Abstract
Rice (Oryza sativa L) is one of the most important staple food worldwide with a global production estimated at around 800 million metric tons for paddy rice in 2021. However, this production is hampered by several factors, such as land salinity. In this study, 230 F2:3 lines of the Sahel 328/NERICA-L-9 mapping populations were evaluated for their tolerance to salinity at the young seedling stage at an electrical conductivity (EC) equivalent to 12 dSm−1. All parameters investigated were negatively affected under saline conditions compared to the control. Of these lines, 10 had a salt injury score (SIS) lower than that of the tolerant control, FL478 and 17 than the donor parent NERICA-L-9. About 4684 informative SNPs and 230 lines were used to construct the genetic linkage map. Twenty quantitative trait loci (QTLs) with LOD > 3 that were related to SIS, root length, and shoot length were identified in this study. Twelve new QTLs associated with salt tolerance, qLR2.1, qLR2.2, qLR3.1, qLR3.2, qLR3.3, qLR5, qLR7.4, qLR10, qLR11, qLF6, qSES10 and qSES12, located on chromosomes 2, 3, 5, 7, 10, 11 and 12, respectively were discovered in this study. These QTLs were mapped on the 12 linkage groups (LG), with LG9 having the lowest number of molecular markers (160 SNPs), while LG2 was the largest with 498 markers. These markers may be useful in rice breeding programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world’s population is estimated at 7.875 billion, of which Sub-Saharan Africa (SSA) alone has 1.104 billion people (United Nations Population Fund 2021). At the same time, the total land available for agriculture is declining due to factors, such as climate change and industrialization. This imposes a demand on the reduced arable land area to be productive enough to feed the continuously increasing world population. Currently, global paddy production is estimated at 800 million tons (FAO 2021). In Senegal, the production is estimated at 742,348 tons, which is only enough to feed just under 35% of the population leading to import about 1.07 million tons of white rice a year. This low production is due to a wide range of constraints such as disease pressure, cold, heat, drought, salinity among others. Salinity is among the major factors that limit rice productivity in Senegal and around the world, rendering 800 million hectares—out of the available 12.7 billion hectares of global arable lands—almost unproductive (Yadav et al. 2011). Soil salinity causes significant damage to the ecosystem by reducing productivity, degrading water quality, reducing biodiversity, causing erosion, increasing the concentrations of toxic ions, and reducing soil fertility (FAO 2021). Yadav et al. (2011) estimated that revenue losses due to salinity stress in soil could be as high as US$ 11.4 billion in irrigated and US$ 1.2 billion in rainfed ecosystems.
Rice (Oryza sativa L) is one of the most sensitive cereals to soil salinization. It can withstand some salinity during the vegetative phase, but it is very sensitive at the seedling and reproductive stages (Jahan et al. 2020). Salt tolerance at the seedling stage and reproductive phase are weakly correlated (Bimpong et al. 2016; Amoah et al. 2020). Although salinity at the seedling stage could affect plant development, and indirectly, the grain yield, at the reproductive phase, it directly impacts yield. It has been reported that rice yields decrease by 50% at an electrical conductivity (EC) of 9.5 dS/m under humid conditions, while similar losses have been observed under arid conditions at only 3.5 dS/m (Bimpong et al. 2016). Several natural and artificial methods, such as good water management by leaching, the use of organic matter and bacteria, and the application of gypsum have been implemented to reduce soil salinity. However, most of these methods may become costly over time and are usually not sustainable or effective in the long term. The development of new rice cultivars that are tolerant to salinity could be a sustainable and cost-effective alternative that can help boost yields in saline areas. However, breeding protocols for salinity-tolerant cultivars complicated due to the complexity of the genetics and the physiology of the tolerance mechanisms (Munns and Tester 2008; Baby et al. 2010). Field screening and identification of genotypes tolerant to salinity at the reproductive stage is further complicated by the existence of soil heterogeneity and complex interaction of other stress factors. However, through the protocol developed by Gregorio et al. (1997), it is possible to evaluate large populations for tolerance to salinity at the seedling stage, which is devoid of any form of heterogeneity. In this protocol, evaluation is performed under homogeneous conditions based on recording a visual salt injury score (SIS) during the early seedling growth phase; observations last just about 3 weeks.
Basing crucial breeding decisions, such as selection of progenies for advancing generations, on phenotype alone could be misleading. Massive improvement in genetic and molecular technology has enabled the discovery of molecular markers of high utility at relatively cheaper costs. An example of such marker system is the single nucleotide polymorphism (SNP), which is routinely applied to marker-assisted selection (MAS) in crop improvement. Foreground selection, where progenies possessing the trait of interest from the donor parent are selected for breeding is a crucial step in MAS. This step involves screening population sets and their parents using SNPs that are diagnostic for the particular trait of interest, otherwise known as “trait-based SNPs”. The development of such SNPs allows for the identification of reliable quantitative trait loci (QTLs) as a starting point. Several biparental populations ranging from F2 to Fx (where ‘x’ is the xth generation) have been used in the past to map QTLs. By using an F5:6 population of IR29/Hasawi, Bimpong et al. (2014) were able to identify seven QTLs related to traits such as fresh and dry weight, and plant height in rice. In a related study, a robust QTL named Saltol was mapped to the short arm of chromosome 1 using the same parents IR29 and Hasawi (Singh et al. 2007; Thomson et al. 2010). From the F4 population of Sahel 317/Madina Koyo, 13 QTLs were identified from their SIS, shoot, and root length, and shoot dry weight (Amoah et al. 2020). Though most of these authors used parents that were tolerant to salinity, the resulting rice cultivars often contained many undesirable traits. such as grain color, presence of awns, lodging, easy shattering, and low yield. Despite their tolerance, these identified lines did not generally meet the needs of farmers and consumers. Thus, crossing Sahel 328 (high yield potential and aromatic) and NERICA-L-9 (good phenotype and tolerant to salinity stress) could be useful in the identification of new and important QTLs, while generating an elite rice population from which a salt-tolerant, yet quality and high-yielding line(s), could be identified. The objective of this study was to identify new lines with QTLs for salinity tolerance at the seedling stage by using an F2:3 population of Sahel 328/ NERICA-L-9.
