Abstract
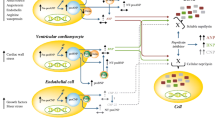
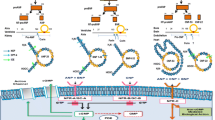
Heart failure (HF) is a worldwide disease with high levels of morbidity and mortality. The pathogenesis of HF is complicated and involves imbalances in hormone and electrolyte. B-type natriuretic peptide (BNP) has served as a biomarker of HF severity, and in recent years, it has been used to treat the disease, thanks to its cardio-protective effects, such as diuresis, natriuresis, and vasodilatation. In stage C/D HF, symptoms are severe despite elevated BNP. Disturbances in Ca2+ homeostasis are often a dominating feature of the disease, causing Ca2+-regulatory protein dysfunction, including reduced expression and activity of sarcoplasmic reticulum Ca2+-ATPase2a (SERCA2a), impaired ryanodine receptors (RYRs) function, intensive Na+-Ca2+ exchanger (NCX), and downregulation of S100A1. The relationship between natriuretic peptides (NPs) and Ca2+-regulatory proteins has been widely studied and represents important mechanisms in the etiology of HF. In this review, we present evidence that BNP may regulate Ca2+-regulatory proteins, in particular, suppressing SERCA2a and S100A1 expression. However, relationships between BNP and other Ca2+-regulatory proteins remain vague.



Similar content being viewed by others
References
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 18(8):891–975. https://doi.org/10.1002/ejhf.592
Metra M, Teerlink JR (2017) Heart failure. Lancet 390(10106):1981–1995. https://doi.org/10.1016/s0140-6736(17)31071-1
Wu A (2018) Heart Failure. Ann Intern Med 168(11):ITC81–ITC96. https://doi.org/10.7326/AITC201806050
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C (2017) 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation 136(6):e137–e161. https://doi.org/10.1161/cir.0000000000000509
Baughman KL (2002) B-type natriuretic peptide -- a window to the heart. N Engl J Med 347(3):158–159. https://doi.org/10.1056/NEJMp020057
de Lemos JA, McGuire DK, Drazner MH (2003) B-type natriuretic peptide in cardiovascular disease. Lancet 362(9380):316–322. https://doi.org/10.1016/s0140-6736(03)13976-1
Daniels LB, Maisel AS (2007) Natriuretic peptides. J Am Coll Cardiol 50(25):2357–2368. https://doi.org/10.1016/j.jacc.2007.09.021
Nakagawa O, Ogawa Y, Itoh H, Suga S, Komatsu Y, Kishimoto I, Nishino K, Yoshimasa T, Nakao K (1995) Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an "emergency" cardiac hormone against ventricular overload. J Clin Invest 96(3):1280–1287. https://doi.org/10.1172/JCI118162
Wiese S, Breyer T, Dragu A, Wakili R, Burkard T, Schmidt-Schweda S, Fuchtbauer EM, Dohrmann U, Beyersdorf F, Radicke D, Holubarsch CJ (2000) Gene expression of brain natriuretic peptide in isolated atrial and ventricular human myocardium: influence of angiotensin II and diastolic fiber length. Circulation 102(25):3074–3079. https://doi.org/10.1161/01.cir.102.25.3074
Sudoh T, Maekawa K, Kojima M, Minamino N, Kangawa K, Matsuo H (1989) Cloning and sequence analysis of cDNA encoding a precursor for human brain natriuretic peptide. Biochem Biophys Res Commun 159(3):1427–1434. https://doi.org/10.1016/0006-291x(89)92269-9
Zhang ZL, Li R, Yang FY, Xi L (2018) Natriuretic peptide family as diagnostic/prognostic biomarker and treatment modality in management of adult and geriatric patients with heart failure: remaining issues and challenges. J Geriatr Cardiol 15(8):540–546. https://doi.org/10.11909/j.issn.1671-5411.2018.08.008
Liang F, O’Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, Apple FS, Maisel AS, Pollitt NS, Protter AA (2007) Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol 49(10):1071–1078. https://doi.org/10.1016/j.jacc.2006.10.063
de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E (2001) The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 345(14):1014–1021. https://doi.org/10.1056/NEJMoa011053
Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H et al (1991) Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest 87(4):1402–1412. https://doi.org/10.1172/JCI115146
Yamamoto K, Burnett JC Jr, Jougasaki M, Nishimura RA, Bailey KR, Saito Y, Nakao K, Redfield MM (1996) Superiority of brain natriuretic peptide as a hormonal marker of ventricular systolic and diastolic dysfunction and ventricular hypertrophy. Hypertension 28(6):988–994. https://doi.org/10.1161/01.hyp.28.6.988
Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, PA MC, Breathing Not Properly Multinational Study I (2002) Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 347(3):161–167. https://doi.org/10.1056/NEJMoa020233
Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr (2002) Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 40(5):976–982. https://doi.org/10.1016/s0735-1097(02)02059-4
Ledwidge M, Gallagher J, Conlon C, Tallon E, O'Connell E, Dawkins I, Watson C, O'Hanlon R, Bermingham M, Patle A, Badabhagni MR, Murtagh G, Voon V, Tilson L, Barry M, McDonald L, Maurer B, McDonald K (2013) Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 310(1):66–74. https://doi.org/10.1001/jama.2013.7588
Berger R (2002) B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 105(20):2392–2397. https://doi.org/10.1161/01.cir.0000016642.15031.34
Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS (2004) Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 350(7):655–663. https://doi.org/10.1056/NEJMoa031994
Doust JA, Pietrzak E, Dobson A, Glasziou P (2005) How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ 330(7492):625. https://doi.org/10.1136/bmj.330.7492.625
McDonagh TA, Robb SD, Murdoch DR, Morton JJ, Ford I, Morrison CE, Tunstall-Pedoe H, McMurray JJV, Dargie HJ (1998) Biochemical detection of left-ventricular systolic dysfunction. Lancet 351(9095):9–13. https://doi.org/10.1016/s0140-6736(97)03034-1
Logeart D, Thabut G, Jourdain P, Chavelas C, Beyne P, Beauvais F, Bouvier E, Solal AC (2004) Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol 43(4):635–641. https://doi.org/10.1016/j.jacc.2003.09.044
Verdiani V, Nozzoli C, Bacci F, Cecchin A, Rutili MS, Paladini S, Olivotto I (2005) Pre-discharge B-type natriuretic peptide predicts early recurrence of decompensated heart failure in patients admitted to a general medical unit. Eur J Heart Fail 7(4):566–571. https://doi.org/10.1016/j.ejheart.2004.12.006
Anand IS (2003) Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the valsartan heart failure trial (Val-HeFT). Circulation 107(9):1278–1283. https://doi.org/10.1161/01.cir.0000054164.99881.00
Chen HH, Grantham JA, Schirger JA, Jougasaki M, Redfield MM, Burnett JC Jr (2000) Subcutaneous administration of brain natriuretic peptide in experimental heart failure. J Am Coll Cardiol 36(5):1706–1712. https://doi.org/10.1016/s0735-1097(00)00911-6
Chen HH, Schirger JA, Chau WL, Jougasaki M, Lisy O, Redfield MM, Barclay PT, Burnett JC Jr (1999) Renal response to acute neutral endopeptidase inhibition in mild and severe experimental heart failure. Circulation 100(24):2443–2448. https://doi.org/10.1161/01.cir.100.24.2443
Chen HH (2007) Heart failure: a state of brain natriuretic peptide deficiency or resistance or both! J Am Coll Cardiol 49(10):1089–1091. https://doi.org/10.1016/j.jacc.2006.12.013
Hawkridge AM, Heublein DM, Bergen HR 3rd, Cataliotti A, Burnett JC Jr, Muddiman DC (2005) Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci U S A 102(48):17442–17447. https://doi.org/10.1073/pnas.0508782102
Forfia PR, Lee M, Tunin RS, Mahmud M, Champion HC, Kass DA (2007) Acute phosphodiesterase 5 inhibition mimics hemodynamic effects of B-type natriuretic peptide and potentiates B-type natriuretic peptide effects in failing but not normal canine heart. J Am Coll Cardiol 49(10):1079–1088. https://doi.org/10.1016/j.jacc.2006.08.066
Jiang J, Pristera N, Wang W, Zhang X, Wu Q (2010) Effect of sialylated O-glycans in pro-brain natriuretic peptide stability. Clin Chem 56(6):959–966. https://doi.org/10.1373/clinchem.2009.140558
Semenov AG, Tamm NN, Seferian KR, Postnikov AB, Karpova NS, Serebryanaya DV, Koshkina EV, Krasnoselsky MI, Katrukha AG (2010) Processing of pro-B-type natriuretic peptide: furin and corin as candidate convertases. Clin Chem 56(7):1166–1176. https://doi.org/10.1373/clinchem.2010.143883
Diez J (2017) Chronic heart failure as a state of reduced effectiveness of the natriuretic peptide system: implications for therapy. Eur J Heart Fail 19(2):167–176. https://doi.org/10.1002/ejhf.656
Tsutamoto T, Wada A, Maeda K, Hisanaga T, Maeda Y, Fukai D, Ohnishi M, Sugimoto Y, Kinoshita M (1997) Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation 96 (2):509-516.
