hydrobiologia 724: 127–140.
González Sagrario, M. A., D. Rodríguez Golpe, L. La Sala, G. Sánchez Vuichard, P. Minotti & H. O. Panarello, 2018. Lake size, macrophytes, and omnivory contribute to food web linkage in temperate shallow eutrophic lakes. Hydrobiologia 818: 87–103.
Hann, B. J. & L. Zrum, 1997. Littoral microcrustaceans (Cladocera, Copepods) in a prairie coastal wetland: seasonal abundance and community structure. Hydrobiologia 357: 37–52.
He, X. J. & W. X. Wang, 2006. Releases of ingested phytoplankton carbon by Daphnia magna. Freshwater Biology 51: 649–665.
Hill, W. R., J. Rinchard & S. Czesny, 2011. Light, nutrients and the fatty acid composition of stream periphyton. Freshwater Biology 56: 1825–1836.
Hu, E., H. He, Y. Su, E. Jeppesen & Z. Liu, 2016. Use of multi-carbon sources by zooplankton in an oligotrophic lake in the Tibetan Plateau. Water 8: 565.
Jaschinski, S., D. C. Brepohl & U. Sommer, 2008. Carbon sources and trophic structure in an eelgrass Zostera marina bed, based on stable isotope and fatty acid analyses. Marine Ecology Progress Series 358: 103–114.
Jeppesen, E., J. P. Jensen, M. Søndergaard, T. Lauridsen, L. J. Pedersen & L. Jensen, 1997. Top-down control in freshwater lakes: the role of nutrient state, submerged macrophytes and water depth. Hydrobiologia 342: 151–164.
Jeppesen, E., T. L. Lauridsen, T. Kairesalo & M. Perrow, 1998. Impact of submerged macrophytes on fish–zooplankton interactions in lakes. In Jeppesen, E., M. Søndergaard, M. Søndergaard & K. Christoffersen (eds.), The Structuring Role of Submerged Macrophytes in Lakes. Springer-Verlag, New York: 91–114.
Jeppesen, E., M. Søndergaard, M. Søndergaard, K. Christoffersen, J. Theil-Nielsen & K. Jürgens, 2002. Cascading trophic interactions in the littoral zone: an enclosure experiment in shallow Lake Stigsholm, Denmark. Archiv für Hydrobiologie 153: 533–555.
Jones, J. I. & S. Waldron, 2003. Combined stable isotope and gut contents analysis of food webs in plant-dominated, shallow lakes. Freshwater Biology 48: 1396–1407.
Kirchman, D. L., L. Mazzella, R. S. Alberte & R. Mitchell, 1984. Epiphytic bacterial production on Zostera marina. Marine Ecology Progress Series 15: 117–123.
McCutchan Jr., J. H., W. M. Lewis, C. Kendall & C. C. McGrath, 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102: 378–390.
Mendonça, R., S. Kosten, G. Lacerot, N. Mazzeo, F. Roland, J. P. Ometto, E. A. Paz, C. P. Bove, N. C. Bueno, J. H. C. Gomes & M. Scheffer, 2013. Bimodality in stable isotope composition facilitates the tracing of carbon transfer from macrophytes to higher trophic levels. Hydrobiologia 710: 205–218.
Middelburg, J. J., C. Barranguet, H. T. Boschker, P. M. Herman, T. Moens & C. H. Heip, 2000. The fate of intertidal microphytobenthos carbon: An in situ 13C-labeling study. Limnology and Oceanography 45: 1224–1234.
Montiel-Martínez, A., J. Ciros-Pérez & G. Corkidi, 2015. Littoral zooplankton-water hyacinth interactions: habitat or refuge? Hydrobiologia 755: 173–182.
Morán, X. A. G., H. W. Ducklow & M. Erickson, 2013. Carbon fluxes through estuarine bacteria reflect coupling with phytoplankton. Marine Ecology Progress Series 489: 75–85.
Nõges, T., H. Luup & T. Feldmann, 2010. Primary production of aquatic macrophytes and their epiphytes in two shallow lakes (Peipsi and Võrtsjärv) in Estonia. Aquatic Ecology 44: 83–92.
Pace, M. L., K. G. Porter & Y. S. Feig, 1983. Species- and age-specific differences in bacterial resource utilization by two co-occurring cladocerans. Ecology 64: 1145–1156.
Pace, M. L., J. J. Cole, S. R. Carpenter, J. F. Kitchell, J. R. Hodgson, M. C. Van de Bogert, D. L. Bade, E. S. Kritzberg & D. Bastviken, 2004. Whole-lake carbon-13 additions reveal terrestrial support of aquatic food webs. Nature 427: 240–243.
Parnell, A., 2016. simmr: A Stable Isotope Mixing Model. https://CRAN.R-project.org/package=simmr.
Penhale, P. A. & W. O. Smith, 1977. Excretion of dissolved organic carbon by eelgrass (Zostera marina) and its epiphytes. Limnology and Oceanography 22: 400–407.
Peterson, B. J., J. E. Hobbie & J. F. Haney, 1978. Daphnia grazing on natural bacteria. Limnology and Oceanography 23: 1039–1044.
Rautio, M. & W. F. Vincent, 2006. Benthic and pelagic food resources for zooplankton in shallow high-latitude lakes and ponds. Freshwater Biology 51: 1038–1052.
