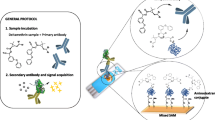
Abstract
Fishes display a wide variation in their physiological responses to stress, which is clearly evident in the plasma corticosteroid changes, chiefly cortisol levels in fish. In the present study, we describe a novel label-free immunosensor for detecting plasma cortisol levels. The method is based on immunologic reactions and amperometric measurement using cyclic voltammetry. For the immobilization of the antibody on the surface of sensing electrode, we used a self-assembled monolayer of thiol-containing compounds. Using this electrode, we detect the CV signal change caused by the generation of antigen–antibody complex. The immunosensor showed a response to cortisol levels, and the anodic peak value linearly decreased with a correlation coefficient of 0.990 in diluted plasma. The specificity of the label-free immunosensor system was investigated using other steroid hormones, such as 17α, 20β-dihydroxy-4-pregnen-3-one, progesterone, estriol, estradiol, and testosterone. The specific detection of cortisol was suggested by a minimal change from −0.32 to 0.51 μA in the anodic peak value of the other steroid hormones. The sensor system was used to determine the plasma cortisol levels in Nile tilapia (Oreochromis niloticus), and the results were compared with those of the same samples determined using the conventional method (ELISA). A good correlation was obtained between values determined using both methods (correlation coefficient 0.993). These findings suggest that the proposed label-free immunosensor could be useful for rapid and convenient analysis of cortisol levels in fish plasma samples.






Similar content being viewed by others
References
Barreto RE, Volpato GL (2006) Stress responses of the fish Nile tilapia subjected to electroshock and social stressors. Braz J Med Biol Res 39:1605–1612
Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effect of corticosteroids. Annu Rev Fish Dis 1:3–26
Barton BA, Morgan JD, Vijayan MM (2002) Physiological and condition-related indicators of environmental stress in fish. In: Adams SM (ed) Biological indicators of stress, 2nd edn. American Fisheries Society, Bethesda, pp 111–148
Barton BA, Ribas L, Acerete L, Tort L (2005) Effects of chronic confinement on physiological responses of juvenile gilthead sea bream, Sparus aurata L., to acute handling. Aquac Res 36:172–179
Chang C, Chou C, Lee M (2005) Determining leaching of bisphenol A from plastic containers by solid-phase microextraction and gas chromatography-mass spectrometry. Anal Chem Acta 539:41–47
Choi SH, Lee JW, Sim SJ (2005) Enhanced performance of a surface plasmon resonance immunosensor for detecting Ab–GAD antibody based on the modified self-assembled monolayers. Biosens Bioelectron 21:378–383
Delaney MA, Klesius PH, Shelby RA (2005) Cortisol response of Nile Tilapia, Oreochromis niloticus (L.), to temperature changes. J Appl Aquac 16:95–104
Douxfils J, Lambert S, Mathieu C, Milla S, Mandiki SNM, Henrotte E, Wang N, Dieu M, Raes M, Rougeot C, Kestemont P (2014) Influence of domestication process on immune response to repeated emersion stressors in Eurasian perch (Perca fluviatilis, L.). Comp Biochem Physiol A Mol Integr Physiol 173:52–60
Endo H, Igarashi M, Banda A, Ohnuki H, Ushio H, Hayashi T, Ren H, Yoshizaki G (2011) Electrode-based immunologic assay system to monitor oocyte maturation-inducing hormone in fish. Int J Environ Anal Chem 91:174–184
Endo H, Muramatsu T, Yoshizaki G, Ren H, Ohnuki H (2012) Development of a label-free immunosensor system for detecting oocyte maturation-inducing hormone in fish. Fish Sci 78:391–398
Farrell AP (2011) Encyclopedia of fish physiology: from genome to environment, 1st edn. Academic Press, Waltham