Semenov AG, Tamm NN, Apple FS, Schulz KM, Love SA, Ler R, Feygina EE, Katrukha AG (2017) Searching for a BNP standard: Glycosylated proBNP as a common calibrator enables improved comparability of commercial BNP immunoassays. Clin Biochem 50(4-5):181–185. https://doi.org/10.1016/j.clinbiochem.2016.11.003
Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, Januzzi JL Jr, Mark DB, Pina IL, Passmore G, Whellan DJ, Yang H, Cooper LS, Leifer ES, Desvigne-Nickens P, O’Connor CM (2017) Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 318(8):713–720. https://doi.org/10.1001/jama.2017.10565
O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM (2011) Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 365(1):32–43. https://doi.org/10.1056/NEJMoa1100171
Sackner-Bernstein JD, Skopicki HA, Aaronson KD (2005) Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation 111(12):1487–1491. https://doi.org/10.1161/01.CIR.0000159340.93220.E4
Yancy CW, Krum H, Massie BM, Silver MA, Stevenson LW, Cheng M, Kim SS, Evans R, Investigators FI (2008) Safety and efficacy of outpatient nesiritide in patients with advanced heart failure: results of the second follow-up serial infusions of nesiritide (FUSION II) trial. Circ Heart Fail 1(1):9–16. https://doi.org/10.1161/CIRCHEARTFAILURE.108.767483
McKie PM, Schirger JA, Benike SL, Harstad LK, Slusser JP, Hodge DO, Redfield MM, Burnett JC Jr, Chen HH (2016) Chronic subcutaneous brain natriuretic peptide therapy in asymptomatic systolic heart failure. Eur J Heart Fail 18(4):433–441. https://doi.org/10.1002/ejhf.468
Sodi R, Dubuis E, Shenkin A, Hart G (2008) B-type natriuretic peptide (BNP) attenuates the L-type calcium current and regulates ventricular myocyte function. Regul Pept 151(1-3):95–105. https://doi.org/10.1016/j.regpep.2008.06.006
Lehnart SE, Schillinger W, Pieske B, Prestle J, Just H, Hasenfuss G (1998) Sarcoplasmic reticulum proteins in heart failure. Ann N Y Acad Sci 853:220–230. https://doi.org/10.1111/j.1749-6632.1998.tb08270.x
Arai M, Matsui H, Periasamy M (1994) Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ Res 74(4):555–564. https://doi.org/10.1161/01.res.74.4.555
Zhang P, Toyoshima C, Yonekura K, Green NM, Stokes DL (1998) Structure of the calcium pump from sarcoplasmic reticulum at 8-A resolution. Nature 392(6678):835–839. https://doi.org/10.1038/33959
Nagai R, Zarain-Herzberg A, Brandl CJ, Fujii J, Tada M, MacLennan DH, Alpert NR, Periasamy M (1989) Regulation of myocardial Ca2+-ATPase and phospholamban mRNA expression in response to pressure overload and thyroid hormone. Proc Natl Acad Sci U S A 86(8):2966–2970. https://doi.org/10.1073/pnas.86.8.2966
Bers DM (2002) Cardiac excitation-contraction coupling. Nature 415(6868):198–205. https://doi.org/10.1038/415198a
Kho C, Lee A, Hajjar RJ (2012) Altered sarcoplasmic reticulum calcium cycling—targets for heart failure therapy. Nat Rev Cardiol 9(12):717–733. https://doi.org/10.1038/nrcardio.2012.145
Movsesian MA, Colyer J, Wang JH, Krall J (1990) Phospholamban-mediated stimulation of Ca2+ uptake in sarcoplasmic reticulum from normal and failing hearts. J Clin Invest 85(5):1698–1702. https://doi.org/10.1172/JCI114623
del Monte F, Harding SE, Dec GW, Gwathmey JK, Hajjar RJ (2002) Targeting phospholamban by gene transfer in human heart failure. Circulation 105(8):904–907. https://doi.org/10.1161/hc0802.105564
