Abstract
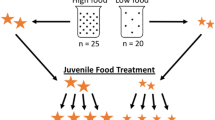
Early life conditions can have lifelong impacts on survival and fitness. Silver spoon effects occur when favorable early life conditions provide advantages through adulthood, even if conditions worsen. Alternatively, compensatory growth allows organisms initially reared in unfavorable conditions to grow more quickly than expected should conditions improve. We examined these possibilities in the American Toad, Anaxyrus americanus, by rearing larvae in low and high competition environments and then transferring them to either high- or low-resource levels. We measured growth one-week post-transfer and toadlet size after metamorphosis. We also dissected larvae and toadlets from each treatment to examine effects on organ size. Before transfer, larvae reared with low competition grew significantly faster than those reared with high competition. Consistent with the silver spoon hypothesis, these larvae ate significantly more food, continued to grow faster post-transfer, and metamorphosed into larger toadlets with smaller livers and larger guts than those initially reared with high competition, despite having the same food availability within a resource level. Overall, our study demonstrates that larvae initially reared in favorable conditions maintain a growth advantage through metamorphosis even if resource levels decline. Future studies should explore the effects of this advantage post-metamorphosis and into adulthood.





Similar content being viewed by others
Data availability
The data sets generated and analyzed during this study are available in the Dryad repository https://doi.org/10.5061/dryad.pvmcvdngc.
References
Ab Ghani, N. I. & J. Merila, 2015. Population divergence in compensatory growth responses and their costs in sticklebacks. Ecology and Evolution 5: 7–23.
Ali, M., A. Nicieza & R. J. Wootton, 2003. Compensatory growth in fishes: a response to growth depression. Fish and Fisheries 4: 147–190.
Bates, D. & M. Maechler, 2009. lme4: Linear mixed-effects models using S4 classes. R package version 1.1-21.
Beiswenger, R. E., 1975. Structure and function in aggregations of tadpoles of the American Toad, Bufo americanus. Herpetologica 31: 222–233.
Boone, M. D., 2005. Juvenile frogs compensate for small metamorph size with terrestrial growth: overcoming the effects of larval density and insecticide exposure. Journal of Herpetology 39: 416–423.
Bouchard, S. S., C. J. O’Leary, L. J. Wargelin, W. B. Rodriguez, K. X. Jennings & K. M. Warkentin, 2015. Alternative competition-induced digestive strategies yield equal growth, but constrain compensatory growth in red-eyed treefrog larvae. Journal of Experimental Zoology 323: 778–788.
Bouchard, S. S., C. J. O’Leary, L. J. Wargelin, J. F. Charbonnier & K. M. Warkentin, 2016. Post-metamorphic carry-over effects of larval digestive plasticity. Functional Ecology 30: 379–388.
Burton, T., S. S. Killen, J. D. Armstrong & N. B. Metcalfe, 2011. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proceedings of the Royal Society B Biological Sciences 278: 3465–3473.
Buston, P. M. & J. Elith, 2011. Determinants of reproductive success in dominant pairs of clownfish: a boosted regression tree analysis. Journal of Animal Ecology 80: 528–538.
Cabrera-Guzman, E., M. R. Crossland, G. P. Brown & R. Shine, 2013. Larger body size at metamorphosis enhances survival, growth and performance of young cane toads (Rhinella marina). PLoS ONE 8: e70121.
Capellan, E. & A. G. Nicieza, 2007. Non-equivalence of growth arrest induced by predation risk or food limitation: context-dependent compensatory growth in anuran tadpoles. Journal of Animal Ecology 76: 1026–1035.
Chen, W., L. Zhang & X. Lu, 2011. Higher pre-hibernation energy storage in anurans from cold environments: a case study on a temperate frog Rana chensinensis along a broad latitudinal and altitudinal gradients. Annales Zoologici Fennici 48: 214–220.
Chin, E. H., A. L. Storm-Suke, R. J. Kelly & G. Burness, 2013. Catch-up growth in Japanese quail (Coturnix japonica): relationships with food intake, metabolic rate and sex. Journal of Comparative Physiology B 183: 821–831.
Cooper, E. B. & L. E. B. Kruuk, 2018. Ageing with a silver-spoon: a meta-analysis of the effect of developmental environment on senescence. Evolution Letters 2: 460–471.