Materials and methods
Plant material
The population used in this study is composed of lines derived from a cross between a donor parent, NERICA-L-9, and a recipient parent, Sahel 328, obtained at the Sahel Regional station of Africa Rice Center (Saint-Louis – Senegal) during the dry season of 2019. This research station is in the village of Ndiaye (16°32.141 N; 15°11.545W). NERICA-L-9 (WAS 122-IDSA-10-WAS-3-1-TGR 3) is derived from a cross between TOG5681/3*IR64, developed at WARDA (now AfricaRice) in the early 2000s. It was identified to have good adaptability to salinity at the early seedling stage during the screening trials at the Sahel station, Senegal (data unpublished). The female parent (Sahel 328), with pedigree name, WAS 197-B-4-1-5, was developed by crossing IR 31851-96-2-3-2-1 (Sahel 134) and IR 66231-37-1-2 at the WARDA and released in 1997 in Senegal. This cultivar is adapted to irrigation conditions and is characterized by a high yield potential around 10 t/ha, a seeding-maturity cycle of 116 days, with a strong aroma. The cross between the parents, Sahel 328 × NERICA L-9, was carried out in a greenhouse during the rainy season of 2018, at the AfricaRice Sahel Centre at Ndiaye, Senegal. These crosses generated about 30 F1 progenies. All 30 were sown together with the two parents in buckets during the dry season of 2019. The plants were watered daily with tap water. Seven F1 plants were retained as true F1s after screening with a panel of 24 QA/QC SNP markers. Each of the seven F1 plant was harvested separately and advanced to raise a total of 230 F2:3 families which were used for the rapid screening for salt tolerance at the seedling stage.
Screening for tolerance to salinity at the early seedling stage
Screening the 230 F2:3 families for tolerance to salinity at the early seedling stage was conducted at the Sahel Regional station of AfricaRice during the dry season of 2022 under greenhouse conditions following a protocol developed by Gregorio et al. (1997). Typically, the temperature within the greenhouse varied between 20 and 44°C with an average of 24°C. The humidity inside the greenhouse ranged between 40 and 53% with an average of 46%. All the seeds were disinfected with 70% ethanol for 30 s, rinsed with sterile distilled water, and then pre-germinated on tissue paper in Petri dishes under moist conditions. After 3 days, pre-germination seedlings were transferred to Styrofoam floats on top of 22-L tanks filled only with distilled water. Each styrofoam float was considered as a block and consisted of 12 lanes, each containing eight slots/wells. The two parents and the checks, (IR29, sensitive) and FL478 (tolerant), were repeated in each Styrofoam float. The experiment was laid out using an ‘Alpha lattice’ design with four replicates (two stresses, two controls). After 3 days, the distilled water was replaced with Yoshida’s solution (Yoshida et al. 1976) with slight modifications (Singh and Flowers 2010). The controls were maintained in the Yoshida’s solution with slight modifications while 5.13 mM of NaCl (3 g/L) were added to obtain an EC equivalent to 6 dS/m for the plants growing in the stressed conditions. Three days later, the EC was increased to 12 dS/m by adding 6 g/L of NaCl (cooking salt). The pH was adjusted to 5 daily by adding HCl or NaOH to the solution as necessary.
Evaluation of agro-morphological traits
Twenty-one days after sowing, agro-morphological traits were recorded. The shoot length (from the base to the tip of the longest leaf) and root length (from the base to the tip of the longest root) were measured on three plants using a double decimeter ruler (MD-146108, Maped), avoiding those on the border. The weights of the fresh and dry leaves and roots were measured using a precision balance (model Ex.324, Ohaus). Leaves and roots were dried in an oven (FD240, Binder) at 50 °C for 24 h. Two salt injury score (SIS) values were recorded on the 15th and the 21st day after implementing the experiment according to the protocol of Gregorio et al. (1997). The SIS was based on the visual appearance of the leaves as described in the Standard Evaluation System (SES) (IRRI 2014).
The percentages of reduction of the different traits investigated were obtained by the following equations:
Shoot length reduction (%)\(=\left(\frac{\text{SLc}-\text{SLs}}{\text{SLc}}\right)\times 100\) Where SLc = Shoot length under control; SLs = Shoot length under stress.
Root length reduction (%)\(=\left(\frac{\text{RLc}-\text{RLs}}{\text{RLc}}\right)\times 100\)Where RLc = Root length under control; RLs = Root length under stress.
Shoot fresh weight reduction (%)\(=\left(\frac{\text{SFWc}-\text{SFWs}}{\text{SFWc}}\right)\times 100\)Where SFWc = Shoot fresh weight under control; SFWs = Shoot fresh weight under stress.
Root fresh weight reduction (%)\(=\left(\frac{\text{RFWc}-\text{RFWs}}{\text{RFWc}}\right)\times 100\)Where RFWc = Root fresh weight under control; RFWs = Root fresh weight under stress.
Shoot dry weight reduction (%)\(=\left(\frac{\text{SDWc}-\text{SDWs}}{\text{SDWc}}\right)\times 100\)Where SFWc = Shoot dry weight under control; SFWs = Shoot fresh weight under stress.
Root dry weight reduction (%)\(=\left(\frac{\text{RDWc}-\text{RDWs}}{\text{RDWc}}\right)\times 100\)Where RDWc = Root dry weight under control; RDWs = Root dry weight under stress.
DNA extraction and genotyping
Leaf discs of about 6 mm in diameter were collected from 21-day-old plants and placed in 96-well plates. To avoid necrosis and decay, these samples were pre-dried in an oven (FD240, Binder) at 50 °C for 24 h. DNA extraction (CTAB method, Murray and Thompson 1980) and genotyping (using SNPs) were carried out at IGSS (now SEQART AFRICA) a platform hosted by the Biosciences Eastern and Central Africa BecA, International Livestock Research Institute ILRI, in Nairobi Kenya. This genotyping platform uses DArTseq™ technology from DArT (Diversity Arrays Technology Pty Ltd) in Australia.
