Abstract
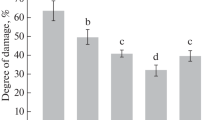
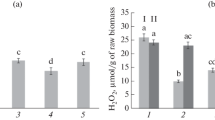
Microbes- and microbe-associated molecular patterns-induced stomatal closure have been known to be connected with the early defense responses and the improvement of instantaneous water use efficiency (WUEi) in plants. Being a commercially available microorganism, Bacillus subtilis can promote plant growth and induce disease resistance. However, its effects on stomatal movement and WUEi in plants have been largely unexplored. Here, we showed that B. subtilis induced stomatal closure in a dose- and time-dependent manner when applied to isolated epidermal peels and intact leaves of broad bean. Pharmacological study further revealed that the B. subtilis-induced stomatal closure in epidermal peels was mediated mainly by reactive oxygen species production via NADPH oxidases. Furthermore, foliar application of B. subtilis significantly reduced stomatal aperture, stomatal conductance (gs), transpiration rate (E) and net photosynthesis rate (Pn) of leaves of broad bean at 8 and 24 h, reductions in which were reversed after 48 h. As a consequence, the WUEi of plants treated with B. subtilis for 8–144 h was higher than that in the control. The chlorophyll fluorescence and content analysis further demonstrated that B. subtilis could enhance plant photosynthetic activities by increasing leaf photosynthetic efficiency and chlorophyll content. These results suggest that foliar spray of B. subtilis can improve WUEi of crop plant via the regulations of stomatal movement and photosynthetic activity during a special time period.




Similar content being viewed by others
Abbreviations
- CAT:
-
Catalase
- Ci :
-
Intercellular CO2 concentration
- DPI:
-
Diphenylene iodonium chloride
- E:
-
Transpiration rate
- Fv/Fm:
-
Maximum quantum yield of photosystem II (PSII)
- gs :
-
Stomatal conductance
- NPQ:
-
Non-photochemical quenching
- Pn :
-
Net photosynthetic rate
- ΦPSII:
-
Effective quantum yield of PSII
- qP:
-
Photochemical quenching
- ROS:
-
Reactive oxygen species
- SHAM:
-
Salicylhydroxamic acid
- WUEi :
-
Instantaneous water use efficiency
References
Acharya BR, Assmann SM (2009) Hormone interactions in stomatal function. Plant Mol Biol 69:451–462
Boller T, He SY (2009) Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 324:742–744
Chaves M, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Chen L, Dodd IC, Davies WJ, Wilkinson S (2013) Ethylene limits abscisic acid- or soil drying-induced stomatal closure in aged wheat leaves. Plant, Cell Environ 36:1850–1859
Cona A, Rea G, Angelini R, Federico R, Tavladoraki P (2006) Functions of amine oxidases in plant development and defence. Trends Plant Sci 11:80–88
Cuevas JC, Sánchez DH, Marina M, Ruiz OA (2004) Do polyamines modulate the Lotus glaber NADPH oxidation activity induced by the herbicide methyl viologen? Funct Plant Biol 31:921–928
Davies WJ, Wilkinson S, Loveys B (2002) Stomatal control by chemical signalling and the exploitation of this mechanism to increase water use efficiency in agriculture. New Phytol 153:449–460
Deng B, Jin X, Yang Y, Lin Z, Zhang Y (2014) The regulatory role of riboflavin in the drought tolerance of tobacco plants depends on ROS production. Plant Growth Regul 72:269–277
Desikan R, Cheung MK, Clarke A, Golding S, Sagi M, Fluhr R et al (2004) Hydrogen peroxide is a common signal for darkness-and ABA-induced stomatal closure in Pisum sativum. Funct Plant Biol 31:913–920
Desikan R, Last K, Harrett-Williams R, Tagliavia C, Harter K, Hooley R et al (2006) Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J 47:907–916
Farquhar G, Richards R (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Funct Plant Biol 11:539–552
Flexas J, Medrano H (2002) Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Ann Bot 89:183–189
Gao J, Wang N, Wang G-X (2013) Saccharomyces cerevisiae-induced stomatal closure mainly mediated by salicylhydroxamic acid-sensitive peroxidases in Vicia faba. Plant Physiol Biochem 65:27–31
Grimmer MK, Foulkes MJ, Paveley ND (2012) Foliar pathogenesis and plant water relations: a review. J Exp Bot 63:4321–4331
He J, Yue X, Wang R, Zhang Y (2011) Ethylene mediates UV-B-induced stomatal closure via peroxidase-dependent hydrogen peroxide synthesis in Vicia faba L. J Exp Bot 62:2657–2666
Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424:901–908
Hoque TS, Uraji M, Ye W, Hossain MA, Nakamura Y, Murata Y (2012) Methylglyoxal-induced stomatal closure accompanied by peroxidase-mediated ROS production in Arabidopsis. J Plant Physiol 169:979–986
Hunsche M, Bürling K, Saied A, Schmitz-Eiberger M, Sohail M, Gebauer J, Noga G, Buerkert A (2010) Effects of NaCl on surface properties, chlorophyll fluorescence and light remission, and cellular compounds of Grewia tenax (Forssk.) Fiori and Tamarindus indica L. leaves. Plant Growth Regul 61:253–263
Iriti M, Picchi V, Rossoni M, Gomarasca S, Ludwig N, Gargano M et al (2009) Chitosan antitranspirant activity is due to abscisic acid-dependent stomatal closure. Environ Exp Bot 66:493–500
Joo JH, Wang S, Chen J, Jones A, Fedoroff NV (2005) Different signaling and cell death roles of heterotrimeric G protein α and β subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17:957–970
Jourdan E, Henry G, Duby F, Dommes J, Barthelemy JP, Thonart P et al (2009) Insights into the defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Mol Plant Microbe Interact 22:456–468
