Abstract
A fundamental problem for any animal is how to weigh the benefits of making a rapid decision against the costs of making a poor decision, because time for detecting and evaluating all options is often restricted. For nest-site selection in birds, an important cost of a speedy decision would be nest predation, which is a major factor lowering reproductive success. I tested whether shorter time available for assessment of nest sites would lead to a decision with higher probability of nest predation. Where boreal owls (Aegolius funereus) had nested successfully in a box in the previous season, I manipulated nest box availability by offering a dyad of nest boxes. One box (kept or exchanged) was in the original nest tree and one box (new or taken from the original tree) was in a new tree for the season, each box containing either “post-nesting residue” from the successful nesting or new wood shavings. Hence, the owls could assess the risk of nest predation at a familiar site relative to that at a new site. The timing of nest box installation and relocation was such that time for assessment varied among localities, from the whole non-breeding season to just a few days prior to laying in spring. Owls that had had longer time in which to make their assessment and selection were less likely to have their nest predated by pine martens (Martes martes). Boreal owls are non-migratory and probably gained information on the relative safety of the two options by a Bayesian-like updating process in the days, weeks or months before the decision had to be made. A migratory cavity-nester exposed to the same landscape of nest predation would be more time-constrained and forced to rely on the win-stay loose-shift tactic, which underperforms relative to Bayesian-like updating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A fundamental problem for any animal is how to weigh the benefits of making a rapid decision against the costs of making a poor decision, because time for detecting and evaluating all options is often restricted. This speed-accuracy trade-off (Busemeyer and Townsend 1993) has been documented for several scenarios and taxa, at both the individual level and at the group level (Chittka et al. 2009). Most studies have dealt with humans, who often can deliberatively choose between speed and accuracy (e.g. Osman et al. 2000). Foraging birds, bees and wasps have been found to make less optimal choice when time is constrained (Langen 1999; Chittka et al. 2003; Dyer and Chittka 2004; Furuichi and Kasuya 2013). This may even explain mimicry; to avoid spending costly time on discrimination between defended models and edible imperfect mimics predators may ignore all prey with similar appearance (Chittka and Ossorio 2007). Further, birds may be less selective in their mate choice if the cost of searching increases; in female pied flycathers (Ficedula hypoleuca) restricted sampling of potential mates led to imperfect choice of mate (Slagsvold and Dale 1994). Finally, collective decisions on selection of nest site in bees and ants were less discriminative when time was critical and thus restricted (Franks et al. 2003; Passino and Seeley 2006).
An unexplored field in the framework of the speed-accuracy trade-off is nest-site selection in wild birds. An important potential cost of speedy selection of nest site would be nest predation, which is a major factor lowering reproductive success, and thus important in the evolution of avian behaviour (e.g., Sonerud 1985a; Marzluff 1988; Pöysä 1999; Martin et al. 2000; Forstmeier and Weiss 2004; Caro 2005; Eggers et al. 2006; Lima 2009, Chalfoun and Schmidt 2012). Therefore, one would expect birds to select nest sites that minimize the probability of nest predation (e.g., Caro 2005; Schmidt et al. 2006; Chalfoun and Martin 2010). It is still unclear how birds assess risk of nest predation (Lima 2009).
The cost for a bird of making an incorrect decision when selecting a nest-site is fairly straightforward to measure when expressed as lowered reproductive success, and in the extreme case as killing of the adult bird. In comparison, the cost of deciding between nest site options involves time and energy spent on sampling of information and assessment, which is difficult to measure in wild birds, even by simply using time spent as a proxy. However, measuring time available for sampling, assessing and selecting a nest site would be feasible by studying birds dependent on pre-existing nests made by other birds rather than studying birds building their own nest. Moreover, it would be particularly feasible by studying birds reusing cavities made by woodpeckers, and by using nest boxes as woodpecker cavity surrogates in managed forests without sufficient natural cavities, because this would allow an experimental design. One way to test whether shorter time available for assessing the potential nest sites increases the probability of making a sub-optimal decision would be to expose birds within each territory to two nest boxes, one at a familiar site and one at a new site, and among territories vary the amount of time this dyad of nest boxes is available for assessment.
Boreal owls (Aegolius funereus) are nocturnal small owls (male body mass c. 100 g) that occur in boreal and alpine forests in the Paleartic and the Nearctic, and subsist mainly on small mammals (Cramp 1985). They nest mostly in cavities excavated by large woodpeckers, such as the black woodpecker (Dryocopus martius) in the western Palearctic, but readily accept nest boxes (e.g., Cramp 1985; Sonerud 1985a). In the western Palearctic boreal owls are exposed to nest predation by the pine marten (Martes martes) (Sonerud 1985a,b, 1989, 1993, 2022; Johnsson 1993; Zarybnicka et al. 2015), which is a medium-sized tree-climbing mustelid (body mass c. 1 kg) with relatively large home range. At 60–61°N in Norway and Sweden the home range of pine martens was on average 7 km2 (Brainerd 1997), thus larger than the home range of boreal owl males, which on average was c. 2 km2 Sonerud et al. 1986; Jacobsen and Sonerud 1987; Sørås et al. 2020). Pine martens have a generalist diet (Helldin 1999). They spend most time on the ground and prey mainly on small mammals (Pulliainen and Ollimäki 1996; Helldin 2000), but visit tree-cavities year-round for roosting, denning and food storing (Sonerud 1985b; Brainerd et al. 1995), and will take any suitable prey that may happen to be there, including eggs and nestlings. Pine martens are relatively long lived; in Sweden 51% of trapped individuals were older than 1 year, 29% were older than 2 years, and the oldest were 9 years (Helldin 1999). Similar age distributions of trapped pine martens were found in older studies from eastern Europe (see Sonerud 1985a).
