Abstract
Background: Familial adenomatous polyposis (FAP) predisposes individuals to duodenal adenomas. This study describes the histopathological features of endoscopic and surgical specimens from the duodenum, as well as genotype-phenotype associations. Methods: All known FAP patients were included from the Danish Polyposis Register. FAP patients were defined as having more than 100 cumulative colorectal adenomas and/or having a known germline pathogenic variant in the APC gene. Endoscopic procedures, histopathology, and genetics were evaluated. Results: Of 500 FAP patients, 70.6% underwent esophagogastroduodenoscopy (EGD) at least once. Of these, 59.2% presented with detectable duodenal adenomas. The most severe morphology was tubular in 62.7% patients, tubulovillous in 25.4%, and villous in 12.0%, while the most severe dysplasia was low-grade in 67.5% patients, high-grade in 25.4%, and 6.7% had adenocarcinoma. In 6.2% of FAP patients, duodenal resection was recommended, including 29% with duodenal adenocarcinoma. The risk of duodenal surgery was 1.31 per 1,000 person-years (median age: 53 years). The predominant reason for surgery was extensive polyposis (67.7%). Of the patients who underwent duodenal resection, a median of six (IQR: 4–8) EGDs were performed within five years prior to surgery, but 67.6% and 83.9% never underwent a duodenal polypectomy or endoscopic mucosa resection, respectively. Of note, seventeen of 500 patients (3.4%) developed duodenal adenocarcinoma, of which 47% were advanced at diagnosis. Genetic evaluations revealed various pathogenic variants in the APC gene, with no strong genotype-phenotype association. Conclusions: The prevalence of duodenal adenomas and cancer in FAP warrants vigilant endoscopic surveillance. Nevertheless, the need for duodenal surgery persists and should together with endoscopic practice be monitored in national registers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Familial adenomatous polyposis (FAP) is an autosomal dominantly inherited disorder, which in addition to colorectal polyposis predisposes individuals to duodenal adenomatosis and cancer [1,2,3,4,5]. In the twentieth century, prophylactic colectomy was introduced for FAP patients and it decreased the risk of colorectal cancer and resulted in prolonged life expectancy [6]. Consequently, the importance of duodenal manifestations has increased. To prevent the development of duodenal cancer, it is essential to identify high-risk cases early on and refer them for surgical intervention before they undergo malignant transformation. Presently, the Spigelman classification system offers a comprehensive approach for endoscopic staging of duodenal adenomatosis and for assessing the risk of duodenal cancer. This system integrates factors such as the number and size of the adenomas, along with their morphology and the extent of dysplasia [7]. Although the Spigelman classification has been validated, it tends to underestimate the importance of ampullary lesions and does not closely correlate with the risk of duodenal cancer [8,9,10,11]. Additionally, it requires obtaining biopsies from duodenal lesions that many endoscopists would prefer to remove completely, either by simple polypectomy or endoscopic mucosal resection (EMR) [12,13,14].
Duodenal resection is indicated in the event of a localized duodenal cancer, as well as prophylactically in cases with severe polyposis and/or an assumed high risk of cancer where endoscopic surveillance and treatment is considered insufficient [15, 16]. However, the threshold for duodenal resection is not clearly defined and the need for prophylactic surgical resection might be reduced with increased use of invasive endoscopic techniques.
In Denmark, all known patients with FAP have been meticulously registered in the Danish Polyposis Register for the last 40 years, which includes data about endoscopic and surgical procedures, as well as genetic reports [17]. The register is free of referral and selection bias, which helps to ensure reliable estimations for the risk of needing duodenal surgery, as well as preoperative endoscopic interventions and other risk factors.
We evaluated the histopathological severity of duodenal polyposis in the surgical specimens and compared it with previous endoscopic examinations, as well as genotypes. Furthermore, we examined whether the need for duodenal resections has been reduced in recent decades, possibly as a benefit of endoscopic interventions.
Methods
The Danish Polyposis Register was established in 1971 and became nationwide in 1974 [17]. It comprises all Danish FAP patients. Endoscopic, surgical, and histopathological reports are all included, together with pedigrees and genetic test results. We conducted a cohort study of all known patients with FAP. No ethics approval or informed written consent were needed as this was a cohort study.