Kautz B, Noga G, Hunsche M (2014) Sensing drought- and salinity-imposed stresses on tomato leaves by means of fluorescence techniques. Plant Growth Regul 73:279–288
Kerstiens G, Tych W, Robinson MF, Mansfield TA (2002) Sodium-related partial stomatal closure and salt tolerance of Aster tripolium. New Phytol 153:509–515
Khokon MAR, Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC et al (2010a) Yeast elicitor-induced stomatal closure and peroxidase-mediated ROS production in Arabidopsis. Plant Cell Physiol 51:1915–1921
Khokon MAR, Uraji M, Munemasa S, Okuma E, Nakamura Y, Mori IC et al (2010b) Chitosan-induced stomatal closure accompanied by peroxidase-mediated reactive oxygen species production in Arabidopsis. Biosci Biotech Biochem 74:2313–2315
Khokon M, Jahan MS, Rahman T, Hossain MA, Muroyama D, Minami I et al (2011a) Allyl isothiocyanate (AITC) induces stomatal closure in Arabidopsis. Plant, Cell Environ 34:1900–1906
Khokon MAR, Okuma E, Hossain MA, Munemasa S, Uraji M, Nakamura Y et al (2011b) Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant, Cell Environ 34:434–443
Koers S, Guzel-Deger A, Marten I, Roelfsema MRG (2011) Barley mildew and its elicitor chitosan promote closed stomata by stimulating guard-cell S-type anion channels. Plant J 68:670–680
Kumar AS, Lakshmanan V, Caplan JL, Powell D, Czymmek KJ, Levia DF et al (2012) Rhizobacteria Bacillus subtilis restricts foliar pathogen entry through stomata. Plant J 72:694–706
Lawlor D, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant, Cell Environ 25:275–294
Li B, Yang Y, Yu C, Li S, Chen J, Liu X, Qin H, Wang D (2014a) Partial suppression of l-galactono-1,4-lactone dehydrogenase causes significant reduction in leaf water loss through decreasing stomatal aperture size in Arabidopsis. Plant Growth Regul 72:171–179
Li Y, Xu S-S, Gao J, Pan S, Wang G-X (2014b) Chlorella induces stomatal closure via NADPH oxidase-dependent ROS production and its effects on instantaneous water use efficiency in Vicia faba. PLoS One 9:e93290
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Liu F, Jensen CR, Shahanzari A, Andersen MN, Jacobsen SE (2005) ABA regulated stomatal control and photosynthetic water use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Sci 168:831–836
Luis A, Corpas FJ, Sandalio LM, Palma JM, Gómez M, Barroso JB (2002) Reactive oxygen species, antioxidant systems and nitric oxide in peroxisomes. J Exp Bot 53:1255–1272
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126:969–980
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mori IC, Pinontoan R, Kawano T, Muto S (2001) Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol 42:1383–1388
Mott KA (2009) Opinion: stomatal responses to light and CO2 depend on the mesophyll. Plant, Cell Environ 32:1479–1486
Murata Y, Pei ZM, Mori IC, Schroeder J (2001) Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD (P) H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13:2513–2523
Nejad AR, van Meeteren U (2008) Dynamics of adaptation of stomatal behaviour to moderate or high relative air humidity in Tradescantia virginiana. J Exp Bot 59:289–301
Ongena M, Duby F, Jourdan E, Beaudry T, Jadin V, Dommes J et al (2005) Bacillus subtilis M4 decreases plant susceptibility towards fungal pathogens by increasing host resistance associated with differential gene expression. Appl Microbiol Biot 67:692–698
Ongena M, Jourdan E, Adam A, Paquot M, Brans A, Joris B et al (2007) Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ Microbiol 9:1084–1090
Pérez-García A, Romero D, De Vicente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotech 22:187–193
Rivero RM, Shulaev V, Blumwald E (2009) Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol 150:1530–1540
Sawinski K, Mersmann S, Robatzek S, Böhmer M (2013) Guarding the Green: pathways to stomatal immunity. Mol Plant-Microbe Interact 26:626–632
Signarbieux C, Feller U (2011) Non-stomatal limitations of photosynthesis in grassland species under artificial drought in the field. Environ Exp Bot 71:192–197
Suhita D, Raghavendra AS, Kwak JM, Vavasseur A (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate-and abscisic acid-induced stomatal closure. Plant Physiol 134:1536–1545
Trejo CL, Davies WJ, Ruiz L (1993) Sensitivity of stomata to abscisic acid (an effect of the mesophyll). Plant Physiol 102:497–502
Underwood W, Melotto M, He SY (2007) Role of plant stomata in bacterial invasion. Cell Microbiol 9:1621–1629
Vranova E, Inzé D, Van Breusegem F (2002) Signal transduction during oxidative stress. J Exp Bot 53:1227–1236
Yu D, Kim S, Lee H (2009) Stomatal and non-stomatal limitations to photosynthesis in field-grown grapevine cultivars. Biol Plantarum 53:133–137
Zeng W, He SY (2010) A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol 153:1188–1198
Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126:1438–1448
Zhang H, Xie X, Kim MS, Kornyeyev DA, Holaday S, Paré PW (2008) Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J 56:264–273
Acknowledgments
This work is supported by the National High-Tech R & D Program (863 Program) for the 12th Five-Year Plan (2011AA100503), Natural Science Fund of China (31330010) and Zhejiang Provincial Natural Science Foundation of China(LZ13C030002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Xu, S., Gao, J. et al. Bacillus subtilis-regulation of stomatal movement and instantaneous water use efficiency in Vicia faba . Plant Growth Regul 78, 43–55 (2016). https://doi.org/10.1007/s10725-015-0073-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-015-0073-7