Abstract
Ecological niche modelling (ENM) has been used to quantify the potential occurrence of species, by identifying the main environmental factors that determine the presence of species across geographical space. We provide a large-scale survey of the distribution of ostracod species in South America, by using the domains of 25 river basins. From 221 known ostracod species, we estimate the potential distribution of 61 species, using ENM. Ten clusters of potential distribution patterns were found. Clusters 8 and 9 grouped most of the species, which presented high similarity of niche between them. Heterocypris paningi Brehm, 1934 (group 1) obtained higher niche variability. The minimum temperatures of the coldest month and the mean elevation of the river basin were most important to predict the potential distribution of ostracods of most groups. South America has a complex pattern of elevation, which affects species distributions indirectly through changes in local factors. For instance, the Andes mountains might impose a barrier for ostracod distribution in the southern part of South America because of the low temperatures and precipitation. The ENM indicated that some regions and/or basins of South America might be susceptible to the entry of several ostracod species, presently absent, including non-native species.








Similar content being viewed by others
References
Allan, J. D., 1995. Stream Ecology. Chapman & Hall, London: 388.
Allouche, O., A. Tsoar & R. Kadmon, 2006. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). Journal of Applied Ecology 43: 1223–1232.
Almeida, N. M., V. G. Ferreira, J. Higuti & K. Martens. On two new species of Cypricercus Sars 1895 (Crustacea, Ostracoda) from Brazil. Zootaxa (submitted).
Araújo, M. B. & M. New, 2007. Ensemble forecasting of species distributions. Trends Ecology Evolution 22: 42–47.
Battauz, Y. S., S. B. J. Paggi & C. Paggi, 2017. Macrophytes as dispersal vectors of zooplankton resting stages in a subtropical riverine floodplain. Aquatic Ecology 51: 191–201.
Brochet, A. L., M. Gauthier-Clerc, M. Guillemain, H. Fritz, A. Waterkeyn, Á. Baltaná & A. J. Green, 2010. Field evidence of dispersal of branchiopods, ostracods and bryozoans by teal (Anas crecca) in the Camargue (southern France). Hydrobiologia 637: 255–261.
Busby, J. R., 1991. BIOCLIM—a bioclimate analysis and prediction system. In Margules, C. R. & M. P. Austin (eds), Nature Conservation: Cost Effective Biological Surveys and Data Analysis. CSIRO, Melbourne: 64–68.
Campos, R., F. M. Lansac-Tôha, E. O. Conceição, K. Martens & J. Higuti, 2018. Factors affecting the metacommunity structure of periphytic ostracods (Crustacea, Ostracoda): a deconstruction approach based on biological traits. Aquatic Sciences 80: 1–16.
Capinha, C. & P. Anastácio, 2010. Assessing the environmental requirements of invaders using ensembles of distribution models. Diversity and Distributions 17: 13–24.
Carpenter, G., A. N. Gillison & J. Winter, 1993. Domain: a flexible modelling procedure for mapping potential distributions of plants and animals. Biodiversity Conservation 2: 667–680.
Cassemiro, F. A. S., D. Bailly, W. J. Graça & A. A. Agostinho, 2018. The invasive potential of tilapias (Osteichthyes, Cichlidae) in the Americas. Hydrobiologia 817: 133–154.
Cohen, A. C. & J. G. Morin, 1990. Patterns of reproduction in ostracodes: a review. Journal of Crustacean Biology 10: 184–211.
Conceição, E. O., J. Higuti, R. Campos & K. Martens, 2018. Effects of flood pulses on persistence and variability of pleuston communities in a tropical floodplain lake. Hydrobiologia 807: 175–188.
Cusminsky, G. C., P. A. Pérez, A. Schwal & R. Whatley, 2005. Recent lacustrine ostracods from patagonia, argentina. Revista Española de Micropaleontología 37: 431–450.
Daday, E., 1905. Untersuchungen über die Süsswasser-Mikrofauna Paraguays. Zoologica, OriginalAbhandlungen aus dem Gesamtgebiete der Zoologie 18: 1–374.
Delorme, L. D., 2001. Ostracoda. In Thorp, J. H. & A. P. Covich (eds), Ecology and Classification of North American Freshwater Invertebrates. Academic Press, San Diego: 811–848.