Identification of QTLs and genetic map construction
Markers derived from the DArTseq were processed to remove un-callable SNPs and genotypes using the FlapJack software (Milne et al. 2010). Genotypes with more than 30% missing data, SNP loci with more than 20% missing data, and rare SNPs with less than 5% minor allele frequencies (MAF) were removed. Of the 57,974 markers at the baseline, only 4684 informative SNPs and 234 lines from the Sahel 328/NERICA-L-9 population were considered for further analysis following filtering and quality control. Missing data were imputed using KDCompute, an online analysis plugin that runs on R scripts.
Statistical analysis
For agro-morphological traits, statistical analyses were performed using Breeding View 1.7.0.0 software. After verification of the normality and homogeneity variance of the data residus, an analysis of variance was performed on shoot length, root length, shoot fresh weight, root fresh weight, shoot dry weight, and root dry weight using a linear mixed model. A combined analysis was performed to assess the significance of the genotype and the environmental interaction. Single-site analyses (saline and control) were performed if the G × E interaction was found to be significant. In this model (combined analysis), genotypes, environment, and G × E were considered to be the fixed factors, while replication and blocking of samples were considered to be random factors. A comparison of mean values using LSD (least significant difference) was calculated for all normally distributed and significant variables. Histograms were constructed using Statview 5.0.1 software. As the SIS appears to follow a multinomial distribution, an ordinal regression was carried out to identify the best genotypes. Odds ratios were used to identify the best genotypes. These were compared with the donor parent NERICA-L-9 and the international tolerant check FL478. The analysis of the SIS was carried out using the R statistical package (R Core Team 2024).
The QTL analysis was done by Windows QTL Cartographer Version 2.5_011 (Wang et al. 2012) using the default settings for Composite Interval Mapping (CIM) (map function = Haldane, Model = 6, Regression method = backward) with a permutation of 1000. A QTL was declared present if the probability of the association between phenotype and adjacent markers was lower than 5%. In addition, the LOD must be greater than or equal to 3. The coefficient of determination, R2, was used to determine the contribution to phenotypic variation associated with each QTL. The QTLs were named according to the nomenclature proposed by McCouch et al. (1997). QTL names start with a lowercase “q”, followed by 2–3 initials of the corresponding measured traits (in uppercase), and then the chromosome number. If there were more than one QTL on a chromosome, a number was added to distinguish them. Graphical representations of QTL positions were generated using mapChart 2.32 (Voorrips 2009).
Original data of this study including morphological and SNPs data are available at Mendeley data (https://data.mendeley.com/drafts/3rz5b6n7h4).
Results
Performance of lines under saline and non-saline conditions
Analysis of variance (ANOVA) revealed a highly significant difference (P < 0.001) in all the traits investigated for the different setups (saline and control; referred to as environments), genotypes, and genotypes × salinity levels (G × E) (Table 1). This indicated that the lines respond differently in the presence or absence of salinity. The F2:3 population responded differently to salinity, where some exhibited better adaptability than others (Fig. 1).
Salt injury score (SIS) Out of the 230 lines evaluated for tolerance to salinity, 59 lines representing 26% of the total had lower or comparable SIS (3–5) as compared to the tolerant control, FL478, and were classified as tolerant to salinity (Fig. 2). On the basis of odds ratio, seventeen and 10 genotypes were identified as tolerant compared to their donor parent NERICA-L-9 and FL478, respectively (Table 2). The mean SIS for the entire F2:3 population was 5.8.
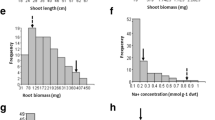
Shoot length The average shoot length of the lines under non-saline conditions was 30.146 cm, compared to 15.385 cm under saline conditions (Table 3; Fig. 3). The percentage reduction in the shoot length of the lines was smaller than that recorded for the donor parent, NERICA-L-9 (49% vs. 51%). However, the latter was slightly greater than the reduction noted for Sahel 328 (47%). Out of the 230 lines tested under saline conditions, 193 (84%) had shoot lengths greater than or comparable to those of the male parent, NERICA-L-9. In addition, 135 lines (59%) had shoot lengths greater than or comparable to the tolerant control, FL478.
Root length Root length decreased moderately under saline conditions. The average root length of the population was 10.989 cm in the control as compared to 9.066 cm under stress. The average percentage reduction in root length of the progenies was 15% as compared to 27% for the donor parent, NERICA-L-9 (Table 3; Fig. 4). Among the 230 lines tested at salinity, 193 (84%) had root lengths greater than or comparable to the male parent, NERICA-L-9. Furthermore, 82 lines (36%) had root lengths greater than or similar to the tolerant control, FL478.
Fresh shoot and root weights The reduction of shoots fresh weight of the progenies was almost identical to the donor parent (75% vs. 76%). However, this reduction was slightly less in the female parent, Sahel 328 (71%) (Table 1; Fig. 4). Out of the 230 lines tested under salinity, 134 (58%) had shoot fresh weight greater than or comparable to the female parent, NERICA-L-9. Additionally, 182 lines (79%) showed shoot fresh weight greater than or equal to the tolerant control, FL478. The roots were also affected by salt stress. The fresh weight of the progenies was reduced by 51%, while that of the donor parent was reduced by 55% (Table 3; Figs. 5 and 6). Among the 230 lines tested under salinity, 194 (84%) had fresh root weight greater than or comparable to the male parent, NERICA-L-9. Likewise, 29 (13%) showed fresh root weight greater than or equal to the tolerant control, FL478.
Shoot and root dry weights The reduction in shoot dry weights was almost identical among the progenies and the donor parent. These lines showed a 53% reduction in shoot dry weight, while the donor parent recorded a 53.335% reduction (Table 3, Fig. 7). Of the 230 lines tested at salinity, 182 (79%) had a shoot dry weight greater than or comparable to that of the male parent, NERICA-L-9. In addition, 75 lines (33%) had shoot dry weight greater than or equal to the tolerant control, FL478. The reduction in roots was also similar in both lines and the donor parent. The lines presented a reduction of 53% as compared to 52.71% for NERICA-L-9 (Table 3). Out of the 230 lines tested under saline conditions, 203 (88%) had a root dry weight greater than or comparable to that of the male parent, NERICA-L-9. In addition, 36 lines (16%) showed a root dry weight greater than or equal to the tolerant control, FL478.