Pine marten predation of boreal owl nests occurs mostly during the 4–6 weeks long period of egg laying and incubation (Sonerud 1985a, 2022; Zarybnicka et al. 2015). As explained in detail by Sonerud (2022), this non-random distribution of predation over the nesting period may partly be due to spatial memory, i.e. that the individual pine marten regularly revisits cavities it has previously found (Sonerud 1985a, 2022), and partly due to the site-effect, i.e. that some nest sites are simply more exposed to pine martens than others (cf. Martin et al. 2000). When a potential nest predator approaches or enters the trunk of the nest tree most incubating or brooding boreal owl females rapidly jump up in the cavity entrance and if needed fly away, and thereby survive nest predation (Sonerud 1985b; Sonerud et al. 1988).
A pine marten would be unaware of a nest box in its home range the day the box is installed, and unaware of a nest box where a successful nesting takes place. Pine martens visit nest boxes year-round for other purposes than searching for nests to predate, and would therefore also revisit a box that they initially found empty (Sonerud 1985a,b; Brainerd et al. 1995). Therefore, as explained in detail by Sonerud (2021a), a box in the tree where the previous nest escaped predation would be relative safe the year after, and thereafter predictably become less safe as more years elapse without nesting (cf. Sonerud 1985a). Correspondingly, a box in a new tree for the season would also be relative safe from pine marten predation (cf. Sonerud 1985a), but less safe in the years that follow. In the first season, the latter would have some unpredictable probability of depredation due to the site effect (sensu Martin et al. 2000), because it may have happened to be installed where the pine marten travels.
To test whether a shorter time available for assessment of nest sites would lead to a decision with poorer nesting success, scored as nest predation, I used data from a long-term field experiment that exposed boreal owls to a dyad of nest boxes at each locality where the previous nesting was successful; one box (kept or exchanged with another at exactly the same site) in the original nest tree and one box (new or taken from the original nest tree) in a new tree for the season, with each box containing either “post-nesting residue” from the successful nesting or new wood shavings. The owls could then assess the risk of nest predation at the new site relative to that at the site where a box had been present continuously for several years. The time available for assessment was taken as the time elapsed from the day the boxes in a dyad were relocated to the day the first egg was laid, and there was no a priori prediction of which option would be the most risky. Among localities the length of time this dyad was available before the breeding season varied from the whole non-breeding season to just a few days prior to laying in spring. I predicted that as the time available for assessment of the two options became longer the decision would result in a lower probability of nest predation.
Methods
Study area
I conducted the field work during 1985–2007 within 60°00´-62°03`N and 11°03´-12°23´E in Hedmark county (from 2020 part of Innlandet county) in south-eastern Norway (Fig. 1). The study area is situated in the boreal zone and covered by coniferous forest (Norway spruce (Picea abies) and Scots pine (Pinus sylvestris)) that is managed for commercial harvesting by unselective clear-cutting, regeneration by planting, and thinning by selective cutting.
Study species
In the western Palearctic, boreal owls show strong numerical response to microtine rodents (Hörnfeldt et al. 1990; Korpimäki and Hakkarainen 1991), so in my study area very few territories support nesting each year, and in most only 1–2 broods fledge during each 3–4 years microtine population cycle (Sonerud 1985a). Adult males are usually locally resident, whereas adult females may disperse widely between successive nesting attempts in response to the regionally asynchronous 3–4 years microtine rodent population fluctuations (e.g., Löfgren et al. 1986; Korpimäki et al. 1987; Sonerud et al. 1988; Korpimäki 1993; Hakkarainen et al. 2001). Nest site selection seems to depend on the male (Hakkarainen and Korpimäki 1998; Hakkarainen et al. 2001; Sonerud 2021a), who provides all prey for the family until the nestlings are no longer being brooded by the female, and most or all prey thereafter until the young become independent (Eldegard and Sonerud 2009, 2010, 2012). The young fledge when 4–5 weeks old (Eldegard and Sonerud 2012). After having been successfully used for nesting, a nest box contains an accumulated layer of feces and compressed pellets of undigested prey remains from the nestlings (Cramp 1985), here termed “post-nesting residue”. In my study area 93% of the cases of nest predation occurred during the 4–6 weeks long period of egg laying and incubation (Sonerud 1985a, 2022). Therefore, until the next nesting attempt the presence of “post-nesting residue” in a box would be a quite reliable cue that the previous nesting had escaped predation. Nevertheless, young boreal owl males seemed to use the presence of new wood shavings (see below) as a proxy for a new, and thus relatively safe, cavity (Sonerud 2021a).
Experimental procedure
First, I installed nest boxes separately > 2 km apart (see Sonerud (2021a) for details). I visited the boxes several times between late March and early July to record the onset and outcome of nesting attempts by boreal owls, i.e. date of egg laying and whether the nest was taken by a predator (see Sonerud (1985a,b) for methods). The term locality denotes one box until the first successful nesting there by boreal owl, and a dyad of boxes in the years thereafter.
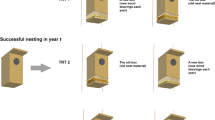
Second, I established a dyad of nest boxes as follows (see Sonerud (2021a) for details): The box in which boreal owls had nested successfully (in year t) was either relocated with its content of “post-nesting residue” while a box lined with new wood shavings was installed in its place (treatment 1), or left in place with its original content of “post-nesting residue” while a new one with new wood shavings was installed in another tree (treatment 2), or the box in which boreal owls had nested successfully was exchanged with a new one while another new box was installed in another tree, both with new wood shavings (treatment 3). I did this relocation between September (in year t) and April (in year t + 1). If none of the two boxes was used for nesting by boreal owls in the following season (year t + 1), I relocated the box in the new tree once more to a further new tree, and renewed the content in the box(es) with wood shavings, between September (in year t + 1) and April (in year t + 2). This procedure was repeated each year until boreal owls nested in one of the two boxes (up to 12 years). In this way, boreal owls always had the choice between a box in a new tree for the season and a box in the original tree, each containing either “post-nesting residue” or new wood shavings. Because the presence of “post-nesting residue” would indicate that the previous nesting was successful (see above), the manipulation of the box content would control for any use of “post-nesting residue” as a cue for a safe nest site. Note that there was a continuous presence of nest box in the original nest tree from the day the first box was installed there, and that this first box was exchanged with a new one at exactly the same site in treatments 1 and 3 (see above). The successful nesting was in season number 1–15 after the box was installed, and the focal nesting was in season number 2–20 after the first box was installed (see Sonerud (2021a) for details).
