Abstract
Background
Patients with familial adenomatous polyposis (FAP) have a lifetime risk of developing duodenal adenomas approaching 100%, and the relative risk for duodenal cancer compared with the general population is high. We conducted a retrospective study to investigate the progression of non-ampullary duodenal adenomas (NADAs) and risk factors for advanced lesions in patients with FAP.
Methods
Of 248 patients with 139 pedigrees at 2 institutes, we assessed 151 patients with 100 pedigrees with a pathogenic germline variant in the adenomatous polyposis coli gene, excluding mosaic variants. We evaluated the prevalence of NADAs in patients with FAP, the progression of these adenomas to advanced adenoma during the observation period, and the risk factors for the lifetime development of high-grade dysplasia (HGD), large (≥ 10 mm) duodenal adenomas, and Spiegelman stage IV.
Results
During the median observation period of 7 years, the incidences of patients with NADAs, with more than 20 polyps, with polyps ≥ 10 mm, with HGD, and with stage IV at the last esophagogastroduodenoscopy were increased 1.6-fold, 1.7-fold, 5-fold, 22-fold, and 9-fold, respectively. Intramucosal cancer occurred in three patients (2%), but no patients developed invasive cancer during the observation period because we performed endoscopic intervention for advanced adenomas. Stage progression was observed in 71% of 113 patients. Stage IV was more common in women, patients with a history of colectomy, and those with a 3’ side mutation in their adenomatous polyposis coli gene.
Conclusions
NADAs in patients with FAP frequently become exacerbated. Our findings suggest that patients with FAP who develop duodenal adenomas should be surveyed to prevent the development of duodenal cancer.
Similar content being viewed by others
Background
Familial adenomatous polyposis (FAP) is an autosomal dominant disorder caused by germline mutations in the adenomatous polyposis coli (APC) gene. It is characterized by multiple adenomas throughout the colon and rectum and results in colorectal cancer (CRC) in almost 100% of patients if left untreated.
FAP is associated with multiple lesions other than those in the large intestine, and the lifetime risk of developing duodenal adenomas approaches 100% [1,2,3,4,5,6]. Unlike adenomas in the large intestine, duodenal adenomas do not necessarily become cancerous. Nevertheless, the risk of duodenal cancer in patients with FAP is 250.0 to 330.8-fold higher than that of the general population [7, 8]. Therefore, patients with FAP constitute a high-risk group for duodenal cancer. The lifetime risk of developing duodenal cancer ranges from 3 to 5% [9,10,11] and is higher in other reports [1, 4, 12, 13]. Duodenal cancer is also the third leading cause of death in patients with FAP, accounting for the deaths of approximately 3% of these patients [14, 15]. Although half of duodenal cancer occur in the ampulla and periampullary region, we should consider ampullary and non-ampullary duodenal adenomas (NADAs) separately because ampullary adenoma rarely progresses, and when it does, it is slow [16]. Although a therapeutic strategy for ampullary adenoma has almost been established, no strategy for NADAs has been established.
CRC that occurs in patients with FAP can be managed by prophylactic total colectomy or intensive endoscopic intervention [17], leading to reduced mortality; thus, the prognosis of FAP has dramatically improved. With respect to duodenal cancer, the severity of NADAs is classified by Spigelman score. The risk of developing duodenal cancer is stratified according to its stage [18]. Because polyps larger than 10 mm and with high-grade dysplasia (HGD) are generally called “advanced adenoma” and are considered a precursor of cancer, we performed endoscopic interventions for these advanced adenomas. However, the extent to which NADAs worsen to advanced adenoma is unclear. Therefore, we proposed intensive downstaging polypectomy for all detected NADAs in our prospective study [19]. By examining the changes that occur in NADAs over time, we may more effectively stratify the risk of developing advanced duodenal adenomas. Therefore, we conducted a retrospective study to investigate the progression of NADAs and risk factors for advanced lesions in patients with FAP.