Díaz, A. R. & E. C. Lopretto, 2011. The genus Chlamydotheca Saussure (Crustacea: Ostracoda) in northeastern Argentina. Nauplius 19: 97–107.
Diniz-Filho, J. A. F., L. M. Bini, T. F. Rangel, R. D. Loyola, C. Hof, D. Nogués-Bravo & M. B. Araújo, 2009. Partitioning and mapping uncertainties in ensembles of forecasts of species turnover under climate change. Ecography 32: 897–906.
Diniz-Filho, J. A. D., P. Marco & B. A. Hawkins, 2010a. Defying the curse of ignorance: perspectives in insect macroecology and conservation biogeography. Insect Conservation and Diversity 3: 172–179.
Diniz-Filho, J. A. F., J. C. Nabout, L. M. Bini, R. D. Loyola, T. F. Rangel, D. Nogues-Bravo & M. B. Araújo, 2010b. Ensemble forecasting shifts in climatically suitable areas for Tropidacris cristata (Orthoptera: Acridoidea: Romaleidae). Insect Conservation and Diversity 3: 213–221.
Elith, J., C. H. Graham, R. P. Anderson, M. Dudík, S. Ferrier, A. Guisan, R. J. Hijmans, F. Huettman, J. R. Leathwick, A. Lehmann, J. Li, L. G. Lohmann, B. A. Loiselle, G. Manion, C. Moritz, M. Nakamura, Y. Nakazawa, J. M. Overton, A. T. Peterson, S. J. Phillips, K. Richardson, R. Scachetti-Pereira, R. E. Schapire, J. Soberón, S. Williams, M. S. Wisz & N. E. Zimmermann, 2006. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29: 129–151.
Farber, O. & R. Kadmon, 2003. Assessment of alternative approaches for bioclimatic modellingmodelling with special emphasis on the Mahalanobis distance. Ecological Modelling 160: 115–130.
Ferreiro, N., C. Feijoó, A. Giorgi & L. Leggieri, 2011. Effects of macrophyte heterogeneity and food availability on structural parameters of the macroinvertebrate community in a Pampean stream. Hydrobiologia 664: 199–211.
Gama, M., D. Crespo, M. Dolbeth & P. M. Anastácio, 2017. Ensemble forecasting of Corbicula fluminea worldwide distribution: projections of the impact of climate change. Aquatic Conservation: Marine and Freshwater Ecosystems 27: 675–684.
Giannini, T. C., A. M. Saraiva & I. Alves-dos-Santos, 2010. Ecological niche modeling and geographical distribution of pollinator and plants: a case study of Peponapis fervens (Smith, 1879) (Eucerini: Apidae) and Cucurbita species (Cucurbitaceae). Ecological Informatics 5: 59–66.
Giannini, T. C., R. Lira-Saade, R. Ayala, A. M. Saraiva & I. Alves-dos-Santos, 2011. Ecological niche similarities of Peponapis bees and non-domesticated Cucurbita species. Ecological Modelling 222: 2011–2018.
Gower, J. C., 1971. A general coefficient of similarity and some of its properties. Biometrics 27: 857–874.
Hansen, J. P., J. Sagerman & S. A. Wikstrom, 2010. Effects of plant morphology on small-scale distribution of invertebrates. Marine Biology 157: 2143–2155.
Hay, S. E., K. M. Jenkins & R. T. Kingsford, 2018. Diverse invertebrate fauna using dry sediment as a refuge in semi-arid and temperate Australin rivers. Hydrobiologia 806: 95–109.
Higuti, J. & K. Martens, 2014. Five new species of Candoninae (Crustacea, Ostracoda) from the alluvial valley of the Upper Paraná River (Brazil, South America). European Journal of Taxonomy 106: 1–36.
Higuti, J., L. F. M. Velho, F. A. Lansac-Tôha & K. Martens, 2007. Pleuston communities are buffered from regional flood River pulses: the example of ostracods in the Paraná River floodplain, Brazil. Freshwater Biology 52: 1930–1943.
Higuti, J., C. Meisch & K. Martens, 2009. On Paranacypris samambaiensis gen. nov., sp. nov. (Crustacea, Ostracoda), the first South American psychrodromid from the alluvial valley of the Upper Paraná River, Brazil. Journal of Natural History 43: 769–783.