I installed the spatially new box in the same species of tree as the original nest tree and in a habitat as similar as possible, and made sure that it could not be seen from any previous nest box position or from the box in the original tree. These constraints defined the distance between the two boxes in the dyad, which ranged 40–600 m when box selection occurred (see below). The two boxes in a dyad had identical shape and size. Also, they were worn to a similar degree, because boxes were stored outdoor when not being used in the study.
I defined a box in a dyad as being selected when at least one boreal owl egg had been laid there. Three cases where the box that was not selected by boreal owls became occupied by another bird species were excluded from the analyses because there was doubt as to whether the boreal owl made its selection first, and thus whether both boxes were available when the owl initiated breeding. I excluded one additional case because the nest was deserted during incubation, so could in theory have been predated later.
If the owls selected the box in a new tree and nested successfully, this tree became the original tree in the next treatment at the locality. I then removed the box in the former original tree. If the nest was predated or deserted, I left the boxes in place until a successful nesting took place in one of them, and then continued the experiment as described above. The latter was the case for 77 of the totally 239 nesting attempts recorded in this study (at 100 localities, with 1–7 attempts per locality). This lead to 81 successful nests followed by a treatment resulting in a box selection, of which 4 were excluded from the analysis (see above). The number of cases used in the analysis was 34, 30 and 13 in treatments 1, 2 and 3, respectively. These 77 cases were from 42 different localities (Fig. 1).
In the 77 cases where the owls had selected one of the two boxes in a dyad and I was able to score whether the nest was predated, the time elapsed since the successful nesting in the original nest tree was on average 2.4 ± 0.2 years, and the distance between the two boxes was on average 177 ± 12 m.
Of localities with data on boreal owl box selection from the same year only 10% were situated closer to each other than 3 km, which is the approximate diameter of an average pine marten home range regarded as a circle (Sonerud 2021a). Thus, almost all cases of box selection were sufficiently separated to be located in the home ranges of different pine marten individuals (see Sonerud (2021a) for details). Because the average home range size of boreal owl males in my study area (see above) corresponds to a circle with radius c. 0.8 km, probably none, or at the most only a few, boreal owl males assessed more than one nest box dyad.
When conducting the field work for this study I was naïve to the hypothesis tested here. I became aware of the possibility of using the data to test the present hypothesis well after all field work was completed. Within each year, the timing of the relocation of boxes may be regarded as random because I did the relocation when time allowed between September and April. Thus, the data collection for this study was blind.
Identity, age and residential status of the owls
I made effort to capture the breeding owls at both the pre-treatment and the treatment nest, as explained in detail by Sonerud (2021a). I captured males at night in mist nets or a swing-door trap when they delivered prey to nestlings, and most females during daytime by hand when they were incubating or brooding. I defined resident owls as those that had previously nested at the same locality or in a neighbour territory, and immigrants as those that did not wear a ring when captured at the treatment nest at localities where the owl of the same sex at the associated pre-treatment nest had been captured and ringed. Among the males, I defined 12 as residents and 38 as immigrants, and among the females I defined 2 as residents and 57 as immigrants (see Sonerud (2021a) for details).
I scored the trapped owls as young (≤ 2 yrs) or old (≥ 3 yrs) based on the moulting pattern of the primaries, as explained by Sonerud (2021a). Of the 55 males that I captured at the treatment nest, I scored 32 as young and 23 as old, while of the 62 females that I captured at the treatment nest, I scored 30 as young and 32 as old. The proportion of the nesting males that I scored as old was higher among those defined as residents than among those defined as immigrants (75% vs. 34%, χ2 = 6.27, df = 1, p = 0.012).
I did not attempt to trap males before hatching for ethical reasons. Because 12 of the 13 cases of nest predation occurred during incubation (92%, cf. Sonerud 1985a, 2022), I did not capture any male from predated nests. This precluded an analysis of the effect of male age or residential status on the probability of nest predation. On the other hand, it also means that the nocturnal human activity involved in trapping of males did not cause the recorded nest predation. Similarly, only 3 of the 62 captured females had later their nest depredated, precluding an analysis of the effect of female age on the probability of nest predation. However, the owls´ age in relation to the time they had available for assessing the options could be analysed for the cases that did not lead to nest predation.
Statistical analyses
The sample unit was each boreal owl nesting event recorded in the dyads. The response variable was whether the boreal owl nest was predated or not (binomial distribution). The explanatory variables were as follows: (1) Time available for assessment of the boxes in a dyad (range 10–229 days). (2) Distance between the two boxes (range 40–600 m). (3) Whether the box in a new nest tree for the season or the box in the original nest tree was selected. (4) Time elapsed since the successful nesting at the locality (range 1–12 years). (5) Whether the selected box contained “post-nesting residue” or new wood shavings. Note that time available for assessment of the boxes in a dyad (variable 1) did not significantly affect whether the owls selected the box in the original nest tree or the box in a new tree for the season (variable 3) (see Sonerud 2021a).