Methods
Patients
Patients with FAP who visited the Osaka International Cancer Institute and Ishikawa Gastrointestinal Clinic from January 1998 to September 2018 were retrospectively assessed for enrollment in this study. The diagnostic criteria for FAP in this study were (i) the presence of either ≥ 100 adenomas in the colon and rectum or 10 to 99 adenomas with a family history of FAP and (ii) a pathogenic germline variant in APC. Patients with mosaic variants in APC were excluded. Esophagogastroduodenoscopy (EGD) was generally recommended for all patients with FAP. Patients who had not undergone EGD at our institutions and patients with a history of duodenal surgery, such as gastric surgery or pancreatoduodenectomy, were not enrolled in this retrospective study because we could not ascertain the progression of the duodenal neoplasms in such patients. Most of the cases in this study were also analyzed in the study by Shimamoto et al. (Genotype-phenotype correlation for life-threatening complications in patients with familial adenomatous polyposis, accepted in Cancer Science).
Extraction of clinical data and definition of measured variables
We collected clinical data from the patients’ medical and endoscopic records. The genetic information used in this study comprised APC pathogenic variant and pedigree data aggregated at the Medical Research Support Co., Ltd. (Osaka, Japan), a data center operated by academic doctors. The genetic information was aggregated and controlled at the center in accordance with strict guidelines. All patients received genetic counseling and provided informed consent for APC genetic testing.
The observation period was defined as the duration from the day on which the first EGD was performed to the day on which the last EGD was performed according to the patient’s medical record or until immediately before the treatment intervention, if any. We generally performed annual surveillance EGD and targeted biopsies of polyps. We considered treatment interventions for patients with an advanced duodenal adenoma, namely > 10 mm or HGD, defined as lesions with severe atypia according to the previous criteria, because advanced adenoma is generally considered a precursor of invasive cancer in colorectal polyp management, and we needed to prevent the development of duodenal cancer to secure the patients’ safety.
Based on the number of colorectal adenomas, severe FAP was defined as the inability to visualize a patient’s normal mucosa macroscopically because of the profusion of colorectal adenomas, and sparse FAP was considered typical FAP (which is not severe FAP). Attenuated FAP was defined as the presence of 10 to 99 colorectal adenomas. Patients were considered to have Helicobacter pylori infection when a positive result was obtained by a serological test, urea breath test, rapid urease test, or histological examination, and when they had a history of eradication therapy for H. pylori.
Endoscopic system and settings
The endoscopic system consisted of a video processor (CV-260, CV-260SL, or CV-290; Olympus Co., Tokyo, Japan and VP-4450HD or VP-7000; Fujifilm Co., Tokyo, Japan) and a light source (CLV-260, CLV-260SL, or CLV-290; Olympus Co. and LL-4450, XL-4450, LL-7000, or BL-7000; Fujifilm Co.). A videoendoscope (GIF-H260Z, Q240Z, H290Z, PCF-Q260JI, or PCF-H290TL/I; Olympus Co. and EG-L590ZW, EG-L600ZW, or EGL600ZW7; Fujifilm Co.) was also used. Observations were performed by white light imaging and non-magnifying narrow-band imaging and/or with chromoendoscopy using indigo carmine with or without magnification.
Histological examination
Lesions thought to be neoplastic or cancerous (well-demarcated lesions, large lesions, lesions with a depression at the center, and lesions with an irregular shape) were selected for biopsy. Biopsy tissue samples or endoscopic resection samples were collected and diagnosed. In patients who underwent intervention for duodenal neoplasms without prior biopsy, we collected information obtained by the following intervention. According to the Japanese classification of colorectal carcinoma, a histopathological examination was performed by two pathologists with expertise in gastrointestinal pathology. According to the Japanese classification, noninvasive cancer was evaluated as severe atypia by the Spigelman classification, which corresponds to Western standards. Because the grading of dysplasia according to the Vienna classification changed from mild/moderate/severe to low-grade/high-grade in 2000, our patients straddled the two eras. Our pathologists followed the mild/moderate/severe grading classification for a while, and then we adopted the original Spigelman stage (SS), using mild/moderate/severe in the analysis. We thus refer to severe atypia as “HGD” in this study. The most severe histopathological diagnosis during the observation period was adopted as the final histopathological diagnosis.