Crespi, E. J. & R. W. Warne, 2013. Environmental conditions experienced during the tadpole stage alter post-metamorphic glucocorticoid response to stress in an amphibian. Integrative and Comparative Biology 53: 989–1001.
Dash, M. D. & A. K. Hota, 1980. Density effects on the survival, growth rate, and metamorphosis of Rana tigrina tadpoles. Ecology 61: 1025–1028.
De Block, M., M. A. McPeek & R. Stoks, 2008. Stronger compensatory growth in a permanent-pond Lestes damselfly relative to temporary-pond Lestes. Oikos 117: 245–254.
Descamps, S., S. Boutin, D. Berteaux, A. G. McAdam & J. Gaillard, 2008. Cohort effects in red squirrels: the influence of density, food abundance and temperature on future survival and reproductive success. Journal of Animal Ecology 77: 305–314.
Distel, C. A. & M. D. Boone, 2010. Effects of aquatic exposure to the insecticide carbaryl are species-specific across life stages and mediated by heterospecific competitors in anurans. Functional Ecology 24: 1342–1352.
Dmitriew, C. & L. Rowe, 2011. The effects of larval nutrition on reproductive performance in a food-limited adult environment. PLoS ONE 6: e17399.
Douhard, M., M. Festa-Bianchet, J. Landes & F. Pelletier, 2019. Trophy hunting mediates sex-specific associations between early-life environmental conditions and adult mortality in bighorn sheep. Journal of Animal Ecology 88: 734–745.
Grafen, A., 1988. On the uses of data on lifetime reproductive success. In Clutton-Brock, T. (ed), Reproductive Success. University of Chicago Press, Chicago: 454–471.
Gramapurohit, N. P., 2009. Catch-up growth during juvenile life can compensate for the small metamorphic size in Euphlyctis cyanophlyctis. Current Science 97: 1243–1246.
Green, A. W. & L. L. Bailey, 2015. Reproductive strategy and carry-over effects for species with complex life histories. Population Ecology 57: 175–184.
Hales, C. N. & D. J. P. Barker, 1992. Type 2 non-insulin-dependent diabetes mellitus the thrifty phenotype hypothesis. Diabetologia 35: 595–601.
Hector, K. L. & S. Nakagawa, 2012. Quantitative analysis of compensatory and catch-up growth in diverse taxa. Journal of Animal Ecology 81: 583–593.
Hector, K. L., P. J. Bishop & S. Nakagawa, 2012. Consequences of compensatory growth in an amphibian. Journal of Zoology 286: 93–101.
Hopwood, P. E., A. J. Moore & N. J. Royle, 2014. Effects of resource variation during early life and adult social environment on contest outcomes in burying beetles: a context-dependent silver spoon strategy? Proceedings of the Royal Society B Biological Sciences 281: 20133102.
Horiuchi, S. & Y. Koshida, 1989. Effects of foodstuffs on intestinal length in larvae of Rhacophorus arboreus (Anura: Rhacophoridae). Zoological Science 6: 321–328.
Hu, F., E. J. Crespi & R. J. Denver, 2008. Programming neuroendocrine stress axis activity by exposure to glucocorticoids during postembryonic development of the frog, Xenopus laevis. Endocrinology 149: 5470–5481.
Jasienski, M., 2008. The potential for recovery growth in stunted larvae of Rana sylvatica and its decline with developmental stages in R. temporaria. Amphibia-Reptilia 29: 399–404.
Kayes, S. M., R. L. Cramp & C. E. Franklin, 2009. Metabolic depression during aestivation in Cyclorana alboguttata. Comparative Biochemistry and Physiology A 154: 557–563.
Lindstrom, J., 1999. Early development and fitness in birds and mammals. Trends in Ecology and Evolution 14: 343–347.
Madsen, T. & R. Shine, 2000. Silver spoons and snake body sizes: prey availability early in life influences long-term growth rates of free-ranging pythons. Journal of Animal Ecology 69: 952–958.
Mangel, M. & S. B. Munch, 2005. A life-history perspective on short- and long-term consequences of compensatory growth. American Naturalist 166: E155–E176.