Definitions
FAP patients were defined as having 100 cumulative colorectal adenomas or more and/or having a known germline pathogenic variant in the APC gene (pathogenic or likely pathogenic, using American College of Medical Genetics and Genomics/the Association for Molecular Pathology APC gene-specific guidelines) [18]. Patients with more than 100 colorectal adenomas and a known non-APC-related genetic etiology were excluded from the register and this study.
FAP cohort
The cohort consisted of all verified FAP patients registered in the Danish Polyposis Register up until April 22nd, 2021. Patients needed to have been alive on January 1st 1990 and should not have undergone duodenal surgery or developed duodenal cancer before initiation of the study. Patients with a duodenal resection (Whipple procedure or total pancreatectomy) due to pancreatic premalignant lesions or cancer were excluded. Since 1968, all Danish individuals have had a unique, 10-digit personal identification number [19]. We submitted the identification numbers of the FAP patients to Statistics Denmark, which enabled us to extract a complete list of endoscopic and surgical procedures from the National Patient Register (Supplementary Material 1), alongside the histopathological results from the Danish Pathology Register (Supplementary Material 2). Additionally, genotypes and indications for surgery were provided by the Polyposis Register.
Outcomes
The primary outcome was duodenal resection due to duodenal adenomatosis or cancer defined as risk per 1,000 person-years. Pancreatic indications were excluded. Secondary outcomes included the risk of developing duodenal adenomas and their morphology and grade of dysplasia. Additionally, the associations between surgical and endoscopic findings, in terms of adenoma morphology, grade of dysplasia, and adenocarcinoma, were analyzed, together with the risk of duodenal surgery. The most severe morphology and grade of dysplasia in each patient were counted. The most severe morphology was defined as villous, followed by tubulovillous, then tubular. Surgical and endoscopic modalities for the treatment of duodenal adenomas were assessed. Finally, the genotypes for all patients with duodenal resections were noted. According to the regulations of Statistics Denmark, absolute numbers of groups smaller than three were omitted.
Statistical methods
Follow-up of patients started on the date of their FAP diagnosis or on January 1st, 1990, whichever was most recent. Follow-up ended on the date of duodenal resection, death, loss to follow-up, or the end of the study, whichever occurred first. Baseline characteristics of the cohort were described using medians and interquartile ranges (IQR) for numerical variables and counts and proportions for categorical variables. A two-sided P value < 0.05 was considered significant. R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria) was used to perform all statistical analyses.
Results
Characteristics of patients with familial adenomatous polyposis
The cohort comprised 500 eligible FAP patients; 235 were female (47%) (Table 1). The genotype was known in 439 (87.8%) patients, and 176 were probands (35%). Of the 500 patients, 17 (3.4%) developed duodenal cancer (adenocarcinoma), 14 of which were identified in biopsies taken during an esophagogastroduodenoscopy (EGD). The remaining three cases of adenocarcinomas were found in the resected specimens in patients with high-grade dysplasia (HGD) in the endoscopic biopsies prior to surgery.
Endoscopy
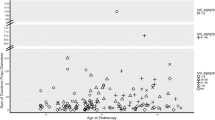
During the follow-up period, 60.8% (304/500) of patients received at least one EGD, 53.0% (265/500) an EGD with biopsies of duodenal polyps, and 70.6% (353/500) either at least one EGD or an EGD with biopsies. Of those who did not receive an EDG, two out of three were either below the age where duodenal surveillance is initiated or died due to CRC before initiating duodenal surveillance. Duodenal polypectomy was performed in 9.4% of the FAP patients (47/500), while 4.8% (24/500) underwent endoscopic mucosal resection (EMR)/endoscopic submucosal dissection (ESD)/argon plasma coagulation of duodenal lesions (Fig. 1).
In 59.2% (209/353) of patients who underwent at least one EGD, the histopathology from either an endoscopic biopsy or a resection showed adenoma. The most severe morphology in these patients included 62.7% (131/209) with tubular adenomas, 25.4% (53/209) with tubulovillous adenomas, and 12.0% (25/209) with villous adenomas (Fig. 2). There was low-grade dysplasia (LGD) in 67.5% (141/209) and HGD in 25.4% (53/209) of patients (Fig. 2). The histopathological diagnosis was adenocarcinoma in 3.9% of FAP patients who received at least one EGD (14/353), corresponding to 6.7% of patients with known duodenal adenomatosis (14/209). Of the 14 patients with malignant histopathology, 57.1% (8/14) did not undergo surgery due to disseminated disease.