Spigelman classification
When NADAs were found, they were scored using the Spigelman classification to indicate the severity of duodenal polyposis [18]. The Spigelman classification includes the number of polyps, maximum diameter, tissue structure, and degree of atypia. Stages 0 to IV were determined by the total score obtained using the above-mentioned criteria. When a detected duodenal polyp was not histopathologically diagnosed as an adenoma, it was not considered to be a NADA.
Statistical analysis
We evaluated the prevalence of NADAs in patients with FAP, the progression of these adenomas during the observation period, the risk factors for the lifetime development of advanced adenoma, and SS IV until the end of the observation period.
The prevalence of NADAs in patients with FAP was indicated by the number and percentage of patients with NADAs. The progression of NADAs during the observation period was assessed with the SS, which includes the number of adenomas, size of adenomas, and development of severe dysplasia. Finally, we evaluated the lifetime risk of developing an advanced duodenal adenoma, and SS IV, which were reported to be risk factors for developing duodenal cancer [18, 20].
For the analysis of SS progression, patients for whom the observation period was < 5 years without SS progression were excluded because the observation period was considered too short. We then examined the progression of patients with SS 0 to III at the first EGD because there was no room for progression for patients with stage IV at the first EGD. We also examined the progression of patients with SS 0 and I at the first EGD, who were recommended to undergo follow-up at 5-year intervals according to the guidelines. Furthermore, we examined the occurrence of HGD according to the SS at the first EGD.
HGD, a tumor size ≥ 10 mm, the surveillance period, a history of colon cancer, age, the SS at the first EGD, classic FAP, and the location of several mutations in APC were evaluated as risk factors for disease progression because they were relevant to the severity of duodenal polyposis and reported to be risk factors for duodenal cancer [2,3,4,5,6, 21,22,23]. We also investigated whether APC mutations at codons 1250 to 1464, which are common in patients with severe FAP, were associated with the incidence and severity of NADAs [23]. Because we used only the protein truncation test for mutation detection in APC in patients whose first endoscopy procedure was performed before 2009, we could not obtain detailed information on the gene mutation site in some cases. We excluded those cases from the analysis of risk factors for disease progression.
Statistical analyses were performed using R software version 3.3.0 (R Foundation for Statistical Computing, Vienna, Austria; http://cran.r-project.org/). The data were analyzed using the χ2 test and Kruskal–Wallis test. Statistical significance was set at P < 0.05.
Results
Patient characteristics at first endoscopy
Among 248 patients from 139 pedigrees diagnosed with FAP from January 1998 to September 2019 at our institutes, 50 patients who had not undergone EGD at our institutes and 4 patients with a history of duodenal surgery were excluded. APC genetic testing was performed for 90% of the remaining 194 patients from 110 pedigrees, among whom 151 patients from 100 pedigrees were mutation-positive (Fig. 1). Table 1 shows the patients’ background characteristics. Female patients were predominant (57%), most patients had a family history of FAP, one-third had a history of colectomy, and a few (7%) had a history of CRC. An ampullary adenoma was found in 22 patients (15%), including 1 patient with a history of papillectomy.
Progression and risk factors for the progression of duodenal adenoma
After excluding 5 patients who underwent only one EGD procedure, we evaluated the remaining 146 patients who underwent two or more EGD procedures. EGD was performed approximately once per year. The median age (range) at the start and end of the observation period was 30 (16–76) and 39 (18–80) years, respectively. The median observation period (range) was 7 (0–19) years and the median number of EGD procedures (range) during the observation period was 7 (2–26).
The prevalence of NADAs at the start of the observation period was 52% (79/151), and histologically, all were tubular adenomas. The prevalence of NADAs at the end of the observation period was 85% (124/146) and this increased 1.6-fold during the observation period. The incidence of patients with more than 20 polyps increased 1.7-fold, with polyps ≥ 10 mm increased 5-fold, with HGD increased 22-fold, and with stage IV at the last EGD increased 9-fold (Table 2; Fig. 2). 30% (21/70) of patients who did not have adenomas at the first EGD had developed at least one NADA by the last EGD. Intramucosal cancer occurred in three patients (2%), but no invasive cancer developed during the observation period because we performed endoscopic interventions for advanced adenomas before developing invasive cancer.