Higuti, J., S. A. J. Declerck, F. A. Lansac-Tôha, L. F. Velho & K. Martens, 2010. Variation in ostracod (Crustacea, Ostracoda) communities in the alluvial valley of the Upper Paraná River (Brazil) in relation to substrate. Hydrobiologia 644: 261–278.
Higuti, J., K. F. Roche & K. Martens, 2017. Checklist of freshwater ostracods (Crustacea, Ostracoda) of the Pantanal of Mato Grosso do Sul, Brazil. Iheringia 107: e2017114.
Hirzel, A. H., J. Hausser, D. Chessel & N. Perrin, 2002. Ecological-niche factor analysis: how to compute habitat suitability maps without absence data? Ecology 83: 2027–2203.
Hutchinson, G. E., 1957. Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology 22: 415–427.
Junk, J. W., 1993. Wetlands of Tropical South America. In Whigham, D., D. Dykyjová & S. Hejný (eds), Wetlands of the world I: Inventory, ecology and management. Kluwer Academic Publisher, Dordrecht.
Klie, W., 1939. Süßwasserostracoden aus Nordostbrasilien: 2. Die Gattung Chlamydotheca. Zoologischer Anzeiger 128: 152–159.
Klie, W., 1940. Süßwasser-Ostracoden aus Nordostbrasilien. 6: Cyprinae mit geißelförmiger Furka. Zoologischer Anzeiger 130: 59–73.
Kline, R. B., 1998. Principles and practice of structural equation modeling. The Guilford Press, New York, NY.
Kock, N. & G. Lynn, 2012. Lateral collinearity and misleading results in variance-based SEM: an illustration and recommendations. Journal of the Association for Information Systems 13: 546–580.
Legendre, P. & L. Legendre, 2012. Numerical Ecology. Elsevier Science BV, Amsterdam.
Lehner, B. & G. Grill, 2013. Global river hydrography and network routing: baseline data and new approaches to study the world’s large river systems. Hydrological Processes 27: 2171–2186.
Lopes, T. M., D. Bailly, B. A. Almeida, N. C. L. Santos, B. C. G. Gimenez, G. O. Landgraf, P. C. L. Sales, M. S. Lima-Ribeiro, F. A. S. Cassemiro, T. F. Rangel, J. A. F. Diniz-Filho, A. A. Agostinho & L. C. Gomes, 2017. Two sides of a coin: effects of climate change on the native and non-native distribution of Colossoma macropomum in South America. PLoS ONE 12: e0179684.
Lourenço-de-Moraes, R., F. M. Lansac-Toha, L. T. F. Schwind, R. L. Arrieira, R. R. Rosa, L. C. Terribile, P. Lemes, T. F. Rangel, J. A. F. Diniz-Filho, R. P. Bastos & D. Bailly, 2019. Climate change will decrease the range size of snake species under negligible protection in the Brazilian Atlantic Forest hotspot. Scientific Reports 9: 8523.
Marmion, M., M. Parviainen, M. Luoto, R. K. Heikkinen & W. Thuiller, 2009. Evaluation of consensus methods in predictive species distribution modelling. Diversity and Distributions 15: 59–69.
Martens, K., 1994. Ostracoda speciation in ancient lakes: a review. Archiv für Hydrobiologie-Beiheft Ergebnisse der Limnologie 4: 203–222.
Martens, K., N. L. Würdig & F. Behen, 1998. Maxillopoda. Non-Marine Ostracoda. In Young, P. S. (ed.), Catalogue of Crustacea of Brazil. Museu Nacional, Série Livros 6, Rio de Janeiro: 45–65.
Martens, K., I. Schön, C. Meisch & D. J. Horne, 2008. Global diversity of ostracods (Ostracoda, Crustacea) in freshwater. Hydrobiologia 595: 185–193.
McGarigal, K., S. Cushman & S. Stafford, 2000. Multivariate Statistics for Wildlife and Ecology Research. Springer, New York: 283p.
McNyset, K. M., 2005. Use of ecological niche modelling to predict distributions of freshwater fish species in Kansas. Ecology of Freshwater Fish 14: 243–255.