Schwinger RH, Munch G, Bolck B, Karczewski P, Krause EG, Erdmann E (1999) Reduced Ca(2+)-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J Mol Cell Cardiol 31(3):479–491. https://doi.org/10.1006/jmcc.1998.0897
Schmitt JP, Ahmad F, Lorenz K, Hein L, Schulz S, Asahi M, Maclennan DH, Seidman CE, Seidman JG, Lohse MJ (2009) Alterations of phospholamban function can exhibit cardiotoxic effects independent of excessive sarcoplasmic reticulum Ca2+-ATPase inhibition. Circulation 119(3):436–444. https://doi.org/10.1161/CIRCULATIONAHA.108.783506
Medeiros A, Biagi DG, Sobreira TJ, de Oliveira PS, Negrao CE, Mansur AJ, Krieger JE, Brum PC, Pereira AC (2011) Mutations in the human phospholamban gene in patients with heart failure. Am Heart J 162(6):1088–1095 e1081. https://doi.org/10.1016/j.ahj.2011.07.028
Schmitt JP (2003) Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 299(5611):1410–1413. https://doi.org/10.1126/science.1081578
Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M (1993) Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res 72(2):463–469. https://doi.org/10.1161/01.res.72.2.463
Hajjar RJ, Kang JX, Gwathmey JK, Rosenzweig A (1997) Physiological effects of adenoviral gene transfer of sarcoplasmic reticulum calcium ATPase in isolated rat myocytes. Circulation 95(2):423–429. https://doi.org/10.1161/01.cir.95.2.423
Frank K, Bolck B, Bavendiek U, Schwinger RH (1998) Frequency dependent force generation correlates with sarcoplasmic calcium ATPase activity in human myocardium. Basic Res Cardiol 93(5):405–411. https://doi.org/10.1007/s003950050109
Schwinger RHG, Böhm M, Schmidt U, Karczewski P, Bavendiek U, Flesch M, Krause E-G, Erdmann E (1995) Unchanged protein levels of SERCA II and phospholamban but reduced Ca2+ uptake and Ca2+-ATPase activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation 92(11):3220–3228. https://doi.org/10.1161/01.cir.92.11.3220
Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H (1994) Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res 75(3):434–442. https://doi.org/10.1161/01.res.75.3.434
Mercadier JJ, Lompre AM, Duc P, Boheler KR, Fraysse JB, Wisnewsky C, Allen PD, Komajda M, Schwartz K (1990) Altered sarcoplasmic reticulum Ca2(+)-ATPase gene expression in the human ventricle during end-stage heart failure. J Clin Invest 85(1):305–309. https://doi.org/10.1172/JCI114429
Cain BS, Meldrum DR, Joo KS, Wang JF, Meng X, Cleveland JC Jr, Banerjee A, Harken AH (1998) Human SERCA2a levels correlate inversely with age in senescent human myocardium. J Am Coll Cardiol 32(2):458–467. https://doi.org/10.1016/s0735-1097(98)00233-2
Brillantes AM, Allen P, Takahashi T, Izumo S, Marks AR (1992) Differences in cardiac calcium release channel (ryanodine receptor) expression in myocardium from patients with end-stage heart failure caused by ischemic versus dilated cardiomyopathy. Circ Res 71(1):18–26. https://doi.org/10.1161/01.res.71.1.18
Bers D (2004) Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol 37(2):417–429. https://doi.org/10.1016/j.yjmcc.2004.05.026
Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR (2000) PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101(4):365–376. https://doi.org/10.1016/s0092-8674(00)80847-8
Fabiato A (1983) Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol 245(1):C1–C14. https://doi.org/10.1152/ajpcell.1983.245.1.C1
Marks AR (2001) Ryanodine receptors/calcium release channels in heart failure and sudden cardiac death. J Mol Cell Cardiol 33(4):615–624. https://doi.org/10.1006/jmcc.2000.1343