Reitsema, R. E., P. Meire & J. Schoelynck, 2018. The future of freshwater macrophytes in a changing world: dissolved organic carbon quantity and quality and its interactions with macrophytes. Frontiers in Plant Science 9: 629.
Richoux, N. B., L. Bergamino, S. Moyo & T. Dalu, 2018. Spatial and temporal variability in the nutritional quality of basal resources along a temperate river/estuary continuum. Organic Geochemistry 116: 1–12.
Sand-Jensen, K. & J. Borum, 1991. Interactions among phytoplankton, periphyton, and macrophytes in temperate freshwaters and estuaries. Aquatic Botany 41: 137–175.
Schoenberg, S. A. & A. E. Maccubbin, 1985. Relative feeding rates on free and particle-bound bacteria by freshwater macrozooplankton. Limnology and Oceanography 30: 1084–1090.
Siehoff, S., M. Hammers-Wirtz, T. Strauss & H. T. Ratte, 2009. Periphyton as alternative food source for the filter-feeding cladoceran Daphnia magna. Freshwater Biology 54: 15–23.
Søndergaard, M., 1983. Heterotrophic utilization and decomposition of extracellular carbon released by the aquatic angiosperm Littorella uniflora (L.) Aschers. Aquatic Botany 16: 59–73.
Taipale, S. J., E. Peltomaa, M. Hiltunen, R. I. Jones, M. W. Hahn, C. Biasi & M. T. Brett, 2015. Inferring phytoplankton, terrestrial plant and bacteria bulk δ13C values from compound specific analyses of lipids and fatty acids. PloS one 10:
Taipale, S. J., A. W. E. Galloway, S. L. Aalto, K. K. Kahilainen, U. Strandberg & P. Kankaala, 2016. Terrestrial carbohydrates support freshwater zooplankton during phytoplankton deficiency. Scientific Reports 6: 30897.
Tang, Y., Y. Su, H. Sun, Z. Liu, H. J. Dumont, J. Hu, Y. Zhang & J. Yu, 2017. Carbon transfer from dissolved organic carbon to the cladoceran Bosmina: a mesocosm study. Knowledge and Management of Aquatic Ecosystems. https://doi.org/10.1051/kmae/2017016.
Tang, Y., X. Yang, R. Xu, X. Zhang, Z. Liu, Y. Zhang & H. J. Dumont, 2019. Heterotrophic microbes upgrade food value of a terrestrial carbon resource for Daphnia magna. Limnology and Oceanography 64: 474–482.
Torremorell, A., G. Pérez, L. Lagomarsino, P. Huber, C. Queimaliños, J. Bustingorry, P. Fermani, M. E. Llames & F. Unrein, 2015. Microbial pelagic metabolism and CDOM characterization in a phytoplankton-dominated versus a macrophyte-dominated shallow lake. Hydrobiologia 752: 203–221.
Van Donk, E. & W. J. van de Bund, 2002. Impact of submerged macrophytes including charophytes on phyto-and zooplankton communities: allelopathy versus other mechanisms. Aquatic Botany 72: 261–274.
Vander Zanden, M. J., T. E. Essington & Y. Vadeboncoeur, 2005. Is pelagic top-down control in lakes augmented by benthic energy pathways? Canadian Journal of Fisheries and Aquatic Sciences 62: 1422–1431.
Vis, C., C. Hudon & R. Carignan, 2006. Influence of the vertical structure of macrophyte stands on epiphyte community metabolism. Canadian Journal of Fisheries and Aquatic Sciences 63: 1014–1026.
Wetzel, R. G., 1983. Attached algal-substrata interactions: fact or myth, and when and how? In Wetzel, R. G. (ed.), Periphyton of Freshwater Ecosystems. Junk Publishers, The Hague, Dr W: 207–215.
Wetzel, R. G., 2005. Periphyton in the aquatic ecosystem and food webs. In Azim, M. E., M. C. J. Verdegem, A. A. van Dam & M. C. M. Beveridge (eds.), Periphyton Ecology Exploitation and Management. CABI, Oxfordshire.
Zhang, Y., X. Liu, M. Wang & B. Qin, 2013. Compositional differences of chromophoric dissolved organic matter derived from phytoplankton and macrophytes. Organic Geochemistry 55: 26–37.
Zhang, X., Y. Tang, E. Jeppesen & Z. Liu, 2017. Biomanipulation-induced reduction of sediment phosphorus release in a tropical shallow lake. Hydrobiologia 794: 49–57.
Acknowledgments
We thank Amy-Jane Beer for language editing, and we are grateful to Luigi Naselli-Flores and the reviewers for providing us with valuable comments and to other participants who collected and analyzed samples during the experimental period. We consider this work a joint contribution from the Department of Ecology and Institute of Hydrobiology, funded by Jinan University, China. This study was funded by the National Natural Science Foundation of China (32071566 and 41471086).
Funding
This study was funded by the National Natural Science Foundation of China (32071566 and 41471086).
Author information
Authors and Affiliations
Contributions
Conceptualization: ZL, YT and LS; methodology: YT, LS, ZJ and LX; writing—original draft preparation: LS; Writing—review and editing: YT, ZL, YS, LS, PZ and QL.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Handling editor: André Padial
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Su, L., Jin, Z., Xie, L. et al. Carbon transfer from the submerged macrophyte Hydrilla verticillata to zooplankton: a 13C-labeled mesocosm study. Hydrobiologia 848, 4179–4188 (2021). https://doi.org/10.1007/s10750-021-04645-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04645-3