Martin, L. J., S. Rainford & B. Blossey, 2015. Effects of plant leaf litter diversity, species, origin and traits on larval toad performance. Oikos 124: 871–879.
McInerney, E. P., A. J. Silla & P. G. Byrne, 2016. The influence of carotenoid supplementation at different life-stages on the foraging performance of the Southern Corroboree frog (Pseudophryne corroboree): a test of the silver spoon and environmental matching hypotheses. Behavioural Processes 125: 26–33.
Metcalfe, N. B. & P. Monaghan, 2001. Compensation for a bad start: grow now, pay later? Trends in Ecology and Evolution 16: 254–260.
Minias, P., R. Wlodarczyk, A. Surmacki & T. Iciek, 2015. Silver spoon effects on plumage quality in a passerine bird. Royal Society Open Science 2: 140459.
Monaghan, P., 2008. Early growth conditions, phenotypic development and environmental change. Philosophical Transactions of the Royal Society B 363: 1635–1645.
Morey, S. & D. Reznick, 2001. Effects of larval density on postmetamorphic Spadefoot Toads (Spea hammondii). Ecology 82: 510–522.
Mueller, T., C. L. Kuell & C. Mueller, 2016. Effects of larval versus adult density conditions on reproduction and behavior of a leaf beetle. Behavioral Ecology and Sociobiology 70: 2081–2091.
Naya, D. E., C. Veloso, P. Sabat & F. Bozinovic, 2009. The effect of short- and long-term fasting on digestive and metabolic flexibility in the Andean toad, Bufo spinulosus. Journal of Experimental Biology 212: 2167–2175.
Neptune, T. C. & S. S. Bouchard, 2020. Predation and competition induce variable organ size trade-offs in larval anurans. Journal of Zoology 312: 193–204.
Nicieza, A. G. & D. Alvarez, 2009. Statistical analysis of structural compensatory growth: how can we reduce the rate of false detection? Oecologia 159: 27–39.
Orizaola, G., E. Dahl & A. Laurila, 2014. Compensatory growth strategies are affected by the strength of environmental time constraints in anuran larvae. Oecologia 174: 131–137.
Pigeon, G., M. Festa-Bianchet & F. Pelletier, 2017. Long-term fitness consequences of early environment in a long-lived ungulate. Proceedings of the Royal Society B 284: 20170222.
Radder, R. S., D. A. Warner & R. Shine, 2007. Compensating for a bad start: catch-up growth in juvenile lizards (Amphibolurus muricatus, Agamidae). Journal of Experimental Zoology Part A 307A: 500–508.
Relyea, R. A. & J. R. Auld, 2004. Having the guts to compete: how intestinal plasticity explains costs of inducible defences. Ecology Letters 7: 869–875.
R Core Team, 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Roark, A. M., K. A. Bjorndal & A. B. Bolten, 2009. Compensatory responses to food restriction in juvenile green turtles (Chelonia mydas). Ecology 90: 2524–2534.
Roberts, L. J., J. Taylor, P. J. Gough, D. W. Forman & C. G. de Leaniz, 2014. Silver spoons in the rough: can environmental enrichment improve survival of hatchery Atlantic salmon Salmo salar in the wild? Journal of Fish Biology 85: 1972–1991.
Salles, O. C., P. Saenz-Agudelo, G. R. Almany, M. L. Berumen, S. R. Thorrold, G. P. Jones & S. Planes, 2016. Genetic tools link long-term demographic and life-history traits of anemonefish to their anemone hosts. Coral Reefs 35: 1127–1138.
Scharf, I., H. Braf, N. Ifrach, S. Rosenstein & A. Subach, 2015. The effects of temperature and diet during development, adulthood, and mating on reproduction in the red flour beetle. PLoS ONE 10: e0136924.
Schneider, C. A., W. S. Rasband & K. W. Eliceiri, 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675.
Scott, D. E., 1994. The effect of larval density on adult demographic traits in Ambystoma opacum. Ecology 75: 1383–1396.
Scott, D. E., E. D. Casey, M. F. Donovan & T. K. Lynch, 2007. Amphibian lipid levels at metamorphosis correlate to post-metamorphic terrestrial survival. Oecologia 153: 521–532.