Pearson correlation between traits in saline conditions
Overall, the correlation between the SIS and every other trait was negative with high magnitude, including for root length (− 0.3973, P < 0.001), root fresh weight (− 0.1537, P < 0.01), shoot length (− 0.4008, P < 0.001), shoot dry weight (− 0.3943, P < 0.001), and shoot fresh weight (− 0.4490, P < 0.001) (Table 4). Conversely, a positive correlation was observed between the weight of fresh shoot and root length (0.7118, P < 0.001), shoot and root fresh weight (0.4801, P < 0.001), shoot fresh weight and leaf length (0.7649, P < 0.001), and shoot fresh and dry weights (0.7730, P < 0.001).
Construction of the genetic linkage map and QTLs identification
A total of 4,684 polymorphic SNPs between 230 F2:3 families from the Sahel 328/NERICA-L-9 population were used to generate the linkage map (Table 5). The total genetic distance of this map was 1630.32 cM across 12 chromosomes; thus, a marker was identified for every 4.33 cM. Twelve linkage groups (LG) corresponding to the 12 chromosomes were constructed. The LG9 recorded the lowest number of molecular markers with 160 SNPs, while LG2 had the greatest number (498 SNP). LG1 presented the largest genetic distance of 203.86 cM with an average distance of 0.41 cM. In contrast, LG10 with a genetic distance of 93.03 cM was the shortest. LG12 was the most saturated with one molecular marker for every 0.24 cM.
Identification of QTLs
A total of 20 QTLs corresponding to three (shoot length, SIS and root length) out of the seven traits studied were mapped across 10 chromosomes in this study (Fig. 8a–j). Of the 20 QTLs, one was mapped for shoot length, while two and seventeen QTLs were associated with SIS and root length, respectively.
a Chromosome 1 showing quantitative trait locus (QTL) positions in cM b Chromosome 2 showing QTL positions in cM c Chromosome 3 showing QTL positions in cM d Chromosome 5 showing QTL positions in cM e Chromosome 6 showing QTL positions in cM f Chromosome 7 showing QTL positions in cM g Chromosome 9 showing QTL positions in cM h Chromosome 10 showing QTL positions in cM i Chromosome 11 showing QTL positions in cM j Chromosome 12 showing QTL positions in cM. QTLs colors: red = root length, blue = salinity score, green = shoot length
Root length
Of the 17 QTLs identified in relation to root length here, one each was mapped to chromosome 1, 5, 9, 10, 11, and 12, while two, three, two, and four, were mapped to chromosomes 2, 3, 6, 7, respectively (Table 6). The contribution to the observed phenotypic variation for these seventeen QTLs ranged between 0.08 to 78%. qLR3.1 (78.02%), qLR2.1 (77.58%), and qLR3.2 (77.51%) contributed the most phenotypic variance and can be considered as major QTLs. The additive effect of 65% of the QTLs was observed.
Shoot length and salt injury score
A unique QTL related to shoot length was identified on chromosome 6 (Fig. 8e). The contribution of this chromosome to phenotypic variation was 9.1%. A negative additive effect was noted for this QTL. Two QTLs related to SIS were identified on chromosomes 10 and 12 (Table 7, Fig. 8e, j). The contributions of these QTLs, qSES10 and qSES12, to phenotypic variation were 6.66 and 2.41%, respectively. The additive effect of all two QTLs was positive.
Discussion
Effect of salinity on the performance of lines
Salinity can induce irreparable damage in rice, especially during the early seedling and reproductive stages. In the first 3 weeks of crop development, even an EC slightly exceeding 3 dS/m is enough to cause a considerable reduction in the mass of the crop stand (Singh et al. 2004; Bimpong et al. 2016). In this study, 230 F2:3 families were evaluated under saline conditions with EC up to 12 dS/m. Salinity caused a general reduction in all measured traits, including shoot and root lengths, and caused leaf discoloration and necrosis as reported in other research (Khatun and Flowers 1995; Zeng et al. 2001; Islam et al. 2007; Singh and Flowers 2010; Hossain et al. 2014). However, this reduction was not uniform among the 230 progenies investigated, where some showed better adaptability to salinity than the donor parent, NERICA-L-9, suggesting a transgressive segregation pattern among the F2:3 population. A similar observation was made by Pabuayon et al. (2021). Transgressive segregation is reported to be due to complementary gene action from the inheritance of additive alleles from both parents and is associated with morphological traits in plants (Rieseberg et al. 1999). Unlike heterosis, transgressive (extreme) genetic types, which are completely different from the two parents and whose characteristics are repeated from one generation to the next, can be identified and used as cultivars if they meet the breeders’ standards. The difference in G × E interaction for all the parameters studied in this experiment, except for shoot and root dry weight was highly significant, confirmed the effect of salt on these variables. It also indicated the detrimental effect of salinity on agronomic traits, such as shoot length, fresh and dry weight of shoots, as observed previously by Ali and Awan (2004); Maiti et al. (2006); and Al-Amin et al. (2013). These variables can be used as discriminating criteria to identify salinity-tolerant genotypes at the young seedling stage. The percentage reduction in the shoot fresh weight of the different lines varied between 60 and 80%. Hussain et al. (2018), reported a reduction in shoot length of 31.2, 52.4, and 63% in an indica cultivar, Liangyoupeijiu (LYP9), when the EC was 1.0894, 3.2, 4.64 dS/m, respectively. The maximum EC used in this study (4.64) is about 3 times less than that used in our case (12 dS/m). This could explain the extent of shoot length reduction in our study. Some genotypes showed a smaller reduction in shoot length than that noted by Hussain et al. (2018).
This reduction in the aboveground parts as compared to the control reached 24.6, 75.5, and 100% for japonica Nipponbare (NPBA) in the presence of the same EC. Furthermore, Kakar et al. (2019) observed a height of 30.25 cm in the control as compared to 22.71 cm for the cultivar IR86052, when grown in 12 dS/m EC. These results confirm our observations that rice has a differential response in the presence of salt. The reduction in shoot length has been attributed to the inhibition of both cell division and elongation in the presence of salt (Munns et al. 2006).