Duodenal surgery
During the follow-up period, 6.2% (31/500) of FAP patients underwent duodenal resection, corresponding to a risk of 1.31 per 1,000 person-years. The median age at surgery was 53 years (IQR = 41–62 years) and 39% (12/31) of patients were female. A Whipple procedure was performed in 67.7% (21/31), while the remaining patients underwent a pancreas-preserving duodenectomy. The histopathology in the resected specimens included adenocarcinoma in 29% (9/31) of the cases and benign histology in the remaining 22 cases (71%). In three of the nine patients diagnosed with adenocarcinoma, the endoscopic biopsies prior to surgery showed HGD as the most severe morphology. However, upon surgical resection, adenocarcinoma was identified in the specimens. In all benign cases, the histopathology showed adenomas with HGD in 86.3% (19/22) of cases and LGD in 13.6% (3/22) of cases. Over a median follow-up period of 9 years, no patients who underwent a duodenectomy required conversion to a Whipple procedure.
Indication for duodenal surgery
The indication for duodenal resection was extensive duodenal adenomatosis prohibiting safe endoscopic surveillance or treatment in 67.7% (21/31) of patients. In the remaining cases, there was an endoscopic suspicion of either an ampullary (22.6%) or luminal (6.5%) cancer. Of these cases, the suspicion of malignancy was confirmed in 88.9% by histopathological examination of the surgical specimen. The indication was not clear in 3.2% (1/22) of cases. For patients receiving surgery with the indication of extensive polyposis, 9.5% (2/21) were operated upon between 1990 and 1999, 38.1% (8/21) between 2000 and 2009, and 52.3% (11/21) between 2010 and 2019.
Genetics
Patients undergoing duodenal resection or developing unresectable duodenal cancer comprised 28 families; the pathogenic variant was known in 89.7% (35/39) of patients. Three variants (p.(Glu1309Aspfs*4), p.(Glu1156Glyfs*8) and p.(Gln161*)) were detected in more than one family, but otherwise each family carried a different variant. All pathogenic variants were frameshift, nonsense, or splice variants, including single nucleotide variants, smaller or larger deletions or duplications, or large rearrangements (including whole APC gene deletion in one family) (Fig. 3). A need for duodenal resection or unresectable duodenal cancer was identified in six patients from one family who were carrying the c.2626C > T, p.(Arg876*) variant. Two families had a variant that was located 5’ of codon 168 in an area associated with A-FAP, while no variants were detected in other A-FAP regions in the APC gene, including codon 312–412 (alternative part of exon 9) and 3’ of codon 1580 (Fig. 3). Additionally, one family had a variant in the codon 976–1067 region. Most pathogenic variants were localized in exon 16. We failed to identify any firm genotype-phenotype correlation.
Lollipop plot showing the APC (NM_000038.6) single nucleotide variants described in this study in relation to attenuated FAP (AFAP) (light blue) and classical FAP (CFAP) (light brown) regions of the APC gene. The variants include 10 frameshift variants (red), eight nonsense variants (green) and one splice variant (blue). Only one variant (p.(Gln161*)) is located in the AFAP region. Exons are indicated with dashed lines starting with the first coding exon (exon 2)
Endoscopic findings prior to surgery
Prior to the surgical procedure, all patients had received at least one EGD. Of the nine patients with adenocarcinoma in their surgical specimen, 66.7% (6/9) had endoscopic biopsies with adenocarcinoma, while 33.3% (3/9) had HGD in the endoscopic biopsies prior to surgery. In cases with a benign surgical pathology, endoscopic biopsies or polypectomy/EMR specimens before surgery included LGD in 27.3% (6/22) of cases and HGD in 72.7% (16/22) of cases (Table 2). A median of six (IQR: 4–8) EGDs were performed within the five years prior to surgery and 35.5% (11/31) had villous adenomas (Table 2). In 67.6% (21/31) of patients who received a duodenal resection, the patient never underwent a duodenal polypectomy and EMR/ESD/APC were not carried out in 83.9% (26/31) of patients.