Knowledge of all potential nest sites (boxes) before each season minimized the problem of underestimating nest predation by failure to include nests already depredated at the first nest check (see Sonerud 1985a,b). The data on the owls´ probability of having their nest predated were analyzed by using logistic regression in JMP® Pro version 15.0.0 (SAS 2019). I created models with all combinations of the explanatory variables and their interactions, with the limitation that any model contained at the most three variables, or two variables with their interaction, due to limited sample size (77 nests, of which 13 were predated). Candidate models were ranked using the Akaike information criterion corrected for small sample size (AICc), following recommendations by Burnham et al. (2011) and Richards et al. (2011). I considered models with ΔAICc < 2.0 to be well supported and thus competing with the model with lowest AICc value. Among competing models, I considered the one with the lowest number of effects the most parsimonious. I also report AICc weight for all models, and use evidence ratio (ER) when comparing the highest-ranked models, i.e. the ratio between the corresponding AICc weights (see Burnham et al. (2011), Richards et al. (2011) and Cade (2015) for definitions). I followed the advice by Cade (2015) and refrained from model averaging as well as the use of relative weight of a variable (i.e. the sum of AICc weights for all models in a model set in which the variable appeared) for evaluating the relative importance of explanatory variables.
In addition, I tested whether the probability that an owl was young rather than old was related to the time available for assessment of the boxes in a dyad by using logistic regression, where the response variable was whether an owl was scored as young or old, and the explanatory variable was time available for assessment of the boxes in a dyad.
In all models, correlations between the explanatory variables were small or negligible (R2 < 0.05). For the most parsimonious and the highest-ranked model, and for all models containing the focal variable (time available for assessment of the boxes in a dyad), I provide parameter estimates calculated by the Wald test based on standardized continuous variables. Estimates are given with ± 1 SE.
Results
Thirteen of the 77 nesting attempts (17%) were predated. As judged from signs in the nest boxes and around the nest trees, the pine marten accounted for at least 10 (77%), and possibly all, predation events.
The most parsimonious of the 36 candidate models included time available for assessment, whether the owls had selected the box in the new tree for the season or the box in the original tree, and the distance between these trees (Table 1). This model had a markedly higher weight than the second highest ranked (ER = 6.68). A nest was less likely to be predated if the dyad of nest boxes had been available for assessment for longer, if the distance between the two boxes was longer, and if the nest was in the original location previously used rather than in the novel one (Table 2; Fig. 2).
The probability that a boreal owl nest was predated as a function of time elapsed from nest box relocation to start of egg laying, with the curve describing the logistic regression model, separately for the box in the original nest tree (grey columns, solid hatched curve, thin hatched lines for 95% CI) and a box in a new nest tree for the season (white columns, solid curve, thin lines for 95% CI), for the average distance from the new nest tree to the original one (177 m). Time since nest box relocation ranged 10–229 days, and is grouped in 50 days units. The parameter estimates are reported in Table 2
Of the 15 models that included the focal variable (time available for assessment), 11 were among the 15 highest-ranked models, and only 4 were among the 20 lowest-ranked models (Table 1). The effect of the focal variable was significant in five of these 15 models (Table 2, Electronic Supplementary Material (ESM 1) Table S2, S5, S7 and S9), close to significant (0.05 < p < 0.06) in five (Table S1, S3-4, S10 and S14), marginally non-significant (0.06 < p < 0.08) in four (Table S6, S8, S11 and S13), and non-significant in only one (Table S12). The slope estimate for the standardized focal variable varied between − 0.61 and − 0.81 in 12 models (Table S1-S4, S6-S11 and S13-S14), and was − 1.01 and − 1.02 in two models (Table 2 and S5). Only in one model did the slope estimate for the focal variable deviate markedly (-0.30), and this was the only model where the effect was non-significant (Table S12). Thus, the effect of the focal variable was quite robust to the presence of other variables in the model. In the model with only the focal variable, the effect was close to significant (Table S3, ESM 2 Fig. S1).
Of the 15 models that included the focal variable, 12 were higher ranked than the null model, and 3 were lower ranked (Table 1). Of all candidate models, 18 were higher ranked than the null model, and 17 were lower ranked (Table 1).
The shortest time available for assessment was 10 days in the cases where the owls selected the box in the original tree (where there had been continuous presence of nest box), and 36 days in the cases where they selected the box in the new tree for the season (Fig. 3). The latter nest was predated between 63 and 78 days after the box was installed, representing the shortest recorded time from nest box installation to nest predation.
Distribution of boreal owl nests that were predated (filled circles) and nests that escaped predation (open circles) in relation to time elapsed from nest box relocation to start of egg laying and to distance between the two boxes in a dyad, for boxes in the original nest tree (upper panel) and boxes in the new nest tree for the season (lower panel)
In 11 cases, the owls selected the same nest tree in successive treatments; i.e. they selected the original nest tree twice. The probability of the male being the same as the previous one nesting at the same locality was not higher in these cases than in the cases when the original nest tree was not selected twice (0.13 vs. 0.19, P = 0.55, Fisher´s exact test, one-tailed). Moreover, excluding these 11 cases from the analysis did not change the results qualitatively (ESM 3). Thus, the effect of the focal variable remained similar when the sample became restricted when cases of repeated selection of the same original nest tree were excluded.
A nest in the original tree was marginally non-significantly more likely to be predated if the number of years elapsed since the successful nesting was higher (Fig. 4; slope = 1.03 ± 0.57, χ2 = 3.32, df = 1, P = 0.069, whole model (N = 36): χ2 = 3.28, df = 1, P = 0.070. R2 = 0.13, model fit χ2/df = 1.15). Therefore, although the overall probability of nest predation was higher in the new tree than in the original nest tree, the difference between these options decreased with increasing number of years elapsed since the previous nesting in the original tree, and was reversed when this elapse exceeded 4 years (Fig. 4).