Reduction in shoot length was more pronounced in susceptible lines as compared to the tolerant ones under saline conditions (Suplick et al. 2002). Nineteen lines presented lower percentages of shoot reduction than the tolerant control, FL478 (48.9%). These genotypes may be considered tolerant because they are better than FL478, a tolerant control used in research around the world. A shoot length reduction of 19% was found by Titov et al. (2009) and Bimpong et al. (2016) upon growth in soil with EC of 12 dS /m. They noted a 20% reduction in plant height in tolerant lines as compared to 38% in the susceptible ones. Salinity also caused a reduction in root length in this study. However, this reduction was less pronounced as compared to that in the shoots. The percentage reduction in roots varied between 0.12 and 23%. Al Amin et al. (2013) concluded that the reduction was genotype-dependent; they observed a reduction in root length for genotypes, S-611/32 (43.95%) and Iratom-24 (43.80%), while a salt-tolerant genotype, Pokkali, presented a reduction of 8.45%
Both fresh and dry weights of shoot and roots followed a similar reduction pattern as the lengths. The reduction percentage of shoot dry weight of the progeny ranged from 47 to 53, while that of the donor parent was 53%. As for the dry roots, the reduction percentage for the weight of the progenies varied between 52 and 53. The reduction of dry shoot weight may be due to a decrease in chlorophyll content in the presence of salinity. The weight of dry shoots after saline treatment could be an indicator of salinity tolerance. Jahan et al. (2020) reported a shoot fresh weight of 1036.3 mg in the control versus 346.66 mg in the stressed cultivar, a shoot dry weight of 220 mg in the control versus 78.75 mg in the stressed cultivar, a root fresh weight of 530 mg in control versus 232.22 mg in the stressed cultivar, and a root dry weight of 133.3 mg in control versus 36.66 mg in the stressed cultivar when the EC was 100 mM = 10 dS/m. Javed et al. (2006) observed a reduction in fresh and dry biomass under saline conditions. Similarly, Hussain et al. (2018) noted a 63% reduction in the total biomass of the LYP9 cultivar at an EC of 12 dS/m and a 100% reduction in biomass of the NPBA cultivar at the same level of EC. These results confirm that salt can induce a reduction in biomass, which is an important criterion for the selection of salinity-tolerant lines. This reduction in biomass depends on whether or not the cultivar is tolerant to salinity.
In this study, all traits negatively correlated with the SIS, which is consistent with the findings of Rohila et al. (2019). In a related study, Bizimana et al. (2017) observed a strong negative correlation between SIS, leaf length, root length, leaf and root fresh weight, and leaf and root dry weight. The tolerant genotypes (less SIS) recorded higher growth parameters. Based on the SIS, Gregorio et al. (1997) were able to identify genotypes based on the visual appearance of the plants. Furthermore, Islam et al. 2007 distinguished between genotypes based on the scores: tolerant (score 3) and susceptible (score 9). Seventeen tolerant genotypes with low odds ratios were identified using NERICA-L-9 as a reference. Furthermore, using the international reference FL478, 10 genotypes were recorded as tolerant. The odds ratios of these genotypes were close to zero at P < 0.028. This means that the odds of high scoring genotypes are approximately identical to those of the international check FL478. Experimental lines can be considered tolerant if they show less or only as much visual damage as FL478.
Identification of QTLs at seedling stage
Among the seven traits investigated, only three, namely root length, shoot length, and salt injury score were associated with QTLs. In total, the 20 QTLs identified were distributed as follows: 17 associated with root length among which three were major (R2 > 15%), one associated with shoot length, and two with SIS. All these 20 QTLs were compared with those linked to salt tolerance at the young seedling stage in the database (http://www.gramene.org) and those described by Negrão et al. (2011). Of these, 12 (qLR2.1, qLR2.2, qLR3.1, qLR3.2, qLR3.3, qLR5, qLR7.4, qLR10, qLR11, qLF6, qSES10 and qSES12) were considered as news.The QTLs associated with root length were present on almost all chromosomes, except chromosomes 4 and 8. At the young seedling stage, several investigations have described numerous QTLs associated with various physical traits, such as shoot length to root dry weight (Gregorio et al. 1997; Masood et al. 2004; Lee et al. 2006; Ammar et al. 2007; Thomson et al. 2010; Islam et al. 2011; Kanjoo et al. 2011; Lang et al. 2017). Recently, many QTLs were found through new markers, such as SNPs (Kumar et al. 2015; Bizimana et al. 2017). A major effect QTL at the early seedling stage in rice, Saltol, has been reported on the short arm of chromosome 1 between 14.7 and 18.6 cM (Thomson et al. 2010) and later between 10.8 Mb and 16.4Mb (Soda et al. 2013). In this study, out of the 20 QTLs mapped, no QTL was found on the short arm of chromosome 1. Other authors, such as Bimpong et al. (2014), Bizimana et al. (2017), Amoah et al. (2020), and reported the same observations. This discrepancy in our observations may be due to the different parents used here as compared to those used during the mapping of Saltol.
Many QTLs for salinity tolerance have been mapped, mainly on chromosomes 1 and 6, but only a few have been reported on chromosomes 2, 3, 5, 9, 10, and 12 (Negrão et al. 2011). In this study, QTLs were identified on all chromosomes, except chromosomes 4 and 8. Four of the 20 QTLs found in this study contributed to more than 10% of the phenotypic variation in the traits studied. Other authors, such as Bizimana et al. (2017), mapped QTLs with a higher percentage contribution to the phenotypic variation. Moreover, out of the 13 QTLs identified by Amoah et al. (2020), only two contributed morethan10% to the phenotypic variation. The tolerance of rice to salinity is a very complex phenomenon due to the multitude of genes or QTLs involved. The observed lower R2 confirms the complexity of salt tolerance and the polygenic nature of parameters related to salinity, such as root length and SIS (Ashraf 2004; Masood et al. 2004; Bimpong et al. 2014; Bizimana et al. 2017).