Discussion
In this nationwide cohort study of all known Danish FAP patients, we found that during a 30-year period the risk of duodenal surgery was 1.31 per 1,000 person-years with a median age at surgery of 53, and an increasing number of resections being carried out during this period. In 71.0% of FAP patients undergoing duodenal surgery, the indications, as well as the final histopathology, were benign. However, two-out-of-three patients never underwent a duodenal polypectomy before surgery, and only 16% had a duodenal EMR, thus emphasizing that the full potential of endoscopic interventions might not have been thoroughly explored. This also indicates that the described risk of duodenal surgery closely follows the natural progression of developing duodenal adenomatosis over time.
Studies have reported a lifetime risk of duodenal adenomatosis in up to 90% of individuals with FAP [2]. The progression from adenoma to adenocarcinoma in the duodenum, albeit slower than in the colon and rectum, remains a significant cause of morbidity and mortality [3, 6]. A recent study demonstrated that FAP patients had a 14-fold higher risk of developing duodenal/small bowel cancer compared to the general population [1]. Thus, regular surveillance for duodenal lesions is paramount. In addition to surveillance, a growing body of evidence suggests that endoscopic techniques can obviate the need for surgery in a significant proportion of patients [13, 20, 21]. However, challenges remain. While EMR is efficient in removing larger lesions, duodenal EMR has its own set of adverse events such as bleeding, perforation, and post-polypectomy syndrome. Nevertheless, recent studies evaluating the use of cold snares for EMR have shown promising results, with fewer adverse events and few recurrent lesions [22,23,24]. Likewise, duodenal polypectomy, either with hot or cold snares, seems very safe and might remove duodenal lesions before they advance [13, 25]. In our study, we found that only a minority of patients had undergone endoscopic removal of duodenal lesions before surgery. While our study’s analyses cannot definitively determine if some surgeries could have been avoided, the data strongly suggest that most patients did not receive the full benefit of currently available endoscopic therapies and the potential of endoscopic therapy was not integrated in the surveillance of these patients. This is despite of centralized surveillance in four centers for three decades. A further centralization into two centers each covering around 200 patients might be ideal to facilitate dedicated patient care.
Endoscopic techniques, while reducing the need for surgery, cannot always replace it, especially for ampullary lesions extending into the pancreatic or common bile duct. In FAP patients with duodenal lesions, choices often oscillate between the Whipple procedure, known for its comprehensive resection and associated complications, and the less invasive pancreas-preserving duodenectomy. The latter, while preserving pancreatic function, can raise the risk of recurrence and limit lymph node clearance in cases of malignancy [26]. Our study showed that two-thirds of patients underwent a Whipple procedure, probably reflecting the presence or suspicion of a malignant lesion. Notably, while the number of Whipple procedures seems to be on the rise, there is a declining trend in pancreas-preserving duodenectomies. This may complicate post-operative endoscopic management, as deep small bowel enteroscopy is needed to inspect the Roux-en-Y limb because of the Whipple operation. The cause of this trend remains undetermined. It might be influenced by surgical preferences, or the future risk of requiring a Whipple procedure due to ampullary adenomatosis [26].
The FAP patients who received a duodenal resection, together with those who developed disseminated duodenal cancer, represent the most severe phenotype. We analyzed the pathogenic APC variants in all these patients and found that only one family had a variant in the codon 976–1067, which has previously been associated with a 3-4-fold risk of developing duodenal adenomatosis [27]. Furthermore, one variant identified in two families was somewhat surprisingly located in an area of the gene which has previously been associated with a less severe phenotype (attenuated FAP) [28,29,30]. We find that the number of families/patients were too few for us to conclude there is a firm phenotype-genotype correlation; hence, endoscopic surveillance and treatment cannot be stratified according to specific pathogenic variants in the APC gene based on the present data. Nonetheless, we identified 6 cases of duodenal resections/unresectable cancer in one family who was carrying the c.2626 C > T, p.(Arg876*) pathogenetic variant and in this family, intensified surveillance might be justified.