Stage progression was observed in 71% of 113 patients with stage 0 to III at the first EGD during the median observation period (2–19) of 9 years, excluding 4 patients with stage IV at the beginning and 29 patients with an observation period of ≤ 5 years without stage progression (Table 3). In addition, stage progression was recognized in 83% of 72 patients with stage 0 to I at the first EGD during the median observation period (0–19) of 10 years, excluding 9 patients with a short observation period of ≤ 5 years (Table 4). Stage progression among patients with stage 0 to III and stage 0 to I was observed significantly more often in those with classic FAP and a history of colectomy. Among patients with stage 0 to I, those with progression during the observation period were younger at the time of FAP diagnosis (Tables 3 and 4).
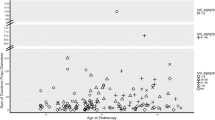
HGD developed at the last EGD in 17.1% (12/70) of patients with stage 0 at the first EGD, 9% (1/11) of those with stage I, 25% (5/20) of those with stage II, 29% (12/41) of those with stage III, and 50% (2/4) of those with stage IV (Fig. 3).
Risk factors for the lifetime development of each event until the end of the observation period
A duodenal polyp ≥ 10 mm developed in 25% of patients and was significantly more common in patients with a history of CRC, a history of colectomy, and a 3’ side mutation (Table 5). HGD occurred in 22% of patients and was significantly more common in patients with a history of colectomy (Table 6). Progression to stage IV was observed in 18% (27/146) of patients. One patient exhibited improvement from stage IV to stage III. Stage IV was significantly more common in women and patients with a history of colectomy, and it was marginally more common in those with a 3’ side mutation (Table 7).
Discussion
In this study, we observed the progression of NADAs to advanced adenoma in patients with FAP. NADAs frequently become exacerbated, which may explain the need for regular surveillance and early intervention to prevent major morbidity. Studies have examined the timing and strategies of endoscopic treatment for NADAs in patients with FAP [9,10,11]; however, a sufficient consensus has not been achieved, and optimal management criteria have not been established. Our results provide important information regarding the management of NADAs in patients with FAP.
The reported prevalence of NADAs in patients with FAP ranges from 30 to 90% but increases after the age of 40 years [24, 25], and the SS also worsens with age [10, 12, 26, 27]. In the present study, the prevalence of NADAs at the start of the observation period was 52%. However, the numbers of patients with NADAs, more than 20 polyps, polyps > 10 mm, HGD, and stage IV at the final EGD were substantially increased during the median 7-year observation period. Although 15% of patients did not develop NADAs during the observation period, the risk of developing NADAs increased over time, as previously reported, and even patients with early-stage NADAs showed frequent progression. Stage progression was observed in 71% of patients, and the same tendency was observed in patients with stage 0 to I at the first EGD. Because the incidence of HGD at the last EGD was higher among patients with a more advanced stage at the first EGD, it is desirable to prevent stage progression. Furthermore, the incidence of HGD in patients with stage 0 at the first EGD was 17% at the last EGD. Therefore, it might be better to consider surveillance EGD for all patients with FAP.
No patients in the present study developed invasive cancer during the study period because we recommended treatment intervention before the development of invasive cancer. Groves et al. [2] reported that SS IV was a risk factor for developing duodenal cancer at the time of the first endoscopy, and was a risk factor in patients who developed duodenal cancer. In other studies, 63% of patients with stage IV disease at the time of the first EGD developed HGD [10, 28], and a ≥ 10-mm adenoma was shown to be a risk factor for HGD [6]. Therefore, advanced duodenal adenomas and SS IV have been cited as the main risk factors for the development of duodenal cancer, and can be considered surrogate measurement indices for the development of invasive cancer in this study. Although the usefulness of SS is controversial, there is currently no alternative measurement [20]. In addition, Thiruvengadam et al. reported that polyp size and HGD were associated with cancer development [20]. Therefore, our assessment using advanced adenoma and SS simultaneously is reasonable.