Out of the 17 QTLs associated with root length, 11 had a negative additive effect, suggesting that the alleles responsible for the improvement in this trait originated from the donor parent, NERICA-L-9. Three QTLs (qLR2.1, qLR3.1, and qLR3.2) contributed more than 15% to phenotypic variation and can be considered as major QTLs. This could be explained by the epistatic interactions due to the alleles from different parents acting together simultaneously to express a given trait. In the Philippines, four QTLs (qRL1.1, qRL1.2, qRL3.1, and qRL11.1) associated with root length were mapped on chromosomes 1, 3, and 11 with LOD between 3.2 and 5.0 (Rahman et al. 2017); Sabouri and Sabouri (2008) mapped them on chromosomes 1, 4, 5, 7, and 9. In the present study, apart from chromosomes 4 and 8, QTLs related to root length were identified on all the remaining chromosomes. The qLR1 mapped in our study occupied the same position as the QTL linked to dry matter. QLR6.1 and qLR6.2 correspond to QTLs linked to sodium concentration. The qLR7.1, qLR7.2 seem to occupy the same location as the QTL corresponding to sodium concentration in leaves. The qRL7.3, qRL9 and qRL12 occupied respectively the same positions as QTLs related to survival days of seedling, Na + concentration in roots and Na + /K + exchange. New QTLs on chromosomes 3 and 11 were discovered. All of these QTLs can be mapped, cloned, and introduced into the genetic background of elite cultivars to increase their tolerance to soil salinization.
Previously several QTLs related to shoot length were found by Rahman et al. (2017) on chromosomes 1 and 12; on chromosomes 1, 6, and 7 by Jahan et al. (2020); on chromosomes 1, 2, 3, 5, and 12 by De Leon et al. (2016); on chromosome 1 by Bimpong et al. (2014); and on chromosomes 3 and 10 by Sabouri and Sabouri (2008). This confirms that several genes control shoot length under salinity conditions. Jahan et al. (2020) identified QTLs related to shoot length on chromosome 6, which is consistent with this study. However, the positions of these previously mapped QTLs differ from the presently identified QTL, qLF6, which occupies a position at 63.45–65.01cM. The additive effect of this QTL is negative, suggesting that the donor parent, NERICA-L-9, was the source of the allele. Bizimana et al. (2017) identified a QTL at position 12.3–27.0 cM on chromosome 6, different to qLF6 identified on same chromosome in the present study. Previously, QTLs related to the SIS were described by Rahman et al. (2017) on chromosomes 1 and 4; by De Leon et al. (2016) on chromosomes 1, 2, 7, 8, 9, and 11; and by Ammar et al. (2007) on chromosomes 1, 3, 4, and 5. Some authors have reported QTLs related to SIS on chromosomes 10 and 12, which is consistent with the observation in this study. Bizimana et al. (2017), identified a QTL on chromosome 12 at the interval 2.9–12.8 cM, which differs from the one mapped in this study. Indeed, the QTL found on chromosome 12 (qSES12) was identified at a range of 52.33–52.57 cM. In addition, Amoah et al. (2020) identified a QTL linked to the SIS on chromosome 10 in the range of 27–35.5 cM. The latter was located on a different interval than the one identified in this study, i.e., qSES10, which was located between 59.47–61.13 cM. Both the QTLs associated with SIS showed a positive additive effect, thus confirming the female (Sahel 328) as the source of the allele. In our study, both QTLs related to SIS showed a positive additive effect, suggesting that the Sahel 328 female was the source of the alleles. Furthermore, Rahman et al. (2017) mapped 6 QTLs related to SIS on chromosomes 1 and 4. These QTLs occupied different chromosomes from those discovered in our study, which were mapped on chromosomes 10 and 12. The QTLs, qSES10 and qSES12, qLF6, can be considered to be newly discovered and might have potential value in breeding programs. The stability of these QTLs appeared to be very important. The presence and position of the QTLs must be confirmed by testing them in the field, as all these QTLs were mapped under hydroponic conditions. Negrão et al. (2011) found that all the significant QTLs in the field did not seem to be in the same location under hydroponic conditions. Therefore, the mapping of QTLs in salty conditions in the field must be carried out in order to verify the stability of these QTLs.
Conclusion
A mapping population of 230 F2:3 families from the Sahel 328/NERICA-L-9 cross was screened for salinity tolerance at the young seedling stage. Agro-morphological traits (leaves length, roots length, leaves fresh weight, roots fresh weight, leaves dry weight, roots dry weight) and the SIS were used for the identification of salinity tolerant lines. All the traits studied were reduced in the presence of salinity equivalent to an electrical conductivity of 12 dS/m. Both the length of the leaves and roots and the weight were reduced. It should be noted that the dry weight of the roots was less affected by salt than the dry weight of the shoots About 92 individuals showed an SIS lower than or equivalent to the tolerant parent (FL478). These can be considered salinity tolerant at the young seedling stage and should be exploited in breeding programs aimed at improving adaptability to salt stress. As a line can show a certain degree of salinity tolerance during the seedling stage, while remaining susceptible during the reproductive stage, it would be judicious to test all the lines identified at the reproductive stage. Those that show some tolerance in both phases (both seedling and reproductive stages) can be evaluated at different sites to see the interaction between genotypes and environment. Twenty QTLs, related to shoot length, root length, and SIS were identified in this study, which are responsible for a phenotypic variation ranging from 0.16 to 78.58%. Of these 20 QTLs, 17 were for root length, two for salinity score, and one for shoot length. Practically, these QTLs were distributed throughout the genome, except for in chromosomes 4 and 8. The alleles of the QTLs originated from both parents, but more than half of them came from the tolerant parent, NERICA-L-9. The QTLs identified in this study could be subjected to a much more finer mapping of the regions, which would help identify the flanking markers, for use in breeding programs, and thus, the QTLs can improve the tolerance of rice cultivars at the seedling stage.