The probability that a boreal owl nest in the box in the original nest tree was predated as a function of the number of years elapsed since the successful nesting in this tree, with the solid curve describing the logistic regression model, and the thin curves the 95% CI. The bars denote the distribution of cases in which the nest was predated (lower row) or escaped predation (upper row), and the dotted horizontal line shows the probability of nest predation in the box in the new tree
Because I did not attempt to capture males before hatching, and because all cases of nest predation except one occurred before hatching, I was unable to test the effect of the owls´ age on the probability of nest predation (see Methods). However, for the owls whose nest escaped predation, I tested whether there was any association between their age and the time they had had available for assessment. The longer the time available for assessment of options, the more likely it was that a young male rather than an old male that had selected the box in the new tree (slope = 1.11 ± 0.53, χ2 = 4.45, df = 1, P = 0.035, whole model (N = 26): χ2 = 5.38, df = 1, P = 0.020, R2 = 0.15, model fit χ2/df = 1.14). This was not the case for the males that selected the box in the original tree (slope = 0.36 ± 0.40, χ2 = 0.81, df = 1, P = 0.37, whole model (N = 29):χ2 = 0.83, df = 1, P = 0.36, R2 = 0.02, model fit χ2/df = 1.18). For females there was no corresponding association between age and the time they had had available for assessment, whether they had selected the box in the new tree (slope = 0.18 ± 0.40, χ2 = 0.19, df = 1, P = 0.66, whole model (N = 30): χ2 = 0.19, df = 1, P = 0.66, R2 = 0.005, model fit χ2/df = 1.33) or the box in the original tree (slope = -0.07 ± 0.34, χ2 = 0.04, df = 1, P = 0.83, whole model (N = 32): χ2 = 0.04, df = 1, P = 0.83, R2 = 0.001, model fit χ2/df = 1.51).
Discussion
Assessment of nest predation risk by boreal owls
The longer time the two boxes in a dyad were available, the lower probability of nest predation the boreal owls experienced if they finally selected one of the boxes. Thus, the less time for sampling of information and assessment, the less optimal choice the owls made, resulting in a larger probability of nest predation. This is in line with the speed-accuracy trade-off (Busemeyer and Townsend 1993), and to the best of my knowledge has not been previously demonstrated for nest site selection in birds. In other words, if the owls had less time to make an assessment and select nest site, taking nest predation risk into consideration mattered less (cf. Mönkkönen et al. 2009). Whether a poor choice under time pressure is an error or a deliberate compromise in the absence of alternatives is unknown (Chittka et al. 2009). One reason for the latter may be that the owls were less likely to find the box in the new tree before they made their choice when boxes were relocated closer to the breeding season. However, time available for assessment did not affect the probability of selecting the box in a new tree for the season rather than the box in the original nest tree (Sonerud 2021a).
With shorter time from box relocation until the breeding season the owls would have less time for learning to avoid unsafe nest sites (cf. Forstmeier and Weiss 2004; Eggers et al. 2006). If pine martens visit cavities at a low rate, an owl may need some time to acquire a reliable estimate of the predation risk in a cavity. After their first nesting boreal owl males are to a large extent locally resident (Löfgren et al. 1986; Korpimäki et al. 1987; Korpimäki 1993), and local resident males may attain an age of at least 11 years (GA Sonerud, personal observation). Therefore, they may monitor the cavities in their home range and assess their relative predation risk, and simply avoid cavities where they once have ever seen a pine marten at any time of the year (cf. Hakkarainen et al. 2001) or even only sensed the smell of one (cf. Amo et al. 2011).
Because I for ethical reasons did not attempt to capture the males before hatching, and because 12 of 13 cases of nest predation occurred before hatching, I was unable to test whether the age (and indirectly the residential status) of the males affected the probability of nest depredation. However, among the males that did not have their nest predated, the longer the time available for assessment of options, the more likely it was that a young male rather than an old one had selected the box in the new tree, whereas such an effect of age was not the case for males that selected the box in the original nest tree. This suggests that older, and presumably resident, males were better able to find the box in the new tree when time was short. Among the females, of which almost all were presumably immigrants, there was no corresponding effect of age. The pattern of selecting between the box in the new tree and the box in the original nest tree differed between young and old males, but not between young and old females, and old males were more likely than young males to select the box in the new tree (Sonerud 2021a).
The probability of predation declined as the distance between the two boxes increased. One interpretation of this pattern is that if the marten found one of the boxes, it would be more likely to find the other if this was located closer, either because the marten used area-concentrated search (Sonerud 1985c), or because it was more likely to encounter the new cavity by chance. The cavity-nesting common goldeneye (Bucephala clangula) faced a decreasing probability of nest predation from pine marten with increasing distance from a box where nest predation had occurred (Dow and Fredga 1983). Moreover, the probability of predation of boreal owl nests was lower in old boxes that had been relocated on average 150 m (range 50–370 m) either c. 6 months earlier or c. 18 months earlier than in control boxes with the same age (Sonerud 1989, 1993).
Overall, the probability of predation was lower in the original nest tree than in the new one, but numerically this switched when 4 years had elapsed since the successful nesting in the original tree. Thus, assessing the relative predation risk in the two boxes was not simple. The previous nest in the original tree was successful, indicating that the pine marten at the time of that nesting had not found the box. Therefore, the next nest in the original tree would be expected to have a low probability of predation if the time elapsed since the previous nesting was short. Although another 2 years had elapsed on average until the boreal owls made their box selection, the box in the original nest tree was still in the safest subset of all boxes installed. In contrast, the boxes installed in a new tree for the season was a random sample from all installed boxes, and according to the site-effect (sensu Martin et al. 2000) more likely to be found by the pine marten, i.e. some of these boxes happened to be installed where the marten travelled.
The probability of a boreal owl nest being predated by pine marten has been found to increase with the functional age of the cavity, i.e. time elapsed since a box was installed in a tree (Sonerud 1985a, 1989, 1993, 2022), although this has been measured on the scale of years. If this pattern also applies on the scale of months and weeks, one would expect the probability of nest predation in the box in the new nest tree to increase with time elapsed from relocation of boxes to start of egg laying. However, the opposite was the case, which strengthens the interpretation that the owls were able to make a better decision the more time they had available. Note that because pine martens visit nest boxes year-round for other purposes than nest predation (Sonerud 1985b; Brainerd et al. 1995), the simple pattern that a box where a nest was found and predated is profitable to revisit and an empty box is unprofitable to revisit is not applicable to predation of nests in boxes by pine marten.