In the present study, we found no correlation between the development of severe duodenal adenomatosis or duodenal cancer and the specific location of the mutation in APC, although the risk factors for advanced duodenal adenoma and SS IV were classic FAP, a history of CRC, a history of colectomy, and a 3’ side mutation. Therefore, it might be better to perform more intensive surveillance for patients with these risk factors instead. In the current study, no patients developed invasive cancer with annual surveillance; thus, annual surveillance may be sufficient, even for high-risk patients. However, for low-risk patients such as those with attenuated FAP and small polyps, the interval of surveillance endoscopy can be prolonged.
In the current study, 18% of the patients progressed to stage IV. Surgical intervention should be considered for such patients because of the risk of the development of duodenal cancer. Because surgical intervention (typically pancreatoduodenectomy) for the treatment of duodenal cancer or stage IV disease can be extremely invasive and is accompanied by severe sequelae, it should be avoided if possible. This is especially true for patients who have undergone total colectomy, which is a standard intervention for classic FAP. Although endoscopic intervention has not been actively recommended because of the high risk of adverse events and frequent recurrences, we have adopted safer endoscopic interventions for the management of duodenal neoplasms in patients with FAP [29]. We also recommended treatment intervention for patients with an advanced duodenal adenoma in the present study, and no invasive cancer developed in our cohort. This indicates that early intensive endoscopic management can avoid development of duodenal cancer or progression to SS IV in patients with FAP [10, 29,30,31].
Three main limitations of this study should be considered. First, this was a retrospective study involving a small number of patients in a small number of facilities. Therefore, the study findings may not be highly generalizable. However, we enrolled all consecutive patients who met the inclusion criteria, and the ability to collect detailed, high-quality endoscopic data from two linked facilities was advantageous. In addition, we focused on patients who met the diagnostic criteria for FAP and were positive for the APC mutation; therefore, we were able to extract and examine more reliable data. In future studies, we hope that patient data will be collected at multiple facilities using registries. In addition, we hope our data will contribute to a future meta-analysis, which can provide more robust information. Second, the observation period was too short to determine the real lifetime risk of duodenal neoplasms. This might have caused an underestimation of the risk of duodenal neoplasms; therefore, the study findings should be interpreted as at least a progression of duodenal manifestations in most of the patients with FAP. Finally, our study did not include ampullary adenoma to clarify the progression of NADAs separate from ampullary adenoma. Because half of duodenal cancers were reported to develop from ampullary adenomas, we should consider them as well as NADAs for the long-term prognosis of patients with FAP. However, the treatment strategies for ampullary adenoma and NADAs are different, and we needed to investigate them separately.
Conclusions
NADAs in patients with FAP frequently become exacerbated, and all patients with FAP should be surveyed to prevent the development of duodenal cancer. An early, minimally invasive therapeutic intervention should be considered to avoid disease progression.
Data Availability
The following data will be made available with publication: complete deidentified patient data set (contact Yoji Takeuchi; e-mail, yoji.endoscopy@oici.jp). The following supporting documents will be made available with publication: analytic/statistical code (contact Yoji Takeuchi; e-mail, yoji.endoscopy@oici.jp). These data will be made available for any purpose to researchers whose proposed use of the data has been approved.
References
Bülow S, Björk J, Christensen IJ, Fausa O, Järvinen H, Moesgaard F, et al. Duodenal adenomatosis in familial adenomatous polyposis. Gut. 2004;53:381–6.
Groves CJ, Saunders BP, Spigelman AD, Phillips RK. Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10 year prospective study. Gut. 2002;50:636–41.
Saurin JC, Ligneau B, Ponchon T, Leprêtre J, Chavaillon A, Napoléon B, et al. The influence of mutation site and age on the severity of duodenal polyposis in patients with familial adenomatous polyposis. Gastrointest Endosc. 2002;55:342–7.