The evolving role of endoscopic interventions, particularly polypectomy, EMR and endoscopic papillectomy in managing duodenal lesions in FAP cannot be understated [20, 31,32,33]. While they offer significant advantages over surgical modalities, a comprehensive, individualized approach is crucial to ensure optimal patient outcomes [12]. Further studies, preferably comparative, that focus on long-term outcomes and newer endoscopic techniques, are eagerly awaited. Of note, in our study the number of endoscopic resections was limited, hence, the FAP cohort may be considered representing the long-term natural course of duodenal adenomatosis under endoscopic surveillance.
This study is limited by the small number of patients undergoing duodenal resection. Furthermore, our knowledge of the endoscopic surveillance before referral for surgery is limited to procedural codes and details such as Spigelman classification and possible reasons for omitting duodenal surveillance are not available. Likewise, we cannot fully explain why half of the patients with duodenal cancer were disseminated at the time of diagnosis. However, the study’s strengths include a national database free of referral and selection bias, as well as access to pathology reports after both endoscopy and surgery for comparison. We have not included adverse events of endoscopic and surgical interventions as we do not find the coding consistent over the complete study period. Finally, endoscopic technology has been improved considerably during the study period, which might have improved the optical diagnoses.
Our nationwide cohort study encompassing the entire Danish FAP population revealed a risk of duodenal surgery of 1.31 per 1,000 person-years, with patients undergoing surgery at a median age of 53 years. Strikingly, two-thirds of the patients referred for surgical intervention had not previously received a duodenal polypectomy, and even fewer an EMR. Furthermore, most patients were found to have a benign histopathology in their surgical specimen. To ensure the quality of endoscopic surveillance and interventions, we find it essential to monitor endoscopic practice and development of duodenal cancer in national registers.
Data availability
Anonymized and summarized data collected for the study will be made available to other researchers upon publication and following reasonable requests made to the corresponding author.
References
Karstensen JG, Bulow S, Hojen H et al (2023) Cancer in patients with familial adenomatous polyposis: a nationwide Danish cohort study with matched controls. Gastroenterology. https://doi.org/10.1053/j.gastro.2023.05.010. [published Online First: 2023/05/19]
Bulow S, Christensen IJ, Hojen H et al (2012) Duodenal surveillance improves the prognosis after duodenal cancer in familial adenomatous polyposis. Colorectal Dis 14(8):947–952. https://doi.org/10.1111/j.1463-1318.2011.02844.x[published Online First: 2011/10/07]
Ghorbanoghli Z, Bastiaansen BA, Langers AM et al (2018) Extracolonic cancer risk in Dutch patients with APC (adenomatous polyposis coli)-associated polyposis. J Med Genet 55(1):11–14. https://doi.org/10.1136/jmedgenet-2017-104545[published Online First: 2017/05/12]
Groves CJ, Saunders BP, Spigelman AD, Phillips RK (2002) Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10 year prospective study. Gut 50(5):636–641. https://doi.org/10.1136/gut.50.5.636[published Online First: 2002/04/16]
Karstensen JG, Wullum L, Andersen KK et al (2023) Psychiatric and educational aspects of familial adenomatous polyposis - a nationwide Danish cohort study with matched non-exposed individuals. Am J Gastroenterol. https://doi.org/10.14309/ajg.0000000000002612[published Online First: 2023/11/30]
Karstensen JG, Burisch J, Pommergaard HC et al (2019) Colorectal Cancer in Individuals With Familial Adenomatous Polyposis, Based on Analysis of the Danish Polyposis Registry. Clin Gastroenterol Hepatol ;17(11):2294 – 300 e1. https://doi.org/10.1016/j.cgh.2019.02.008 [published Online First: 2019/02/12]