Meisch, C., R. J. Smith & K. Martens, 2019. Subjective global checklist of the extant non-marine Ostracoda (Crustacea). European Journal of Taxonomy 492: 1–135.
Mesquita-Joanes, F., A. J. Smith & F. A. Viehberg. 2012. The ecology of Ostracoda across levels of biological organisation from individual to ecosystem: a review of recent developments and future potential. In Horne, D. J., J. A. Holmes, J. Rodríguez-Lázaro & F. A. Viehberg (eds.), Ostracoda as proxies for quaternary climate change. Developments in Quaternary Science Series, Elsevier, Amsterdam, 17: 15–35.
Moreno, E., C. Pérez-Martínez & J. M. Conde-Porcuna, 2016. Dispersal of zooplankton dormant propagules by wind and rain in two aquatic systems. Limnetica 35: 323–336.
Müller, G. H., 1912a. Ostracoda. Das Tierreich, Berlin: 1–434.
Müller, G. W., 1912b. Crustacea. Ostracoda. In Schulze, F. E. (ed), Das Tierreich. Auftrage Konigl Preuss, Akad Wiss: 1–434.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs & H. Wagner, 2016. Vegan: Community Ecology Package. R Package Version 2.4-1 [available on internet at https://cran.r-project.org/package=vegan]. Accessed 10 Feb 2019.
Paruelo, J. M., A. Beltrán, E. Jobbágy, O. E. Sala & R. A. Golluscio, 1998. The climate of Patagonia: general patterns and controls on biotic processes. Ecología Austral 8: 85–101.
Pereira, D., M. C. D. Mansur, L. D. S. Duarte, A. S. Oliveira, D. M. Pimpão, C. T. Callil, C. Ituarte, E. Parada, S. Peredo, G. Darrigran, F. Scarabino, C. Clavijo, G. Lara, I. C. Miyahira, M. T. R. Rodriguez & L. Lasso, 2014. Bivalve distribution in hydrographic regions in South America: historical overview and conservation. Hydrobiologia 735: 15–44.
Pereira, L. C., F. A. Lansac-Tohâ, K. Martens & J. Higuti, 2017. Biodiversity of ostracod communities (Crustacea, Ostracoda) in a tropical floodplain. Inland Waters 7: 323–332.
Peterson, A. T., M. Papes & J. Saberón, 2008. Rethinking receiver operating characteristic analysis applications in ecological niche modelling. Ecological Modelling 213: 63–72.
Peterson, A. T., J. Soberón, R. G. Pearson, R. P. Anderson, E. Martínez-Meyer, M. Nakamura & M. B. Araújo, 2011. Ecological Niches and Geographic Distributions. Monographs in Population Biology, 49. Princeton University Press, Princeton.
Petitpierre, B., O. Broennimann, C. Kueffer, C. Daehler & A. Guisan, 2017. Selecting predictors to maximize the transferability of species distribution models: lessons from crosscontinental plant invasions. Global Ecology and Biogeography 26: 275–287.
Phillips, S. J., R. P. Anderson & R. E. Schapire, 2006. Maximum entropy modelling of species geographic distributions. Ecological Modelling 190: 231–259.
Pinto, I. D. & C. B. Kotzian, 1961. Novos ostrácodes da família Darwinulidae e a variação das impressões musculares. Boletim do Instituto de Ciências Naturais da Universidade do Rio Grande do Sul 11: 5–64.
Pinto, R. L., C. E. F. Rocha & K. Martens, 2008. On the first terrestrial ostracod of the Superfamily Cytheroidea (Crustacea, Ostracoda): description of Intrepidocythere ibipora n. gen. n. sp. from forest leaf litter in São Paulo State, Brazil. Zootaxa 1828: 29–42.
Pinto, R. L., C. E. F. Rocha, G. Rossetti & K. Martens, 2013. Contribution to the knowledge of the genus Vestalenula Rossetti & Martens, 1998 (Crustacea, Ostracoda, Darwinulidae), with the description of a new species, V. carinata n. sp., from the island of Florianópolis, Brazil. Zootaxa 3666: 62–72.
Poquet, J. M. & F. Mesquita-Joanes, 2010. Combined effects of local environment and continental biogeography on the distribution of Ostracoda. Freshwater Biology 56: 448–469.