Methodological considerations
The experiment allowed the owls to assess the risk of nest predation at a new site relative to that at the site where a box had been available continuously for several years (see Sonerud (2021a) for details). Hence, the time for assessment was the time available for comparing the predation risk in the new tree with the already known predation risk in the original nest tree. An alternative experimental setup may have been to remove the box in the original tree immediately after the successful nesting (and after each following nesting season until the focal nesting event occurred), and put it up again at the same site on the day when a box was put up in a new tree. Such a setup would provide an intermittent nest box presence in the original nest tree rather than a continuous one. Note that the time elapsed from the successful nesting in the original tree until one of the boxes in the dyad was selected ranged up to 12 years, and on average was 2.4 years, so the alternative setup would in sum lead to a long time without a nest box in the original nest tree. If the pine marten at some point had found the box in the original tree, the box would most likely have been incorporated in the pine marten´s later travel routes (cf. Sonerud 1985a). If I had taken down this box after each nesting season, the pine marten may have searched in vain for it, and probably abandoned the original nest tree on later travel routes. Then, a box put up at this site again before the next nesting season may have had the same expected probability of being visited by the pine marten as a box put up in a new tree. Also, for the boreal owl, which prefers newly installed boxes (Sonerud 1985a, 2021a), the two boxes may then have represented equal options. Hence, I regard the setup that I used to better fit the behaviour of pine marten and boreal owl.
The effect of the focal variable was quite robust to the presence of other variables in the model. However, of reasons of principle, a single point of precaution should be declared in the form of the relatively small number of events (13 cases of predation) relative to the number of predictors in the most parsimonious model (3), resulting in a low event-predictor ratio (4.3). Even though a conventional rule-of-thumb recommends an event-predictor ratio to be at least 10 to safeguard against model over-fitting, a recent study indicated that this conservative premise can be relaxed (van Smeden et al. 2016). As the most parsimonious model´s residual structure provided a good fit to the data, I therefore consider this model to be reasonably reliable.
The haste of a migratory cavity-nester
Cavity-nesting birds that migrate are very time-constrained in their assessment and selection of nest site when they return in spring. They may be comparable with the boreal owls that had the shortest time between the appearance of the box in the new tree for the season and laying of the first egg in the box in the original nest tree (10 days), and may have less time to locate and assess potential nest cavities than the minimum interval that I detected for boreal owls of 36 days between appearance of the box in the new tree and laying of the first boreal owl egg in it.
The common goldeneye is a migrating duck that uses the same type of cavities as the boreal owl for nesting, i.e. black woodpecker cavities and nest boxes, and is thus exposed to the same nest predation from the pine marten (e.g., Dow and Fredga 1983). It is noteworthy that common goldeneye females base their cavity selection on private or public information gained in the previous year, i.e. they either return to the cavity where they nested successfully last year, or they lay eggs in a cavity where a conspecific nested successfully last year (Dow and Fredga 1983; Pöysä 1999; Sonerud 2021b). Site fidelity after successful nesting and site shift after nest predation (win-stay, lose-shift (WSLS)), both within and between nesting seasons, is an adaptation to spatially heterogeneous and temporally auto-correlated predation risk (e.g., Dow and Fredga 1983, 1985; Schmidt et al. 2006; Chalfoun and Martin 2010; Chalfoun and Schmidt 2012). Interestingly, when common goldeneye females were forced to select among previously unoccupied boxes, their selection was not correlated with the difference in risk of nest predation between these boxes, as measured by predation of artificial eggs (Pöysä et al. 2001).
Thus, common goldeneyes have no better options than to use the WSLS assessment rule, which would underperform compared to a Bayesian-like assessment rule (Chalfoun and Smith 2012). Boreal owls may use the latter rule in the form of continuous updating of the information on predation risk in available cavities throughout the non-breeding season.
Conclusions
An explicit demonstration of a speed-accuracy trade-off must show that the subject exhibits more or fewer errors because it is acting more or less quickly (Franks et al. 2003). By manipulating the length of time a non-migratory cavity-nesting bird had available for assessing two alternative nest-sites I demonstrated that shorter time until decision led to a higher probability of nest predation. Whether this represents a speed-accuracy trade-off sensu stricto, i.e. a poor decision due to an error under haste, or a compromise based on making the best of a bad job, is unclear (cf. Chittka et al. 2009). It is likely, however, that the boreal owls gained information on the relative safety of the two options by Bayesian-like updating (cf. Luttbeg 1996) in the days, weeks or months before the decision had to be made, and that this information enabled them to make a better choice than a migrant cavity-nester exposed to the same landscape of nest predation risk would do. Further work should use a similar design on other birds, both residents and migrants, and also study how individuals sample and assess the alternatives.
Data Availability
The data that support the plots within this paper and other finding of this study are available from the corresponding author upon request.