Semlitsch, R. D., D. E. Scott & J. Pechmann, 1988. Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology 69: 184–192.
Sheridan, M. A. & Y. H. Kao, 1998. Regulation of metamorphosis-associated changes in the lipid metabolism of selected vertebrates. American Zoologist 38(3): 50–368.
Smith, D. C., 1987. Adult recruitment in chorus frogs: effects of size and date at metamorphosis. Ecology 68: 344–350.
Song, Z., Y. Zou, C. Hu, Y. Ye, C. Wang, B. Qing, J. Komdeur & C. Ding, 2019. Silver spoon effects of hatching order in an asynchronous hatching bird. Behavioral Ecology 30: 509–517.
Stamper, C. E., D. J. Stevens, J. R. Downie & P. Monaghan, 2008. The effects of competition on pre- and post-metamorphic phenotypes in the common frog. Herpetological Journal 18: 187–195.
Stamps, J. A., 2006. The silver spoon effect and habitat selection by natal dispersers. Ecology Letters 9: 1179–1185.
Stoler, A. B. & R. A. Relyea, 2013. Leaf litter quality induces morphological and developmental changes in larval amphibians. Ecology 94: 1594–1603.
Stoler, A. B., J. P. Stephens, R. A. Relyea, K. A. Berven & S. D. Tiegs, 2015. Leaf litter resource quality induces morphological changes in wood frog (Lithobates sylvaticus) metamorphs. Oecologia 179: 667–677.
Taborsky, B., 2006. The influence of juvenile and adult environments on life-history trajectories. Proceedings of the Royal Society B 273: 741–750.
Tarvin, R. D., C. S. Bermudez, V. S. Briggs & K. M. Warkentin, 2015. Carry-over effects of size at metamorphosis in Red-eyed Treefrogs: higher survival but slower growth of larger metamorphs. Biotropica 47: 218–226.
Tejedo, M., F. Marangoni, C. Pertoldi, A. Richter-Boix, A. Laurila, G. Orizaola, A. G. Nicieza, D. Alvarez & I. Gomez-Mestre, 2010. Contrasting effects of environmental factors during larval stage on morphological plasticity in post-metamorphic frogs. Climate Research 43: 31–U46.
Touchon, J. C., M. W. McCoy, J. R. Vonesh & K. M. Warkentin, 2013. Effects of plastic hatching timing carry over through metamorphosis in red-eyed treefrogs. Ecology 94: 850–860.
Van de Pol, M., L. W. Bruinzeel, D. Heg, H. P. Van der Jeugd & S. Verhulst, 2006. A silver spoon for a golden future: long-term effects of natal origin on fitness prospects of oystercatchers (Haematopus ostralegus). Journal of Animal Ecology 75: 616–626.
Warkentin, K. M., 1992. Microhabitat use and feeding rate variation in green frog tadpoles (Rana clamitans). Copeia 1992: 731–740.
Wong, J. W. Y. & M. Koelliker, 2014. Effects of food restriction across stages of juvenile and early adult development on body weight, survival and adult life history. Journal of Evolutionary Biology 27: 2420–2430.
Acknowledgements
We thank the Biology & Earth Science Department at Otterbein University for logistical support of this project. Erin Ulrich was particularly helpful in providing supplies and equipment, and Molly Kukawka, Justin McCurdy, Delaney Galbraith and Emma Kimberly assisted with the experimental set up and data collection. This work was conducted under Wild Animal Permit 17-125 from the Ohio Department of Natural Resources Division of Wildlife and with approval from the Otterbein University Animal Care and Use Committee. All applicable institutional and/or national guidelines for the care and use of animals were followed.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design, as well as the data collection and analysis. The first manuscript was written by SB, and SB commented on previous versions. Both authors participated in manuscript revisions and both approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the contents of this article.
Ethical approval
This work was conducted under Wild Animal Permit 17-125 from the Ohio Department of Natural Resources Division of Wildlife and with approval from the Otterbein University Animal Care and Use Committee. All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Handling editor: Lee B. Kats
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bonifas, S.M., Bouchard, S.S. Competition induces silver spoon effects in developing anuran larvae. Hydrobiologia 848, 1219–1230 (2021). https://doi.org/10.1007/s10750-020-04492-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04492-8