Walweel K, Molenaar P, Imtiaz MS, Denniss A, dos Remedios C, van Helden DF, Dulhunty AF, Laver DR, Beard NA (2017) Ryanodine receptor modification and regulation by intracellular Ca2+ and Mg2+ in healthy and failing human hearts. J Mol Cell Cardiol 104:53–62. https://doi.org/10.1016/j.yjmcc.2017.01.016
Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH (2007) Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res 101(8):819–829. https://doi.org/10.1161/CIRCRESAHA.107.153007
Litwin SE (2006) “Ryanogate”: who leaked the calcium? Circ Res 98(2):165–168. https://doi.org/10.1161/01.RES.0000204551.09930.84
Wehrens XH, Lehnart SE, Reiken SR, Marks AR (2004) Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res 94(6):e61–e70. https://doi.org/10.1161/01.RES.0000125626.33738.E2
Reiken S, Gaburjakova M, Gaburjakova J, He K-l, Prieto A, Becker E, G-h Y, Wang J, Burkhoff D, Marks AR (2001) β-Adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation 104(23):2843–2848. https://doi.org/10.1161/hc4701.099578
Ullrich ND, Valdivia HH, Niggli E (2012) PKA phosphorylation of cardiac ryanodine receptor modulates SR luminal Ca2+ sensitivity. J Mol Cell Cardiol 53(1):33–42. https://doi.org/10.1016/j.yjmcc.2012.03.015
Gyorke I, Hester N, Jones LR, Gyorke S (2004) The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J 86(4):2121–2128. https://doi.org/10.1016/S0006-3495(04)74271-X
Hashambhoy YL, Greenstein JL, Winslow RL (2010) Role of CaMKII in RyR leak, EC coupling and action potential duration: a computational model. J Mol Cell Cardiol 49(4):617–624. https://doi.org/10.1016/j.yjmcc.2010.07.011
Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM (2005) Ca2+/calmodulin–dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res 97 (12):1314-1322. doi:https://doi.org/10.1161/01.res.0000194329.41863.89
Blaustein MP, Lederer WJ (1999) Sodium/calcium exchange: its physiological implications. Physiol Rev 79(3):763–854. https://doi.org/10.1152/physrev.1999.79.3.763
Komuro I, Wenninger KE, Philipson KD, Izumo S (1992) Molecular cloning and characterization of the human cardiac Na+/Ca2+ exchanger cDNA. Proc Natl Acad Sci U S A 89(10):4769–4773. https://doi.org/10.1073/pnas.89.10.4769
Eisner DA, Choi HS, Diaz ME, O'Neill SC, Trafford AW (2000) Integrative analysis of calcium cycling in cardiac muscle. Circ Res 87(12):1087–1094. https://doi.org/10.1161/01.res.87.12.1087
Piacentino V, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR (2003) Cellular Basis of Abnormal Calcium Transients of Failing Human Ventricular Myocytes. Circulation Research 92(6):651–658. https://doi.org/10.1161/01.res.0000062469.83985.9b
Bers D, Christensen D, Nguyen T (1988) Can Ca entry via Na-Ca exchange directly activate cardiac muscle contraction? Journal of Molecular and Cellular Cardiology 20(5):405–414. https://doi.org/10.1016/s0022-2828(88)80132-9
Ottolia M, Torres N, Bridge JH, Philipson KD, Goldhaber JI (2013) Na/Ca exchange and contraction of the heart. J Mol Cell Cardiol 61:28–33. https://doi.org/10.1016/j.yjmcc.2013.06.001
Sipido KR, Volders PG, de Groot SH, Verdonck F, Van de Werf F, Wellens HJ, Vos MA (2000) Enhanced Ca(2+) release and Na/Ca exchange activity in hypertrophied canine ventricular myocytes: potential link between contractile adaptation and arrhythmogenesis. Circulation 102(17):2137–2144. https://doi.org/10.1161/01.cir.102.17.2137
Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM (2001) Arrhythmogenesis and contractile dysfunction in heart failure. Circ Res 88(11):1159–1167. https://doi.org/10.1161/hh1101.091193