Spigelman AD, Williams CB, Talbot IC et al (1989) Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet 2(8666):783–785. https://doi.org/10.1016/s0140-6736(89)90840-4[published Online First: 1989/09/30]
Karstensen JG, Bulow S, Burisch J et al (2022) Validation of the endoscopic part of the Spigelman classification for evaluating duodenal adenomatosis in familial adenomatous polyposis: a prospective study of Interrater and Intrarater Reliability. Am J Gastroenterol 117(2):343–345. https://doi.org/10.14309/ajg.0000000000001582[published Online First: 2021/12/17]
Soons E, Bisseling TM, van Kouwen MCA et al (2021) Endoscopic management of duodenal adenomatosis in familial adenomatous polyposis-A case-based review. United Eur Gastroenterol J 9(4):461–468. https://doi.org/10.1002/ueg2.12071[published Online First: 2021/09/17]
Sourrouille I, Lefevre JH, Shields C et al (2017) Surveillance of duodenal polyposis in familial adenomatous polyposis: should the Spigelman score be modified? Dis Colon Rectum 60(11):1137–1146. https://doi.org/10.1097/DCR.0000000000000903[published Online First: 2017/10/11]
Latchford AR, Neale KF, Spigelman AD et al (2009) Features of duodenal cancer in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol 7(6):659–663. https://doi.org/10.1016/j.cgh.2009.02.028[published Online First: 2009/03/14]
Aelvoet AS, Pellise M, Bastiaansen BAJ et al (2023) Personalized endoscopic surveillance and intervention protocols for patients with familial adenomatous polyposis: the European FAP Consortium strategy. Endosc Int Open 11(4):E386–E93. https://doi.org/10.1055/a-2011-1933[published Online First: 2023/04/27]
Takeuchi Y, Hamada K, Nakahira H et al (2023) Efficacy and safety of intensive downstaging polypectomy (IDP) for multiple duodenal adenomas in patients with familial adenomatous polyposis: a prospective cohort study. Endoscopy 55(6):515–523. https://doi.org/10.1055/a-1983-5963[published Online First: 2022/11/22]
Roos VH, Bastiaansen BA, Kallenberg FGJ et al (2021) Endoscopic management of duodenal adenomas in patients with familial adenomatous polyposis. Gastrointest Endosc 93(2):457–466. https://doi.org/10.1016/j.gie.2020.05.065[published Online First: 2020/06/15]
van Leerdam ME, Roos VH, van Hooft JE et al (2019) Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy ;51(9):877 – 95. https://doi.org/10.1055/a-0965-0605 [published Online First: 2019/07/26]
Yang J, Gurudu SR, Koptiuch C et al (2020) American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc ;91(5):963 – 82 e2. https://doi.org/10.1016/j.gie.2020.01.028 [published Online First: 2020/03/15]
Bulow S, Bulow C, Nielsen TF et al (1995) Centralized registration, prophylactic examination, and treatment results in improved prognosis in familial adenomatous polyposis. Results from the Danish polyposis Register. Scand J Gastroenterol 30(10):989–993. https://doi.org/10.3109/00365529509096343[published Online First: 1995/10/01]
Spier I, Yin X, Richardson M et al (2024) Gene-specific ACMG/AMP classification criteria for germline APC variants: recommendations from the ClinGen InSiGHT Hereditary Colorectal Cancer/Polyposis variant Curation Expert Panel. Genet Med 26(2):100992. https://doi.org/10.1016/j.gim.2023.100992[published Online First: 20231004]
Pedersen CB, Gotzsche H, Moller JO, Mortensen PB (2006) The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull 53(4):441–449 [published Online First: 2006/12/08]
Moussata D, Napoleon B, Lepilliez V et al (2014) Endoscopic treatment of severe duodenal polyposis as an alternative to surgery for patients with familial adenomatous polyposis. Gastrointest Endosc 80(5):817–825. https://doi.org/10.1016/j.gie.2014.03.012[published Online First: 2014/05/13]
Iwata K, Kato M, Sasaki M et al (2023) Intensive endoscopic resection strategy for multiple duodenal polyposis associated with familial adenomatous polyposis. J Gastroenterol Hepatol. https://doi.org/10.1111/jgh.16281[published Online First: 2023/07/10]
Repici A, Capogreco A, Spadaccini M et al (2022) Cold versus hot EMR for large duodenal adenomas. Gut 71(9):1763–1765. https://doi.org/10.1136/gutjnl-2022-327171[published Online First: 2022/07/06]