Giardiello FM, Brensinger JD, Petersen GM. AGA technical review on hereditary colorectal cancer and genetic testing. Gastroenterology. 2001;121:198–213.
Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc. 1999;49:358–64.
Sourrouille I, Lefèvre JH, Shields C, Colas C, Bellanger J, Desaint B, et al. Surveillance of duodenal polyposis in familial adenomatous polyposis: should the Spigelman score be modified? Dis Colon Rectum. 2017;60:1137–46.
Park JG, Park KJ, Ahn YO, Song IS, Choi KW, Moon HY, et al. Risk of gastric cancer among korean familial adenomatous polyposis patients. Report of three cases. Dis Colon Rectum. 1992;35:996–8.
Offerhaus GJ, Giardiello FM, Krush AJ, Booker SV, Tersmette AC, Kelley NC, et al. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102:1980–2.
Campos FG, Martinez CAR, Sulbaran M, Bustamante-Lopez LA, Safatle-Ribeiro AV. Upper gastrointestinal neoplasia in familial adenomatous polyposis: prevalence, endoscopic features and management. J Gastrointest Oncol. 2019;10:734–44.
Dekker E, Boparai KS, Poley JW, Mathus-Vliegen EM, Offerhaus GJ, Kuipers EJ, et al. High resolution endoscopy and the additional value of chromoendoscopy in the evaluation of duodenal adenomatosis in patients with familial adenomatous polyposis. Endoscopy. 2009;41:666–9.
Heiskanen I, Kellokumpu I, Järvinen H. Management of duodenal adenomas in 98 patients with familial adenomatous polyposis. Endoscopy. 1999;31:412–6.
Bülow S, Christensen IJ, Højen H, Björk J, Elmberg M, Järvinen H, et al. Duodenal surveillance improves the prognosis after duodenal cancer in familial adenomatous polyposis. Colorectal Dis. 2012;14:947–52.
Vasen HF, Bulow S, Myrhoj T, Mathus-Vliegen L, Griffioen G, Buskens E, et al. Decision analysis in the management of duodenal adenomatosis in familial adenomatous polyposis. Gut. 1997;40:716–9.
Tomita N, Ishida H, Tanakaya K, Yamaguchi T, Kumamoto K, Tanaka T, et al. Japanese Society for Cancer of the Colon and rectum (JSCCR) guidelines 2020 for the clinical practice of Hereditary Colorectal Cancer. Int J Clin Oncol. 2021;26:1353–419.
Galle TS, Juel K, Bülow S. Causes of death in familial adenomatous polyposis. Scand J Gastroenterol. 1999;34:808–12.
Singh AD, Bhatt A, Joseph A, Lyu R, Heald B, Macaron C, et al. Natural history of ampullary adenomas in familial adenomatous polyposis: a long-term follow-up study. Gastrointest Endosc. 2022;95:455–67e3.
Ishikawa H, Mutoh M, Iwama T, Suzuki S, Abe T, Takeuchi Y, et al. Endoscopic management of familial adenomatous polyposis in patients refusing colectomy. Endoscopy. 2016;48:51–5.
Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783–5.
Takeuchi Y, Hamada K, Nakahira H, Shimamoto Y, Sakurai H, Tani Y, et al. Efficacy and safety of intensive downstaging polypectomy (IDP) for multiple duodenal adenomas in patients with familial adenomatous polyposis: a prospective cohort study. Endoscopy. 2023;55:515–23.
Thiruvengadam SS, Lopez R, O’Malley M, LaGuardia L, Church JM, Kalady M, et al. Spigelman stage IV duodenal polyposis does not precede most duodenal cancer cases in patients with familial adenomatous polyposis. Gastrointest Endosc. 2019;89:345–54.
Matsumoto T, Lida M, Kobori Y, Mizuno M, Nakamura S, Hizawa K, et al. Genetic predisposition to clinical manifestations in familial adenomatous polyposis with special reference to duodenal lesions. Am J Gastroenterol. 2002;97:180–5.