References
Amo L, Visser ME, van Oers K (2011) Smelling out predators is innate in birds. Ardea 99:177–184. https://doi.org/10.5253/078.099.0207
Brainerd SM (1997) Habitat selection and range use by the Eurasian pine marten (Martes martes) in relation to commercial forestry practises in southern boreal Scandinavia. PhD dissertation, Agricultural University of Norway, Ås
Brainerd SM, Helldin JO, Lindström ER, Rolstad E, Rolstad J, Storch I (1995) Pine marten (Martes martes) selection of resting and denning sites in Scandinavian managed forests. Ann Zool Fenn 32:151–157
Busemeyer JR, Townsend JT (1993) Decision field-theory: a dynamic cognitive approach to decision-making in an uncertain environment. Psychol Rev 100:432–459
Caro T (2005) Antipredator defences in birds and mammals. University of Chicago Press, Chicago
Chalfoun AD, Martin TE (2010) Facultative nest patch shifts in response to predation risk in the Brewer´s sparrow: a “win-stay, lose-shift” strategy? Oecologia 163:885–892. https://doi.org/10.1007/s00442-010-1679-0
Chalfoun AD, Schmidt KA (2012) Adaptive breeding-habitat selection: is it for the birds? Auk 12:589–599. https://doi.org/10.1525/auk2012.129.4589
Chittka L, Dyer AG, Bock F, Dornhaus A (2003) Bees trade-off foraging speed for accuracy. Nature 424:388
Chittka L, Osorio D (2007) Cognitive dimensions of predator responses to imperfect mimicry? PLoS Biology 5(12): Article e339
Chittka L, Skorupski P, Raine NE (2009) Speed-accuracy tradeoffs in animal decision making. Trends Ecol Evol 24:400–407
Cramp S (1985) The birds of the western Palearctic, vol IV. Oxford University Press, Oxford
Dow H, Fredga S (1983) Breeding and natal dispersal of the goldeneye, Bucephala clangula. J Anim Ecol 52:681–695. https://doi.org/10.2307/4447
Dow H, Fredga S (1985) Selection of nest sites by a hole-nesting duck, the goldeneye Bucephala clangula. Ibis 127:16–30. https://doi.org/10.1111/j.1474-919X.1985.tb05034.x
Dyer AG, Chittka L (2004) Bumblebees (Bombus terrestris) sacrifice foraging speed to solve difficult discrimination tasks. J Comp Physiol A 190:759–763
Eggers S, Griesser M, Nystrand M, Ekman J (2006) Predation risk induces changes in nest site selection and clutch size in the Siberian jay. Proc Soc R B 273:701–706. https://doi.org/10.1098/rspb.2005.3373
Eldegard K, Sonerud GA (2009) Female offspring desertion and male-only care increase with natural and experimental increase in food abundance. Proc R Soc B 276:1713–1721. https://doi.org/10.1098/rspb.2008.1775
Eldegard K, Sonerud GA (2010) Experimental increase in food supply influences the outcome of within -family conflicts in Tengmalm´s owl. Behav Ecol Sociobiol 64:815–826. https://doi.org/10.1007/s00265-009-0898-z
Eldegard K, Sonerud GA (2012) Sex roles during post-fledging care in birds: female Tengmalm´s owls contribute little to food provisioning.J Ornithol 153:385–398. https://doi.org/10.1007/s10336-011-0753-7
Forstmeier W, Weiss I (2004) Adaptive plasticity in nest-site selection in response to changing predation risk. Oikos 104:487–499. https://doi.org/10.1111/j.0030-1299.1999.12698.x
Franks NR, Dornhaus A, Fitzsimmons JP, Stevens M (2003) Speed versus accuracy in collective decision making. Proc R Soc B: 270:2457–2463
Furuichi S, Kauya E (2013) Hurrying foragers fail to choose the best prey item prey choice in paper wasps depredating conspecific nests. Ethology 119:786–792
Hakkarainen H, Ilmonen P, Koivunen V, Korpimäki E (2001) Experimental increase of predation risk induces breeding dispersal of Tengmalm´s owl. Oecologia 126:355–359. https://doi.org/10.1007/s004420000525
Hakkarainen H, Korpimäki E (1998) Why do territorial male Tengmalm´s owl fail to obtain a mate? Oecologia 114:578–582. https://doi.org/10.1007/s004420050483
Helldin JO (1999) Diet, body condition, and reproduction of Eurasian pine martens Martes martes during cycles in microtine density. Ecography 22:324–336. https://doi.org/10.1111/j.1600-0587.1999.tb00508.x
Helldin JO (2000) Seasonal diet of pine marten Martes martes in southern boreal Sweden.Acta Theriol 45:409–420
Hörnfeldt B, Carlsson B-G, Löfgren O, Eklund U (1990) Effects of cyclic food supply on breeding performance in Tengmalm´s owl (Aegolius funereus). Can J Zool 68:522–530. https://doi.org/10.1139/z90-077
Jacobsen BV, Sonerud GA (1987) Home range of Tengmalm´s owl: a comparison between nocturnal hunting and diurnal roosting.USDA For Serv Gen Tech Rep RM-142:189–192
Johnsson K (1993) The black woodpecker Dryocopus martius as a keystone species in forest. PhD dissertation, Swedish University of Agricultural Sciences, Uppsala
Korpimäki E (1993) Does nest-hole quality, poor breeding success or food depletion drive the breeding dispersal of Tengmalm´sowls? J Anim Ecol 62:606–613. https://doi.org/10.2307/5382
Korpimäki E, Hakkarainen H (1991) Fluctuating food supply affects the clutch size of Tengmalm s owl independent of laying date.Oecologia 85:543–552. https://doi.org/10.1007/BF00323767
Korpimäki E, Lagerström M, Saurola P (1987) Field evidence for nomadism in Tengmalm´s owl Aegolius funereus. Ornis Scand 18:1–4
Langen TA (1999) How western scrub-jays (Aphelocoma californica) select a nut: effects of number of options, variation in nut size,and social competition among foragers. Anim Cogn 2:223–233
Lima SL (2009) Predators and the breeding bird: behavioral and reproductive flexibility under the risk of predation. Biol Rev 85:485–513. https://doi.org/10.1111/j.1469-185X.2009.00085.x
Löfgren O, Hörnfeldt B, Carlsson B-G (1986) Site tenacity and nomadism in Tengmalm´s owl (Aegolius funereus (L.)) in relation to cyclic food production. Oecologia 69:321–326. https://doi.org/10.1007/BF00377051
Luttbeg B (1996) A Comparative Bayes tactic for mate assessment and choice. Behav Ecol 7:451–460. https://doi.org/10.1093/beheco/7.4.451
