Abstract
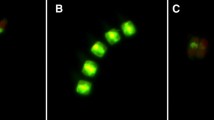
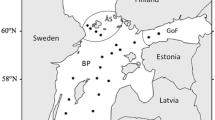
Despite the global importance of phytoplankton primary production, the ecological role of cell death as an important loss process in phytoplankton is poorly understood. To assess the significance of cell death in phytoplankton, we studied cell viability of dominant species in the canyon-shaped eutrophic Římov Reservoir (Czech Republic) at weekly and biweekly intervals from April to October 2011. Surface samples were taken from the lacustrine zone (near the dam, low nutrient level) and transition zone (near the river inflow, high nutrient level) of the reservoir. Moreover, samples from euphotic depth (1% of surface irradiance) were taken from the lacustrine zone. We used the membrane-impermeant nucleic acid dye SYTOX Green to examine seasonal and spatial differences in phytoplankton cell viability. Three species (diatoms Asterionella formosa, Fragilaria crotonensis, and cyanobacterium Aphanizomenon flos-aquae) were studied in detail. There was no difference in Asterionella cell viability among sampling sites. In the lacustrine zone, Fragilaria and Aphanizomenon exhibited lower viability than in the transition zone. In addition, Aphanizomenon viability was significantly lower at the euphotic depth. Nutrient levels were revealed as a factor influencing Fragilaria viability, while light availability was more important for Aphanizomenon. Our results evidenced that the importance of cell death, in particular phytoplankton taxa, varies both spatially and temporally. Moreover, our study indicates that coexisting taxa may differ in their capacity to cope with different environmental stressors.









Similar content being viewed by others
References
Agusti, S., 2004. Viability and niche segregation of Prochlorococcus and Synechococcus cells across the Central Atlantic Ocean. Aquatic Microbial Ecology 36(1): 53–59.
Agusti, S. & M. C. Sanchez, 2002. Cell viability in natural phytoplankton communities quantified by a membrane permeability probe. Limnology and Oceanography 47(3): 818–828.
Agusti, S., M. P. Satta, M. P. Mura & E. Benavent, 1998. Dissolved esterase activity as a tracer of phytoplankton lysis: evidence of high phytoplankton lysis rates in the northwestern Mediterranean. Limnology and Oceanography 43(8): 1836–1849.
Agusti, S., E. Alou, M. V. Hoyer, T. K. Frazer & D. E. Canfield, 2006. Cell death in lake phytoplankton communities. Freshwater Biology 51(8): 1496–1506.
Alonso-Laita, P. & S. Agusti, 2006. Contrasting patterns of phytoplankton viability in the subtropical NE Atlantic Ocean. Aquatic Microbial Ecology 43(1): 67–78.
Berges, J. A. & P. G. Falkowski, 1998. Physiological stress and cell death in marine phytoplankton: induction of proteases in response to nitrogen or light limitation. Limnology and Oceanography 43(1): 129–135.
Berman, T. & D. Wynne, 2005. Assessing phytoplankton lysis in Lake Kinneret. Limnology and Oceanography 50(2): 526–537.
Bidle, K. D. & P. G. Falkowski, 2004. Cell death in planktonic, photosynthetic microorganisms. Nature Reviews Microbiology 2(8): 643–655.
Brussaard, C. P. D. & R. Riegman, 1998. Influence of bacteria on phytoplankton cell mortality with phosphorus or nitrogen as the algal-growth-limiting nutrient. Aquatic Microbial Ecology 14(3): 271–280.
Brussaard, C. P. D., R. Riegman, A. A. M. Noordeloos, G. C. Cadee, H. Witte, A. J. Kop, G. Nieuwland, F. C. Vanduyl & R. P. M. Bak, 1995. Effects of grazing, sedimentation and phytoplankton cell-lysis on the structure of a coastal pelagic food-web. Marine Ecology Progress Series 123(1–3): 259–271.
Brussaard, C. P. D., A. A. M. Noordeloos & R. Riegman, 1997. Phytoplankton cell lysis. Phycologia 36(4): 12.
Brussaard, C. P. D., X. Mari, J. D. L. Van Bleijswijk & M. J. W. Veldhuis, 2005. A mesocosm study of Phaeocystis globosa (Prymnesiophyceae) population dynamics – II. Significance for the microbial community. Harmful Algae 4(5): 875–893.
Davey, H. M., 2011. Life, death, and in-between: meanings and methods in microbiology. Applied and Environmental Microbiology 77(16): 5571–5576.
Davey, H. M. & P. Hexley, 2011. Red but not dead? Membranes of stressed Saccharomyces cerevisiae are permeable to propidium iodide. Environmental Microbiology 13: 163–171.
Dokulil, M. T. & K. Teubner, 2000. Cyanobacterial dominance in lakes. Hydrobiologia 438(1–3): 1–12.