Miyaki M, Yamaguchi T, Iijima T, Takahashi K, Matsumoto H, Yasutome M, et al. Difference in characteristics of APC mutations between colonic and extracolonic tumors of FAP patients: variations with phenotype. Int J Cancer. 2008;122:2491–7.
Groves C, Lamulum H, Crabtee M, Williamson J, Taylor C, Bass S, et al. Mutation cluster region, association between germ-line and somatic mutations and genotype-phenotype correlation in upper gastrointestinal familial adenomatous polyposis. Am J Pathol. 2002;160:2055–61.
Morpurgo E, Vitale GC, Galandiuk S, Kimberling J, Ziegler C, Polk HC Jr. Clinical characteristics of familial adenomatous polyposis and management of duodenal adenomas. J Gastrointest Surg. 2004;8:559–64.
Brosens LAA, Keller JJ, Offerhaus GJ, Goggins M, Giardiello FM. Prevention and management of duodenal polyps in familial adenomatous polyposis. Gut. 2005;54:1034–43.
Gibbons DC, Sinha A, Phillips RK, Clark SK. Colorectal cancer: no longer the issue in familial adenomatous polyposis? Fam Cancer. 2011;10:11–20.
Moozar KL, Madlensky L, Berk T, Gallinger S. Slow progression of periampullary neoplasia in familial adenomatous polyposis. J Gastrointest Surg. 2002;6:831–7.
Yachida T, Nakajima T, Nonaka S, Nakamura K, Suzuki H, Yoshinaga S, et al. Characteristics and clinical outcomes of duodenal neoplasia in japanese patients with familial adenomatous polyposis. J Clin Gastroenterol. 2017;51:407–11.
Hamada K, Takeuchi Y, Ishikawa H, Ezoe Y, Arao M, Suzuki S, et al. Safety of cold snare polypectomy for duodenal adenomas in familial adenomatous polyposis: a prospective exploratory study. Endoscopy. 2018;50:511–7.
Campos FG, Martinez CAR, Bustamante Lopez LA, Kanno DT, Nahas SC, Cecconello I. Advanced duodenal neoplasia and carcinoma in familial adenomatous polyposis: outcomes of surgical management. J Gastrointest Oncol. 2017;8:877–84.
Moussata D, Napoleon B, Lepilliez V, Klich A, Ecochard R, Lapalus MG, et al. Endoscopic treatment of severe duodenal polyposis as an alternative to surgery for patients with familial adenomatous polyposis. Gastrointest Endosc. 2014;80:817–25.
Acknowledgements
The authors thank Angela Morben, DVM, ELS, and J. Ludovic Croxford, PhD, from Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript, and Eri Okuda, Data Manager at the Medical Research Support Co., Ltd., for acquiring the data.
Funding
This study was funded by the Japan Agency for Medical Research and Development (AMED; 22ck0106556h0003).
Author information
Authors and Affiliations
Contributions
Conception and design: HN, YT, HI; Data Acquisition: HN, YS, SI, HY, YE, RI, TT, TY, HI; Drafting of the article: HN, YT; Critical revision for important intellectual content: HI, MM; Final approval of the article: All authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Osaka International Cancer Institute (2018–18229 and 2019–19006). Informed consent was obtained in the form of an opt-out on the institutional websites. The study was performed in accordance with the guidelines of the World Medical Association’s Declaration of Helsinki. All authors consent to publish this manuscript.
Consent for publication
Not applicable.
Competing interests
Yoji Takeuchi has received honoraria for lectures from Olympus, Boston Scientific Japan, Daiichi-Sankyo, Miyarisan Pharmaceutical, Asuka Pharmaceutical, AstraZeneca, EA Pharma, Zeria Pharmaceutical, Fujifilm, Kaneka Medix, and Kyorin Pharmaceutical. The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nakahira, H., Takeuchi, Y., Shimamoto, Y. et al. Progression of duodenal neoplasia to advanced adenoma in patients with familial adenomatous polyposis. Hered Cancer Clin Pract 21, 25 (2023). https://doi.org/10.1186/s13053-023-00264-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13053-023-00264-2