Fast MD, Hosoya S, Johnson SC, Afonso LOB (2008) Cortisol response and immune-related effects of Atlantic salmon (Salmo salar Linnaeus) subjected to short- and long-term stress. Fish Shellfish Immunol 24:194–204
Fischer LM, Tenje M, Heiskanen AR, Masuda N, Castillo J, Bentien A, Émneus J, Jakobsen MH, Boisen A (2009) Gold cleaning methods for electrochemical detection applications. Microelectron Eng 86:1282–1285
Gamperl AK, Vijayan MM, Boutilier RG (1994) Experimental control of stress hormone levels in fishes: techniques and applications. Rev Fish Biol Fisher 4:215–255
Inoue K, Wada M, Higuchi T, Oshio S, Umeda T, Yoshimura Y, Nakazawa H (2002) Application of liquid chromatography-mass spectrometry to the quantification of bisphenol A in human semen. J Chromatogr B Technol Biomed Life Sci 733:97–102
Iwama GK, Thomas PT, Forsyth RB, Vijayan MM (1998) Heat shock protein expression in fish. Rev Fish Biol Fisher 8:35–56
Kagawa H, Young G, Nagahama Y (1983) Changes in plasma steroid hormone levels during gonadal maturation in female goldfish, Carassius auratus. Nippon Suisan Gakkaishi 49:1783–1787
Kobayashi N, Sun P, Fujimaki Y, Niwa T, Nishio T, Goto J, Hosoda H (2002) Generation of a novel monoclonal antibody against cortisol- [C-4]: bovine serum albumin conjugate: application to enzyme-linked immunosorbent assay for urinary and serum cortisol. Anal Sci 18:1309–1314
Li J, Wu Z, Wang H, Shen G, Yu R (2005) A reusable capacitive immunosensor with a novel immobilization procedure based on 1, 6-hexanedithiol and nano-Au self-assembled layers. Sens Actuators B Chem 110:327–334
Mason DW, Williams AF (1980) The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J 187:1–20
Mommsen TP, Vijayan MM, Moon TW (1999) Cortisol in teleosts: dynamics, mechanism of action, and metabolic regulation. Rev Fish Biol Fisher 9:211–268
Muramatsu T, Ohnuki H, Ushio H, Hibi K, Igarashi M, Hayashi T, Ren H, Endo H (2011) Electrochemical flow injection immunoassay for cortisol using magnetic microbeads. Int J Environ Anal Chem 91:161–173
Nikaido Y, Aluru N, McGuire A, Park YJ, Vijayan MM, Takemura A (2010) Effect of cortisol on melatonin production by the pineal organ of tilapia, Oreochromis mossambicus. Comp Biochem Physiol A Mol Integr Physiol 155:84–90
Ogawa K, Ito F, Nagae M, Nishimura T, Yamaguchi M, Ishimatsu A (2011) Effects of acid stress on reproductive functions in immature Carp, CYPRIMUS CARPIO. Water Air Soil Pollut 130:887–892
Piao MH, Noh HB, Rahman MA, Won MS, Shim YB (2008) Label-free detection of bisphenol: a using a potentiometric immunosensor. Electroanalysis 20:30–37
Qiu LP, Wang CC, Hu P, Wu ZS, Shen GL, Yu RQ (2010) A label-free electrochemical immunoassay for IgG detection based on the electron transfer. Talanta 83:42–47
Radi AE, Muñoz-Berbel X, Lates V, Marty JL (2009) Label-free impedimetric immunosensor for sensitive detection of ochratoxin A. Biosens Bioelectron 24:1888–1892
Thavarungkul P, Dawan S, Kanatharana P, Asawatreratanakul P (2007) Detecting penicillin G in milk with impedimetric label-free immunosensor. Biosens Bioelectron 23:688–694
Tschmelak J, Proll G, Gaugiltz G (2004) Verification of performance with the automated direct optical TIRF immunosensor (River analyser) in single and multi-analyte assays with real water samples. Biosens Bioelectron 20:743–752
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Acknowledgments
This research was supported in part by a Grant-in-Aid for Scientific Research (B) from The Ministry of Education, Culture, Sports, Science, and Technology in Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, H., Ohnuki, H., Hibi, K. et al. Development of a label-free immunosensor system for detecting plasma cortisol levels in fish. Fish Physiol Biochem 42, 19–27 (2016). https://doi.org/10.1007/s10695-015-0113-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0113-2