Wei SK, Ruknudin AM, Shou M, McCurley JM, Hanlon SU, Elgin E, Schulze DH, Haigney MC (2007) Muscarinic modulation of the sodium-calcium exchanger in heart failure. Circulation 115(10):1225–1233. https://doi.org/10.1161/CIRCULATIONAHA.106.650416
Sipido K (2002) Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: a new target for therapy? Cardiovascular Research 53(4):782–805. https://doi.org/10.1016/s0008-6363(01)00470-9
Studer R, Reinecke H, Bilger J, Eschenhagen T, Bohm M, Hasenfuss G, Just H, Holtz J, Drexler H (1994) Gene expression of the cardiac Na(+)-Ca2+ exchanger in end-stage human heart failure. Circ Res 75(3):443–453. https://doi.org/10.1161/01.res.75.3.443
Reinecke H, Studer R, Vetter R, Holtz J, Drexler H (1996) Cardiac Na+/Ca2+ exchange activity in patients with end-stage heart failure. Cardiovasc Res 31(1):48–54
Schwinger RHG, Wang J, Frank K, Müller-Ehmsen J, Brixius K, McDonough AA, Erdmann E (1999) Reduced Sodium Pump α1, α3, and β1-Isoform Protein Levels and Na+,K+-ATPase Activity but Unchanged Na+-Ca2+Exchanger Protein Levels in Human Heart Failure. Circulation 99(16)):2105–2112. https://doi.org/10.1161/01.cir.99.16.2105
Most P, Remppis A, Pleger ST, Katus HA, Koch WJ (2007) S100A1: a novel inotropic regulator of cardiac performance. Transition from molecular physiology to pathophysiological relevance. Am J Phys Regul Integr Comp Phys 293(2):R568–R577. https://doi.org/10.1152/ajpregu.00075.2007
Kiewitz R, Lyons GE, Schafer BW, Heizmann CW (2000) Transcriptional regulation of S100A1 and expression during mouse heart development. Biochim Biophys Acta 1498(2-3):207–219. https://doi.org/10.1016/s0167-4889(00)00097-5
Ehlermann P, Remppis A, Guddat O, Weimann J, Schnabel PA, Motsch J, Heizmann CW, Katus HA (2000) Right ventricular upregulation of the Ca(2+) binding protein S100A1 in chronic pulmonary hypertension. Biochim Biophys Acta 1500 (2):249-255. https://doi.org/10.1016/s0925-4439(99)00106-4
Kettlewell S, Most P, Currie S, Koch WJ, Smith GL (2005) S100A1 increases the gain of excitation-contraction coupling in isolated rabbit ventricular cardiomyocytes. J Mol Cell Cardiol 39(6):900–910. https://doi.org/10.1016/j.yjmcc.2005.06.018
Reppel M, Sasse P, Piekorz R, Tang M, Roell W, Duan Y, Kletke A, Hescheler J, Nürnberg B, Fleischmann BK (2005) S100A1 Enhances the L-type Ca2+ current in embryonic mouse and neonatal rat ventricular cardiomyocytes. J Biol Chem 280(43):36019–36028. https://doi.org/10.1074/jbc.M504750200
Reppel M, Fleischmann BK, Reuter H, Pillekamp F, Schunkert H, Hescheler J (2007) Regulation of Na+/Ca2+ exchange current in the normal and failing heart. Ann N Y Acad Sci 1099(1):361–372. https://doi.org/10.1196/annals.1387.065
Most P, Seifert H, Gao E, Funakoshi H, Völkers M, Jr H, Remppis A, Pleger ST, DeGeorge BR, Eckhart AD, Feldman AM, Koch WJ (2006) Cardiac S100A1 protein levels determine contractile performance and propensity toward heart failure after myocardial infarction. Circulation 114(12):1258–1268. https://doi.org/10.1161/circulationaha.106.622415
Bennett MK, Sweet WE, Baicker-McKee S, Looney E, Karohl K, Mountis M, Tang WH, Starling RC, Moravec CS (2014) S100A1 in human heart failure: lack of recovery following left ventricular assist device support. Circ Heart Fail 7(4):612–618. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000849