Franklin, D. J., C. P. D. Brussaard & J. A. Berges, 2006. What is the role and nature of programmed cell death in phytoplankton ecology? European Journal of Phycology 41(1): 1–14.
Franklin, D. J., C. J. Choi, C. Hughes, G. Malin & J. A. Berges, 2009. Effect of dead phytoplankton cells on the apparent efficiency of photosystem II. Marine Ecology Progress Series 382: 35–40.
Franklin, D. J., R. L. Airs, M. Fernandes, T. G. Bell, R. J. Bongaerts, J. A. Berges & G. Malin, 2012. Identification of senescence and death in Emiliania huxleyi and Thalassiosira pseudonana: cell staining, chlorophyll alterations, and dimethylsulfoniopropionate (DMSP) metabolism. Limnology and Oceanography 57(1): 305–317.
Garvey, M., B. Moriceau & U. Passow, 2007. Applicability of the FDA assay to determine the viability of marine phytoplankton under different environmental conditions. Marine Ecology Progress Series 352: 17–26.
Geider, R. J., E. H. Delucia, P. G. Falkowski, A. C. Finzi, J. P. Grime, J. Grace, T. M. Kana, J. La Roche, S. P. Long, B. A. Osborne, T. Platt, I. C. Prentice, J. A. Raven, W. H. Schlesinger, V. Smetacek, V. Stuart, S. Sathyendranath, R. B. Thomas, T. C. Vogelmann, P. Williams & F. I. Woodward, 2001. Primary productivity of planet earth: biological determinants and physical constraints in terrestrial and aquatic habitats. Global Change Biology 7(8): 849–882.
Gorokhova, E., L. Mattsson & A. M. Sundstrom, 2012. A comparison of TO-PRO-1 iodide and 5-CFDA-AM staining methods for assessing viability of planktonic algae with epifluorescence microscopy. Journal of Microbiological Methods 89(3): 216–221.
Hayakawa, M., K. Suzuki, H. Saito, K. Takahashi & S. Ito, 2008. Differences in cell viabilities of phytoplankton between spring and late summer in the northwest Pacific Ocean. Journal of Experimental Marine Biology and Ecology 360(2): 63–70.
Hillebrand, H., C. D. Durselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35(2): 403–424.
Jochem, F. J., 1999. Dark survival strategies in marine phytoplankton assessed by cytometric measurement of metabolic activity with fluorescein diacetate. Marine Biology 135(4): 721–728.
Kalff, J., 2002. Limnology: Inland Water Ecosystems. Prentice Hall, Upper Saddle River.
Kerr, J. F. R., A. H. Wyllie & A. R. Currie, 1972. Apoptosis – basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer 26(4): 239–257.
Kimmel, B. L., O. T. Lind & L. J. Paulson, 1990. Reservoir primary production. In Thornton, K. W., B. L. Kimmel & F. E. Paine (eds), Reservoir Limnology: Ecological Perspectives. Wiley, New York: 133–193.
Kirchman, D. L., 1999. Oceanography – phytoplankton death in the sea. Nature 398(6725): 293–294.
Konopka, A., J. Kromkamp & L. R. Mur, 1987. Regulation of gas vesicle content and buoyancy in light-limited or phosphate-limited cultures of Aphanizomenon flos-aquae (Cyanophyta). Journal of Phycology 23(1): 70–78.
Kopáček, J. & J. Hejzlar, 1993. Semi-micro determination of total phosphorus in fresh-waters with perchloric-acid digestion. International Journal of Environmental Analytical Chemistry 53(3): 173–183.
Lee, D. Y. & G. Y. Rhee, 1997. Kinetics of cell death in the cyanobacterium Anabaena flos-aquae and the production of dissolved organic carbon. Journal of Phycology 33(6): 991–998.
Lorenzen, C. J., 1967. Determination of chlorophyll and phaeo-pigments: spectrophotometric equations. Limnology and Oceanography 12: 2243–2246.
Lund, J. W. G., C. Kipling & E. D. Cren, 1958. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 11(2): 143–170.
Machado, M. D. & E. V. Soares, 2012. Development of a short-term assay based on the evaluation of the plasma membrane integrity of the alga Pseudokirchneriella subcapitata. Applied Microbiology and Biotechnology 95(4): 1035–1042.
Mackereth, F. J. H., J. Heron & J. F. Talling, 1989. Water Analysis: Some Revised Methods for Limnologists. Freshwater Biological Association, Ambleside.
Murphy, J. & J. P. Riley, 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27: 31–36.
Nedoma, J., A. Štrojsová, J. Vrba, J. Komárková & K. Šimek, 2003. Extracellular phosphatase activity of natural plankton studied with ELF97 phosphate: fluorescence quantification and labelling kinetics. Environmental Microbiology 5(6): 462–472.