Wang H, Sidhu M, Gupta S et al (2023) Cold snare EMR for the removal of large duodenal adenomas. Gastrointest Endosc 97(6):1100–1108. https://doi.org/10.1016/j.gie.2023.01.040[published Online First: 2023/02/01]
Dang DT, Suresh S, Vance RB et al (2022) Outcomes of cold snare piecemeal EMR for nonampullary small-bowel adenomas larger than 1 cm: a retrospective study. Gastrointest Endosc 95(6):1176–1182. https://doi.org/10.1016/j.gie.2021.12.018[published Online First: 2022/01/01]
Hamada K, Takeuchi Y, Ishikawa H et al (2018) Safety of cold snare polypectomy for duodenal adenomas in familial adenomatous polyposis: a prospective exploratory study. Endoscopy 50(5):511–517. https://doi.org/10.1055/s-0043-124765[published Online First: 2018/01/20]
Aelvoet AS, Bastiaansen BAJ, Fockens P et al (2022) Pancreas-preserving total duodenectomy for advanced duodenal polyposis in patients with familial adenomatous polyposis: short and long-term outcomes. HPB (Oxford) 24(10):1642–1650. https://doi.org/10.1016/j.hpb.2022.04.004[published Online First: 2022/05/15]
Bertario L, Russo A, Sala P et al (2003) Multiple approach to the exploration of genotype-phenotype correlations in familial adenomatous polyposis. J Clin Oncol 21(9):1698–1707. https://doi.org/10.1200/JCO.2003.09.118[published Online First: 2003/05/02]
Anele CC, Martin I, McGinty Duggan PM et al (2022) Attenuated familial adenomatous polyposis: a phenotypic diagnosis but obsolete term? Dis Colon Rectum 65(4):529–535. https://doi.org/10.1097/DCR.0000000000002217[published Online First: 2021/11/15]
Sieber OM, Segditsas S, Knudsen AL et al (2006) Disease severity and genetic pathways in attenuated familial adenomatous polyposis vary greatly but depend on the site of the germline mutation. Gut 55(10):1440–1448. https://doi.org/10.1136/gut.2005.087106[published Online First: 2006/02/08]
Spier I, Yin X, Richardson M et al Gene-specific ACMG/AMP classification criteria for germline APC variants: recommendations from the ClinGen InSiGHT Hereditary Colorectal Cancer / Polyposis variant Curation Expert Panel. Genet Med 2023:100992. https://doi.org/10.1016/j.gim.2023.100992 [published Online First: 2023/10/06]
Angsuwatcharakon P, Ahmed O, Lynch PM et al (2020) Management of ampullary adenomas in familial adenomatous polyposis syndrome: 16 years of experience from a tertiary cancer center. Gastrointest Endosc 92(2):323–330. https://doi.org/10.1016/j.gie.2020.02.040[published Online First: 2020/03/08]
Vu Trung K, Abou-Ali E, Caillol F et al (2023) Endoscopic papillectomy for ampullary lesions in patients with familial adenomatous polyposis compared with sporadic lesions: a propensity score-matched cohort. Endoscopy 55(8):709–718. https://doi.org/10.1055/a-2029-2935[published Online First: 2023/02/07]
Le Bras P, Cauchin E, De Lange G et al (2024) Impact of endoscopic treatment in severe duodenal polyposis: a National Study in familial adenomatous polyposis patients. Clin Gastroenterol Hepatol. https://doi.org/10.1016/j.cgh.2024.03.007[published Online First: 20240328]
Funding
Open access funding provided by Copenhagen University
Author information
Authors and Affiliations
Contributions
JGK, MDW, TPK, JB, and HCP designed and planned the study. JGK, MDW, SB, TvOH, HH, AMJ, TPK, JB, and HCP conducted the study. JGK drafted the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
JGK is a consultant for SNIPR BIOME. The other authors have no conflicts of interest to declare.
Grant Support
The Misse and Valdemar Risom Foundation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anonymized and summarized data collected for the study will be made available to other researchers upon publication and following reasonable requests made to the corresponding author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karstensen, J., Wewer, M., Bülow, S. et al. Endoscopic indicators in patients with familial adenomatous polyposis undergoing duodenal resections – a nationwide Danish cohort study with long-term follow-up. Familial Cancer (2024). https://doi.org/10.1007/s10689-024-00415-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10689-024-00415-x