QGIS Development Team. 2019. QGIS Geographic Information System. [available on internet at http://qgis.osgeo.org]. Accessed on 02 Feb 2019.
R Development Core Team, 2019. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna [available on internet at http://www.r-project.org/]. Accessed 10 Feb 2019.
Rangel, T. F. & R. D. Loyola, 2012. Labeling ecological niche models. Natureza & Conservacão 10: 119–126.
Rangel, T. F., J. A. F. Diniz-Filho & L. M. Bini, 2010. SAM: a comprehensive application for spatial analysis in macroecology. Ecography 33: 46–50.
Reboita, M. S., M. A. Gan, R. P. Rocha & T. Ambrizzi, 2010. Regimes de precipitação na América do Sul: uma revisão bibliográfica. Revista Brasileira de Metereologia 25: 185–204.
Roessler, E. W., 1985. Estudios taxonomicos, ontogeneticos, ecologicos y etologicos sobre los ostracodos de agua dulce en Colombia. V. Estudio taxonomico del genero Chlamydotheca Saussure 1858, (Ostracoda, Podocopida, Cyprididae). Parte 1. Aspectos morfologicos de una nueva especie colombiana del genero Chlamydotheca. Caldasia 67: 329–354.
Rosa, J., R. Campos, K. Martens & J. Higuti. 2019. Spatial variation of ostracod (Crustacea, Ostracoda) egg banks in temporary lakes of a tropical floodplain. [submitted].
Rossi, V., D. Albini, G. Benassi & P. Menozzi, 2012. To rest in hydration: hatching phenology of resting eggs of Heterocypris incongruens (Crustacea: Ostracoda). Fundamental and Applied Limnology/Archiv für Hydrobiologie 181: 49–58.
Ruaro, R., E. O. Conceição, G. C. Silva, E. G. Cafofo, M. A. Angulo-Valencia, T. Mantovano, A. Pineda, A. C. M. Paula, B. F. Zanco, E. M. Capparros, G. A. Moresco, I. J. Oliveira, J. L. Antiqueira, J. Ernandes-Silva, J. V. F. Silva, J. R. P. Adelino, J. A. Santos, M. J. Ganassin, M. S. Iquematsu, G. O. Landgraf, P. Lemes, F. A. S. Cassemiro, V. Batista-Silva, J. A. D. Diniz-Filho, T. F. Rangel, A. A. Agostinho & D. Bailly, 2019. Climate change will decrease the range of a keystone fish species in La Plata River Basin, South America. Hydrobiologia 836: 1–19.
Sars, G. O., 1901. Contribuitions to the Knowledge of the Fresh-water “Entomostraca” of South America, as Shown by Artificial Hatching from Dried Material, by GO Sars. Part II. “Copepoda-Ostracoda”. Cammermeyers Forlag, Oslo.
Silva, C. V. & R. Henry, 2013. Aquatic macroinvertebrates associated with Eichhornia azurea (Swartz) Kunth and relationships with abiotic factors in marginal lentic ecosystems (São Paulo, Brazil). Brazilian Journal of Biology 73: 149–162.
Sneath, P. H. A. & R. R. Sokal, 1973. Numerical Taxonomy. W. H. Freeman, San Francisco: 573 p.
Soberón, J. & A. T. Peterson, 2005. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodiversity Informatics 2: 1–10.
Stockwell, D. R. B. & D. Peters, 1999. The GARP modelling system: problems and solutions to automated spatial predictions. International Journal of Geographical Information Science 13: 143–158.
Terribile, L. C., J. A. F. Diniz-Filho & P. De Marco, 2010. How many studies are necessary to compare niche-based models for geographic distributions? Inductive reasoning may fail at the end. Brazilian Journal of Biology 70: 263–269.
THE DOCUMENT FOUNDATION. LibreOffice. 2019.LibreOffice. [available on internet at http://www.r-project.org/]. Accessed 10 Feb 2019.
Thomaz, S. M., L. M. Bini & R. L. Bozelli, 2007. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579: 1–13.
Urbina-Cardona, J. N. & O. Flores-Villela, 2010. Ecological-niche modelling and prioritization of conservation-area networks for Mexican herpetofauna. Conservation Biology 24: 1031–1041.