Data availability
Data is available at Mendeley Data, V1, https://doi.org/10.17632/3rz5b6n7h4.1
References
Al-Amin M, Islam M, Begum S et al (2013) Evaluation of rice germplasm under salt stress at the seedling stage through SSR markers. Int J Agric Res Innov Technol 3:52–59. https://doi.org/10.3329/ijarit.v3i1.16093
Ali Y, Awan AR (2004) Influence of salinity at seedling stage and on yield and yield components of different rice lines. Intl J Biol Biotechnol 1(2):175–179
Ammar MHM, Singh RK, Singh AK, Mohapatra T, Sharma TR, Singh NK (2007) Mapping QTLs for salinity tolerance at seedling stage in rice (Oryza sativa L). Afr Crop Sci Conf Proc 8:617–620
Amoah NK, Akromah R, Kena AW, Manneh B, Dieng I, Bimpong IK (2020) Mapping QTLs for tolerance to salt stress at the early seedling stage in rice (Oryza sativa L.) using a newly identified donor ‘Madina Koyo.’ Euphytica. https://doi.org/10.1007/s10681-020-02689-5
Ashraf M (2004) Some important physiological selection criteria for salt tolerance in plants. Flora-Morphol Distrib Funct Ecol Plants 199:361–376. https://doi.org/10.1078/0367-2530-00165
Baby J, Jini D, Sujatha S (2010) Biological and physiological perspectives of specificity in abiotic salt stress response from various rice plants. Asian J Agri Sci 2: 99–105
Bimpong IK, Manneh B, Diop B, Ghislain K, Kofi N, Amoah A (2014) New quantitative trait loci for enhancing adaptation to salinity in rice from Hasawi, a Saudi Landrace into three African cultivars at the reproductive stage. Euphytica 200:45–60. https://doi.org/10.1007/s10681-014-1134-0
Bimpong IK, Manneh B, Sock M, Diaw F, Kofi N, Amoah A, Ismail AM, Gregorio G, Kumar R, Wopereis M (2016) Improving salt tolerance of lowland rice cultivar ‘Rassi’ through marker-aided backcross breeding in West Africa. Plant Sci 242:288–299. https://doi.org/10.1016/j.plantsci.2015.09.020
Bizimana JB, Luzi-Kihupi A, Murori RW, Singh RK (2017) Identification of quantitative trait loci for salinity tolerance in rice (Oryza sativa L.) using IR29/Hasawi mapping population. J Genet 96:571–582. https://doi.org/10.1007/s12041-017-0803-x
De Leon TB, Linscombe S, Subudhi PK (2016) Molecular dissection of seedling salinity tolerance in rice (Oryza sativa L.) using a high-density GBS-based SNP linkage map. Rice. https://doi.org/10.1186/s12284-016-0125-2
FAO (2021) World Food and Agriculture - Statistical Yearbook 2021. Rome. https://doi.org/10.4060/cb4477en
Gregorio GB, Senadhira D, Mendoza RD (1997) Screening rice for salt tolerance.In: IRRI discussion paper series no.22. Manila (Philippines), p 1–30
Hossain H, Rahman MA, Alam MS, Singh RK (2014) Mapping of quantitative trait loci associated with reproductive-stage salt tolerance in Rice. J Agron Crop Sci 201:17–31. https://doi.org/10.1111/jac.12086
Hussain S, Cao X, Zhong C, et al (2018) Sodium chloride stress during early growth stages altered physiological and growth characteristics of rice. Chilean Journal of Agricultural Research, 78(2), 183–197. https://doi.org/10.4067/s0718-58392018000200183
IRRI-SES (Standard Evaluation System for Rice) (2014) IRRI, Manila, p 36
Islam MM, Mondol MNH, Emon RM, Begum SN, Bhowmik SK, Hasan AK (2007) Screening of salt tolerant rice genotypes using SSR markers at seedling stage. Bangladesh J Prog Sci Tech 5(1):45–48
Islam M, Salam M, Hassan L, Collard BCY, Singh RK, Gregorio GB (2011) QTL mapping for salinity tolerance at seedling stage in Rice. Emir J Food Agric 23:137. https://doi.org/10.9755/ejfa.v23i2.6348
Jahan N, Zhang Y, Lv Y, Song M, Zhao C, Hu H, Cui Y, Wang Z, Yang S, Zhang A, Hu J, Ye G, Qian Q, Gao Z, Guo L et al (2020) QTL analysis for rice salinity tolerance and fine mapping of a candidate locus qsl7 for shoot length under salt stress. Plant Growth Regul 90:307–319. https://doi.org/10.1007/s10725-019-00566-3
Javed MA, Ishii T, Kamijima O, Misoo S (2006) Discrepancy of two ecotypes of Oryza sativa L. to salinity at germination and seedling stages. Ann Biol 22(2):201–211
Kakar N, Jumaa SH, Redoña ED, Warburton ML, Reddy KR (2019) Evaluating rice for salinity using pot-culture provides a systematic tolerance assessment at the seedling stage. Rice. https://doi.org/10.1186/s12284-019-0317-7
Kanjoo V, Jearakongman S, Punyawaew K, Siangliw JL, Siangliw M, Vanavichit A, Toojinda T (2011) Co-location of quantitative trait loci for drought and salinity tolerance in rice. Thai J Genet 4(2):126–138
Khatun S, Flowers TJ (1995) Effects of salinity on seed set in rice. Plant Cell Environ 18:61–67. https://doi.org/10.1111/j.1365-3040.1995.tb00544.x
Kumar V, Singh A, Mithra SV, Krishnamurthy SL, Parida SK, Jain S, Tiwari KK, Kumar P, Rao AR, Sharma SK, Khurana JP, Singh NK, Mohapatra T (2015) Genome-wide association mapping of salinity tolerance in rice (Oryza sativa). DNA Res 22:133–145. https://doi.org/10.1093/dnares/dsu046
Lang NT, Phuoc NT, Ha PT, Buu BC (2017) Identifying QTLs associated and marker-assisted selection for salinity tolerance at the seedling, vegetative and reproductive stages in rice (Oryza sativa L.). Int J Environ Agric Biotechnol 2:2927–2935. https://doi.org/10.22161/ijeab/2.6.20
Lee SY, Ahn JH, Cha YS, Yun DW, Lee MC, Ko JC et al (2006) Mapping of quantitative trait loci for salt tolerance at the seedling stage in rice. Mol Cells 21:192–196
Maiti RK, Vidyasagar P, Banerjee PP (2006) Salinity tolerance in rice (Oryza sativa L.) hybrids and their parents at emergence and seedling stage. Crop Res Hisar 31(3):427–433