Fargnoli AS, Katz MG, Williams RD, Kendle AP, Steuerwald N, Bridges CR (2015) Liquid jet delivery method featuring S100A1 gene therapy in the rodent model following acute myocardial infarction. Gene Ther 23(2):151–157. https://doi.org/10.1038/gt.2015.100
Brinks H, Rohde D, Voelkers M, Qiu G, Pleger ST, Herzog N, Rabinowitz J, Ruhparwar A, Silvestry S, Lerchenmuller C, Mather PJ, Eckhart AD, Katus HA, Carrel T, Koch WJ, Most P (2011) S100A1 genetically targeted therapy reverses dysfunction of human failing cardiomyocytes. J Am Coll Cardiol 58(9):966–973. https://doi.org/10.1016/j.jacc.2011.03.054
Pleger ST, Most P, Boucher M, Soltys S, Chuprun JK, Pleger W, Gao E, Dasgupta A, Rengo G, Remppis A, Katus HA, Eckhart AD, Rabinowitz JE, Koch WJ (2007) Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation 115(19):2506–2515. https://doi.org/10.1161/CIRCULATIONAHA.106.671701
Most P, Pleger ST, Völkers M, Heidt B, Boerries M, Weichenhan D, Löffler E, Janssen PML, Eckhart AD, Martini J, Williams ML, Katus HA, Remppis A, Koch WJ (2004) Cardiac adenoviral S100A1 gene delivery rescues failing myocardium. J Clin Investig 114(11):1550–1563. https://doi.org/10.1172/jci21454
Takahashi T, Allen PD, Izumo S (1992) Expression of A-, B-, and C-type natriuretic peptide genes in failing and developing human ventricles. Correlation with expression of the Ca(2+)-ATPase gene. Circulation Research 71 (1):9-17. https://doi.org/10.1161/01.res.71.1.9
Howarth FC, Al-Shamsi N, Al-Qaydi M, Al-Mazrouei M, Qureshi A, Chandranath SI, Kazzam E, Adem A (2006) Effects of brain natriuretic peptide on contraction and intracellular Ca2+ in ventricular myocytes from the streptozotocin-induced diabetic rat. Ann N Y Acad Sci 1084:155–165. https://doi.org/10.1196/annals.1372.007
Fares N, Nader L, Saliba Y, Aftimos G, Gebara V (2010) B-type natriuretic peptide modulates the action potential and the L-type calcium current in adult rat heart muscle cells. J Med Liban 58(4):222–227
de Boer RA, Henning RH, Suurmeijer AJ, Pinto YM, Olthof E, Kirkels JH, van Gilst WH, Crijns HJ, van Veldhuisen DJ (2001) Early expression of natriuretic peptides and SERCA in mild heart failure: association with severity of the disease. Int J Cardiol 78(1):5–12. https://doi.org/10.1016/s0167-5273(00)00440-x
Ohkusa T, Noma T, Ueyama T, Hisamatsu Y, Yano M, Esato K, Nakazawa A, Matsuzaki M (1997) Differences in sarcoplasmic reticulum gene expression in myocardium from patients undergoing cardiac surgery. Quantification of steady-state levels of messenger RNA using the reverse transcription-polymerase chain reaction. Heart and Vessels 12(1):1–9. https://doi.org/10.1007/bf01747496
Leszek P, Szperl M, Klisiewicz A, Janas J, Rozanski J, Rywik T, Piotrowski W, Kopacz M, Korewicki J (2008) Alterations in calcium regulatory protein expression in patients with preserved left ventricle systolic function and mitral valve stenosis. J Card Fail 14(10):873–880. https://doi.org/10.1016/j.cardfail.2008.07.232
Kogler H, Schott P, Toischer K, Milting H, Van PN, Kohlhaas M, Grebe C, Kassner A, Domeier E, Teucher N, Seidler T, Knoll R, Maier LS, El-Banayosy A, Korfer R, Hasenfuss G (2006) Relevance of brain natriuretic peptide in preload-dependent regulation of cardiac sarcoplasmic reticulum Ca2+ ATPase expression. Circulation 113(23):2724–2732. https://doi.org/10.1161/CIRCULATIONAHA.105.608828
Toischer K, Teucher N, Unsold B, Kuhn M, Kogler H, Hasenfuss G (2010) BNP controls early load-dependent regulation of SERCA through calcineurin. Basic Res Cardiol 105(6):795–804. https://doi.org/10.1007/s00395-010-0115-2
Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F, Molkentin JD, Drexler H, Wollert KC (2002) Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci U S A 99(17):11363–11368. https://doi.org/10.1073/pnas.162100799
Ding B, Abe JI, Wei H, Huang Q, Walsh RA, Molina CA, Zhao A, Sadoshima J, Blaxall BC, Berk BC, Yan C (2005) Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation 111(19):2469–2476. https://doi.org/10.1161/01.CIR.0000165128.39715.87
Chan NY, Seyedi N, Takano K, Levi R (2012) An unsuspected property of natriuretic peptides: promotion of calcium-dependent catecholamine release via protein kinase G-mediated phosphodiesterase type 3 inhibition. Circulation 125(2):298–307. https://doi.org/10.1161/CIRCULATIONAHA.111.059097
Beca S, Ahmad F, Shen W, Liu J, Makary S, Polidovitch N, Sun J, Hockman S, Chung YW, Movsesian M, Murphy E, Manganiello V, Backx PH (2013) Phosphodiesterase type 3A regulates basal myocardial contractility through interacting with sarcoplasmic reticulum calcium ATPase type 2a signaling complexes in mouse heart. Circ Res 112(2):289–297. https://doi.org/10.1161/CIRCRESAHA.111.300003
Furukawa K, Ohshima N, Tawada-Iwata Y, Shigekawa M (1991) Cyclic GMP stimulates Na+/Ca2+ exchange in vascular smooth muscle cells in primary culture. J Biol Chem 266(19):12337–12341
Wei J, Watanabe Y, Takeuchi K, Yamashita K, Tashiro M, Kita S, Iwamoto T, Watanabe H, Kimura J (2016) Nicorandil stimulates a Na(+)/Ca(2)(+) exchanger by activating guanylate cyclase in guinea pig cardiac myocytes. Pflugers Arch 468(4):693–703. https://doi.org/10.1007/s00424-015-1763-8
Reppel M, Fleischmann BK, Reuter H, Sasse P, Schunkert H, Hescheler J (2007) Regulation of the Na+/Ca2+ exchanger (NCX) in the murine embryonic heart. Cardiovasc Res 75(1):99–108. https://doi.org/10.1016/j.cardiores.2007.03.018
Ruknudin A, He S, Lederer WJ, Schulze DH (2000) Functional differences between cardiac and renal isoforms of the rat Na+-Ca2+ exchanger NCX1 expressed in Xenopus oocytes. J Physiol 529(Pt 3):599–610. https://doi.org/10.1111/j.1469-7793.2000.00599.x
Ruknudin A, Schulze DH (2002) Phosphorylation of the (Na+/Ca2) exchangers by PKA. Ann N Y Acad Sci 976:209–213. https://doi.org/10.1111/j.1749-6632.2002.tb04743.x
Schillinger W (2002) Importance of sympathetic activation for the expression of Na+-Ca2+ exchanger in end-stage failing human myocardium. Eur Heart J 23(14):1118–1124. https://doi.org/10.1053/euhj.2001.3044
Thireau J, Karam S, Fauconnier J, Roberge S, Cassan C, Cazorla O, Aimond F, Lacampagne A, Babuty D, Richard S (2012) Functional evidence for an active role of B-type natriuretic peptide in cardiac remodelling and pro-arrhythmogenicity. Cardiovasc Res 95(1):59–68. https://doi.org/10.1093/cvr/cvs167
Zhai Y, Luo Y, Wu P, Li D (2018) New insights into SERCA2a gene therapy in heart failure: pay attention to the negative effects of B-type natriuretic peptides. J Med Genet 55(5):287–296. https://doi.org/10.1136/jmedgenet-2017-105120
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights and informed consent
This manuscript does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, J., Xu, T., Zhou, Y. et al. B-type natriuretic peptide and its role in altering Ca2+-regulatory proteins in heart failure—mechanistic insights. Heart Fail Rev 25, 861–871 (2020). https://doi.org/10.1007/s10741-019-09883-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-019-09883-1