Vari, R.P. & L. R. Malabarba, 1998. Neotropical ichthyology: an overview. In Malabarba, L. R., R.E. Reis, R.P. Vari, Z.M.S. Lucena & C. A. S. Lucena (eds), Phylogeny and classification of Neotropical fishes Edipucrs. Porto Alegre: pp. 1–12.
Wrozyna, C., W. E. Piller & M. Gross, 2014. Morphotypes of Cytheridella ilosvayi (Ostracoda) detected by softand hard part analyses. Crustaceana 87: 1043–1071.
Würdig, N. L. & I. D. Pinto, 1990. Diaphanocypris, a new ostracod genus occurring in South and Central America. Pesquisas, Instituto de Geociencias, Universidade federal do Rio Grande do Sul 17: 31–38.
Acknowledgements
We thank the Centre of Research in Limnology, Ichthyology and Aquaculture (Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura - Nupélia) and the Graduate Programme in Ecology of Inland Water Ecosystems (Programa de Pós-Graduação em Ecologia de Ambientes Aquáticos Continentais – PEA) of the State University of Maringá (Universidade Estadual de Maringá - UEM) for the logistic support. We would like to thank the Ministry of Science and Technology (MCT)/National Council for Scientific and Technological Development (CNPq) and Fundação Araucária for financial support. We also thank the National System of Biodiversity Research (SISBIOTA) and Long-Term Ecological Research (LTER -site 6) programmes and Academic Excellency Program (PROEX/Coordination of Improvement of Higher Education Personnel (CAPES). EOC, TM and RC would like to thank CAPES for granting their doctoral scholarships, DB thanks CNPQ/PDS for the post-doctoral fellowship and TFR thanks CNPq for the grant of research productivity. The State University of Maringá and the Royal Belgian Institute of Natural Sciences (RBINS, Brussels) have a bilateral Memorandum of Understanding regarding collaborative Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Koen Martens, Sidinei M. Thomaz, Diego Fontaneto & Luigi Naselli-Flores / Emerging Trends in Aquatic Ecology III
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIFF 16846 kb)
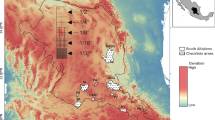
Figure S1 Climatic and environmental suitability patterns of ostracod species from Groups 2 and 3 in South America. Sy = Suitability
Supplementary material 2 (TIFF 15814 kb)
Figure S2 Climatic and environmental suitability patterns of ostracod species from Groups 3, 4, 6 and 7 in South America. Sy = Suitability
Supplementary material 3 (TIFF 17260 kb)
Figure S3 Climatic and environmental suitability patterns of ostracod species from Groups 6 and 7 in South America. Sy = Suitability
Supplementary material 4 (TIFF 16616 kb)
Figure S4 Climatic and environmental suitability patterns of ostracod species from Group 8 in South America. Sy = Suitability
Supplementary material 5 (TIFF 14793 kb)
Figure S5 Climatic and environmental suitability patterns of ostracod species from Group 8 in South America. Sy = Suitability
Supplementary material 6 (TIFF 16880 kb)
Figure S6 Climatic and environmental suitability patterns of ostracod species from Group 9 in South America. Sy = Suitability
Supplementary material 7 (TIFF 17332 kb)
Figure S7 Climatic and environmental suitability patterns of ostracod species from Groups 9 and 10 in South America. Sy = Suitability
Supplementary material 8 (TIFF 5560 kb)
Figure S8 Climatic and environmental suitability patterns of ostracod species from Group 10 in South America. Sy = Suitability
Supplementary material 9 (TIFF 19288 kb)
Figure S9 Spatial mapping of hydrologic and climatic variables in South America. FRMDN = elevation value of the stream, STRORD = order of the stream, TMAX = Maximum temperature of warmest month, TMIN = Minimum temperature of coldest month, PMAX = Precipitation of wettest month, PMIN = Precipitation of driest month
Rights and permissions
About this article
Cite this article
de Oliveira da Conceição, E., Mantovano, T., de Campos, R. et al. Mapping the observed and modelled intracontinental distribution of non-marine ostracods from South America. Hydrobiologia 847, 1663–1687 (2020). https://doi.org/10.1007/s10750-019-04136-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04136-6