Masood MS, Seiji Y, Shinwari ZK, Anwar R (2004) Mapping quantitative trait loci (QTLs) for salt tolerance in rice (Oryza sativa) using RFLPs. Pak J Bot 36(4):825–834
McCouch SR, Cho Y, Yano M, Paul E, Blinstrub M, Morishima H, Kinoshita T (1997) Report on QTL nomenclature. Rice Genet Newsl 14:11–13
Milne I, Shaw P, Stephen G, Bayer M, Cardle L, Thomas WTB et al (2010) Flapjack—graphical genotype visualization. Bioinformatics 26:3133–3134. https://doi.org/10.1093/bioinformatics/btq580
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Munns R, James RA, Läuchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57:1025–1043. https://doi.org/10.1093/jxb/erj100
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326. https://doi.org/10.1093/nar/8.19.4321
Negrão S, Courtois B, Ahmadi N, Abreu I, Saibo N, Oliveira MM (2011) Recent updates on salinity stress in rice: from physiological to molecular responses. Crit Rev Plant Sci 30:329–377. https://doi.org/10.1080/07352689.2011.587725
Pabuayon IC, Kitazumi A, Cushman KR, Singh RK, Gregorio GB, Dhatt B, Zabet-Moghaddam M, Walia H, de los Reyes BG (2021) Novel and transgressive salinity tolerance in recombinant inbred lines of rice created by physiological coupling-uncoupling and network rewiring effects. Front Plant Sci. https://doi.org/10.3389/fpls.2021.615277
R Core Team (2024) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rahman MA, Bimpong IK, Bizimana JB et al (2017) Mapping qtls using a novel source of salinity tolerance from Hasawi and their interaction with environments in Rice. Rice. https://doi.org/10.1186/s12284-017-0186-x
Rohila JS, Edwards JD, Tran GD, Jackson AK, Mcclung AM (2019) Identification of superior alleles for seedling stage salt tolerance in the USDA rice mini-core collection. Plants 8:472. https://doi.org/10.3390/plants8110472
Sabouri H, Sabouri A (2008) New evidence of QTLs attributed to salinity tolerance in rice. Afr J Biotech 7(24):4376–4383
Singh RK, Gregorio GB, Jain RK (2007) QTL mapping for salinity tolerance in rice. Physiol Mol Biol Plants 13:87–99
Singh RK, Flowers TJ (2010) Physiology and molecular biology of the effects of salinity on rice. In: Handbook of plant and crop stress p 929–970. https://doi.org/10.1201/b10329-49
Singh RK, Singh KN, Mishra B, Sharma SK, Tyagi NK (2004) Harnessing plant salt tolerance for overcoming sodicity constraints: an Indian experience. In: Advances in sodic land reclamation, Proc intl conf sustainable management of sodic soils, Lucknow, p 81–120
Soda N, Kushwaha HR, Soni P, Singla-Pareek SL, Pareek A (2013) A suite of new genes defining salinity stress tolerance in seedlings of contrasting rice genotypes. Funct Integr Genomics 13:351–365. https://doi.org/10.1007/s10142-013-0328-1
Suplick Ploense MR, Qian YL, Read JC (2002) Salinity tolerance of Texas bluegrass, Kentucky bluegrass, and their hybrids. Crop Sci 42:2025–2030
Thomson MJ, de Ocampo M, Egdane J, Rahman MA, Sajise AG, Adorada DL, Tumimbang-raiz E, Blumwald E, Seraj ZI, Singh RK, Gregorio GB, Ismail AM (2010) Characterizing the saltol quantitative trait locus for salinity tolerance in Rice. Rice 3:148–160. https://doi.org/10.1007/s12284-010-9053-8
Titov S, Bhowmik SK, Islam MM, Sultana S, Haque S (2009) Phenotypic and genotypic screening of rice genotypes at seedling stage for salt tolerance. Afr J Biotechnol 8(23):6490–6494
United Nations Population Fund (2021) Delivering on the transformative results: UNFPA annual report 2021. United Nations Population Fund. https://www.unfpa.org/sites/default/files/pub-pdf/EN_AR2021.pdf
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and qtls. J Hered 93:77–78
Wang S, Basten CJ, Zeng ZB (2012) Windows QTL Cartographer 2.5. (Department of Statistics, North Carolina State University, Raleigh, NC)
Yadav S, Irfan MD, Ahmad A, Hayat S (2011) Causes of salinity and plant manifestations to salt stress: a review. J Environ Biol 32(5):667–685
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Laboratory manual for physiological studies of rice, 3rd edn. International rice research institute, Manila
Zeng L, Shannon MC, Lesch SM (2001) Timing of salinity stress affects rice growth and yield components. Agric Water Manage 48:191–206
Acknowledgements
We are grateful to Mrs. Faty Diaw, Mrs. Anta Gueye, Mr. Papa A Diakhate, and Mr. Modou S Faye for their assistance in the greenhouse data collection.
Funding
This work was supported by the AfricaRice Sahel Center, KAFACI project (Enhancement of high-yielding rice germplasm and breeding capacity of rice producing countries in Africa by the Africa Rice Development Partnership, Phase II), funded by the South Korean government. Grant Number: KAR201600.
Author information
Authors and Affiliations
Contributions
MS performed the experimentation and data analysis, wrote the original draft, KB and DD performed the conceptualization, reviewed and edited the manuscript. NKAA performed data analysis and reviewed the manuscript. BM and SBL reviewed the project. All the authors agreed to this final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sock, M., Diouf, D., Amoah, N.K.A. et al. Identification of quantitative trait loci for salinity tolerance in rice (Oryza sativa L.) through Sahel 328/NERICA-L-9 mapping population at seedling stage. Genet Resour Crop Evol (2024). https://doi.org/10.1007/s10722-024-02108-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10722-024-02108-x