Martin TE, Scott J, Menge C (2000) Nest predation increases with parental activity: separating nest site and parental activity effects. Proc R Soc B 267:2287–2293. https://doi.org/10.1098/rsbp.2001.1879
Marzluff JM (1988) Do pinyon jays alter nest placement based on prior experience? Anim Behav 36:1–10. https://doi.org/10.1016/50003-3472(88)80244-6
Mönkkönen M, Forsman J, Kananoja T, Ylönen H (2009) Indirect cues of nest predation risk and avian reproductive decisions. Biol Lett 5:176–178. https://doi.org/10.1098/rsbl.2008.0631
Osman A, Lou LG, Muller-Gethmann H, Rinkenauer G, Mattes S, Ulrich R (2000) Mechanisms of speed -accuracy trade-off: evidence from covert motor processes. Biol Psychol 51:173–199
Passino KM, Seeley TD (2006) Modeling and analyzing of nest-site selection by honeybee swarms: the speed and accuracy trade-off. Behav Ecol Sociobiol 59:427–442
Pöysä H (1999) Conspecific nest parasitism is associated with inequality in nest predation in the common goldeneye (Bucephala clangula). Behav Ecol 10:533–540. https://doi.org/10.1093/beheco/10.5.533
Pöysä H, Ruusila V, Milonoff M, Virtanen J (2001) Ability to assess nest predation risk in secondary hole-nesting birds: an experimental study. Oecologia 126:201–207
Pulliainen E, Ollimäki P (1996) A long-term study of the winter food niche of the pine marten Martes martes in northern boreal Finland.Acta Theriol 41:337–352
SAS (2019) JMP® Pro version 15. SAS Institute, Cary, USA
Schmidt KA, Ostfeld RS, Smyth KN (2006) Spatial heterogeneity in predator activity, nest survivorship, and nest-site selection in two forest thrushes. Oecologia 148:22–29. https://doi.org/10.1007/s00442-005-0340-9
Slagsvold T, Dale S (1994) Why do female pied flycatchers mate with already mated males: deception or restricted mate sampling? Behav Ecol Sociobiol 34:239–250
Sonerud GA (1985a) Nest hole shift in Tengmalm´s owl Aegolius funereus as defence against nest predation involving long-term memory in the predator. J Anim Ecol 54:179–192. https://doi.org/10.2307/4629
Sonerud GA (1985b) Risk of nest predation in three species of hole nesting owls influence on choice of nesting habitat and incubation behaviour. Ornis Scand 16:261–269. https://doi.org/10.2307/3676689
Sonerud GA (1985c) Brood movements in grouse and waders as defence against win-stay search in their predators. Oikos 44:287–300. https://doi.org/10.2307/3544702
Sonerud GA (1989) Reduced predation by pine martens on nests of Tengmalm´s owl in relocated boxes.Anim Behav 37:332–334. https://doi.org/10.1016/0003-3472(89)90122-X
Sonerud GA (1993) Reduced predation by nest box relocation: differential effect on natural and artificial Tengmalm´s owl nests. Ornis Scand 24:249–253. https://doi.org/10.2307/3676742
Sonerud GA (2021a) Win – and stay, but too long: cavity selection by boreal owls to minimize nest predation by pine marten. J Ornithol 162:839–855. https://doi.org/10.1007/s10336-021-01876y
Sonerud GA (2021b) Nest box reuse in a migrating bird is independent of nest content. Ornis Fennica 98:105–115
Sonerud GA (2022) Predation of boreal owl nests by pine martens in the boreal forest does not vary as predicted by the alternative prey hypothesis. Oecologia 198:995–1009. https://doi.org/10.1007/s00442-022-05149-0
Sonerud GA, Solheim R, Jacobsen BV (1986) Home-range use and habitat selection during hunting in a male Tengmalm´s owl Aegolius funereus. Fauna norv Ser C, Cinclus, 9:100–106
Sonerud GA, Solheim R, Prestrud K (1988) Dispersal of Tengmalm´s owl Aegolius funereus in relation to prey availability and nesting success. Ornis Scand 19:175–181. https://doi.org/10.2307/3676555
Sørås R, Gundersen OA, Steen R, Sonerud GA (2020) Returning for more prey? Foraging in provisioning male boreal owl (Aegolius funereus). J Ornithol 161:171–181. https://doi.org/10.1007/s10336-019-01710-6
van Smeden M, de Groot JAH, Moons KGM, Collins GS, Altman DG,Eijkemans MJC, Reirsma JB (2016) No rationale for 1 variable per 10 events criterion for binary logistic regression analysis. BMC Med Res Method 16:163. https://doi.org/10.1186/s12874-016-0267-3
Zarybnicka M, Riegert J, Kouba M (2015) Indirect food web interactions affect predation of Tengmalm´s owls Aegolius funereus nests by pine martens Martes martes according to the alternative prey hypothesis. Ibis 157:459–467. https://doi.org/10.1111/ibi.12265
Acknowledgements
I am especially grateful to H. Steen for help and companionship when installing and checking many of the nest boxes in 1990-94. I also would like to thank K. Eldegard, E. Maartmann and D. I. Sonerud for assistance during various parts of the field work, L. E. Loe for statistical advice, R. Økseter for making the map, M. Mjelde and R. Steen for making the figures, and R. Burner, S. Dale, J. Madden, V. Selås, T. Slagsvold and P. Sunde for comments on drafts of the manuscript.
Funding
This work was supported by grants from the Nansen Endowment (grant numbers 102/87, 101/88, 111/89, 82/90, 97/91, 101/92, 203/93, 197b/94 and 87/95).
Open access funding provided by Norwegian University of Life Sciences
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This research adhered to the Association for the Study of Animal Behaviour Guidelines for the Use of Animals in Research, the legal requirements of Norway, and all institutional guidelines. Permit to trap and ring the owls was obtained from the Norwegian bird ringing centre.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sonerud, G.A. Haste makes waste: shorter time for nest-site assessment leads to higher nest predation in a cavity nester. Evol Ecol 36, 879–898 (2022). https://doi.org/10.1007/s10682-022-10194-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-022-10194-5