Peperzak, L. & C. P. D. Brussaard, 2011. Flow cytometric applicability of fluorescent vitality probes on phytoplankton. Journal of Phycology 47(3): 692–702.
Procházková, L., 1959. Bestimmung Der Nitrate Im Wasser. Fresenius Zeitschrift Fur Analytische Chemie 167(4): 254–260.
R Core Team, 2013. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria [available on internet at http://www.R-project.org/].
Reavie, E. D., A. A. Cangelosi & L. E. Allinger, 2010. Assessing ballast water treatments: evaluation of viability methods for ambient freshwater microplankton assemblages. Journal of Great Lakes Research 36(3): 540–547.
Roth, B. L., M. Poot, S. T. Yue & P. J. Millard, 1997. Bacterial viability and antibiotic susceptibility testing with SYTOX Green nucleic acid stain. Applied and Environmental Microbiology 63(6): 2421–2431.
Rychtecký, P. & P. Znachor, 2011. Spatial heterogeneity and seasonal succession of phytoplankton along the longitudinal gradient in a eutrophic reservoir. Hydrobiologia 663(1): 175–186.
Saros, J. E., T. J. Michel, S. J. Interlandi & A. P. Wolfe, 2005. Resource requirements of Asterionella formosa and Fragilaria crotonensis in oligotrophic alpine lakes: implications for recent phytoplankton community reorganizations. Canadian Journal of Fisheries and Aquatic Sciences 62(7): 1681–1689.
Segovia, M. & J. A. Berges, 2009. Inhibition of caspase-like activities prevents the appearance of reactive oxygen species and dark-induced apoptosis in the unicellular chlorophyte Dunaliella tertiolecta. Journal of Phycology 45(5): 1116–1126.
Sigee, D. C., A. Selwyn, P. Gallois & A. P. Dean, 2007. Patterns of cell death in freshwater colonial cyanobacteria during the late summer bloom. Phycologia 46(3): 284–292.
Vandonk, E. & S. S. Kilham, 1990. Temperature effects on silicon-limited and phosphorus-limited growth and competitive interactions among 3 diatoms. Journal of Phycology 26(1): 40–50.
Veldhuis, M. J. W. & G. W. Kraay, 2000. Application of flow cytometry in marine phytoplankton research: current applications and future perspectives. Scientia Marina 64(2): 121–134.
Veldhuis, M. J. W., T. L. Cucci & M. E. Sieracki, 1997. Cellular DNA content of marine phytoplankton using two new fluorochromes: taxonomic and ecological implications. Journal of Phycology 33(3): 527–541.
Veldhuis, M. J. W., G. W. Kraay & K. R. Timmermans, 2001. Cell death in phytoplankton: correlation between changes in membrane permeability, photosynthetic activity, pigmentation and growth. European Journal of Phycology 36(2): 167–177.
Vives-Rego, J., P. Lebaron & G. Nebe-von Caron, 2000. Current and future applications of flow cytometry in aquatic microbiology. FEMS Microbiology Reviews 24(4): 429–448.
Ye, L. L., X. L. Shi, X. D. Wu & F. X. Kong, 2011. Phytoplankton cell lysis after water bloom in a eutrophic freshwater lake Taihu (China). International Review of Hydrobiology 96(6): 709–719.
Zetsche, E. M. & F. J. R. Meysman, 2012. Dead or alive? Viability assessment of micro- and mesoplankton. Journal of Plankton Research 34(6): 493–509.
Znachor, P. & J. Nedoma, 2008. Application of the pdmpo technique in studying silica deposition in natural populations of Fragilaria crotonensis (Bacillariophyceae) at different depths in a eutrophic reservoir. Journal of Phycology 44(2): 518–525.
Znachor, P., V. Visocká, J. Nedoma & P. Rychtecký, 2013. Spatial heterogeneity of diatom silicification and growth in a eutrophic reservoir. Freshwater Biology 58(9): 1889–1902.
Acknowledgments
We gratefully acknowledge all the people who participated in field sampling and laboratory analysis. This study was supported by the Grant Agency of the Czech Republic (Grant numbers P504/11/2177, P504/11/2182) and by the Grant Agency of the University of South Bohemia in České Budějovice (Grant number 142/2010/P). English correction was made by Anton Baer. We also thank Petr Šmilauer and Terezie Rychtecká for their help with LME models and two anonymous reviewers for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisak
Rights and permissions
About this article
Cite this article
Rychtecký, P., Znachor, P. & Nedoma, J. Spatio-temporal study of phytoplankton cell viability in a eutrophic reservoir using SYTOX Green nucleic acid stain. Hydrobiologia 740, 177–189 (2014). https://doi.org/10.1007/s10750-014-1952-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-1952-z