Abstract
Seasonal long-distance migratory behaviour of trillions of animals may in part have evolved to reduce parasite infection risk, and the fitness costs that may come with these infections. This may apply to a diversity of vertebrate migration strategies that can sometimes be observed within species and may often be age-dependent. Herein we review some common age-related variations in migration strategy, discussing why in some animal species juveniles preferentially forego or otherwise rearrange their migrations as compared to adults, potentially as an either immediate (proximate) or anticipatory (ultimate) response to infection risk and disease. We notably focus on the phenomenon of “oversummering”, where juveniles abstain from migration to the breeding grounds. This strategy is particularly prevalent amongst migratory shorebirds and has thus far received little attention as a strategy to reduce parasite infection rate, while comparative intra-specific research approaches have strong potential to elucidate the drivers of differential behavioural strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Every year, trillions of animals, including billions of birds (e.g. Hahn et al. 2009) undertake long-distance migrations. Some of the most eye-catching migrations comprise Monarch Butterflies (Danas plexippus) migrating from Mexico to Canada (Browner 1995), Arctic Terns (Sterna paradisaea) undertaking the longest avian migration connecting Arctic with Antarctic waters (Egevang et al. 2010), Bar-tailed Godwit (Limosa lapponica) migrating from Alaska to New Zealand in an 8 day long, non-stop flight (Battley et al. 2012), or Humpback Whales (Megaptera novaeangliae) migrating from the chilly waters of the Antarctic to tropical regions to calve (Stone et al. 1990). The ultimate drivers for these predictable, long-distance movements are thought to be multifactorial, with key factors considered to include: (i) tracking spatial and temporal variations in food availability, escaping seasonal food shortages and taking advantage of abundant food resources where and when they occur; (ii) seeking physiologically optimal climates, limiting thermoregulatory costs and climactic conditions that are conducive for reproduction and vulnerable neonates; (iii) moving to areas of reduced predation, particularly during reproduction; and (iv) moving to areas of low pathogen and parasite pressure (Alerstam et al. 2003; Altizer et al. 2011; Avgar et al. 2013; Chapman et al. 2015; Gnanadesikan et al. 2017; McKinnon et al. 2010). Despite the growing body of literature exploring the evolutionary reasons for migration in specific species and species groups, the role of parasites (including both micro- and macroparasites) as drivers for migration have seldom been considered (Binning et al. 2017; Moller and Szep 2010; Pedersen et al. 2007; Poulin and Dutra 2021; Shaw et al. 2019) .
Herein, we propose that differential migration strategies between juveniles and adults may have their ultimate explanation in parasite exposure and susceptibility and form particularly interesting systems to elucidate drivers of migration behaviour. Comparative research approaches bear notable promise in elucidating the role of parasites in shaping migration strategies, especially when these can make use of within species variations in migration strategy. Juvenile birds are generally more prone to infection than adults and, in parallel, age-related differences in migration strategy are relatively common. As such, to address the putative role of parasites as a driver for differential juvenile migration, we will first, briefly, review the impacts of infection on migratory birds and how those effects are thought to have shaped migration (Fig. 1). Second, we focus on four distinct, age-related, differential migration strategies where juveniles and adults (i) differentially time their migration, (ii) have a different migration route, (iii) migrate a shorter distance, or (iv) where juveniles forego migration altogether (Figs. 1 and 2). Third, we review evidence pertaining to the higher susceptibility of juveniles to infection compared to adults. Finally, we discuss the extent these differential strategies could be driven by age differences in susceptibility to parasite infection, with a focus on juveniles foregoing migration.
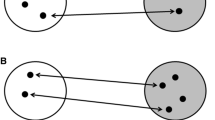
Framework in which we discuss the potential role of infection risk on the evolution of migration allopatry between juvenile and adults. Grey arrows comprise the four main drivers of migration. The disease arrow is expanded to detail the four disease mechanisms thought to play a role in shaping migration strategies of which migration allopatry is one. The four main variants of migration allopatry, or instances wherein juveniles and adults have differing migration strategies, are presented. This includes oversummering as the most extreme case of migration allopatry, where juvenile birds forego migration. To also showcase alternative explanations to disease risk as an ultimate explanation for age-differential migration allopatry, we present other proximate explanations for oversummering. Avian silhouettes generated by M. Wille
Juvenile animals are generally more prone to parasite infection than adults, which may have driven the evolution of different types of age-dependent migration strategies. We distinguish four different types of such “migration allopatry” strategies. Image of Wood Thrush was photographed by, and shared with permission from Mike Melton, Ruddy Turnstone and Sharp-tailed Sandpiper were photographed by, and shared with permission from Kerry Vickers, Honey Buzzard was photographed by and shared with permission from Magnus Hellström, Short-toed Snake Eagle was photographed by Lehava Kiryat Shmona Pikiwiki Israel and distributed under a CC BY 2.5 licence, Eleonora’s Falcon was distributed by Conselleria de Medi Ambient i Mobilitat, Govern des Illes Balears under a CC-BY-SA 3.0 licence, Greater Yellow-legs was photographed by Alan D. Wilson and distributed under a CC BY-SA 2.5, Red Knot was photographed by, and is shared with permission from Sandy Horne
Infection and migratory performance
Migration is an energetically expensive process requiring substantial, costly physiological adjustments, which could potentially be better achieved by trading-off other costly processes, including mechanisms for protection and clearing of parasite infections (Klaassen et al. 2012). A recent meta-analysis reviewing 85 observations extracted from 41 studies spanning a range of taxa including birds, fish and insects demonstrated that parasite infection is associated with behavioural changes that may alter migratory performance. Specifically, infection status and intensity were associated with reduced movement capacity, delayed migration phenology, and lower rates of survival, with infection status also being associated with reduced body stores, which are often needed to help fuelling the migratory journeys (Risely et al. 2018). The costs to avoid, battle or tolerate parasitic infections are diverse and include evolving, maintaining and mounting physiological or behavioural responses (Schmid Hempel 2011). Further, these costs may exist regardless of whether the parasitic infection manifests in clinical disease or is asymptomatic. For instance, the activation and maintenance of an immune response may impose significant nutritional and energetic demands on the host, potentially trading-off with other physiological processes, such as the rate of acquiring body stores to fuel migration (Buehler et al. 2010; Klaassen et al. 2012). An example of such trade-off was demonstrated in the partial migrant, the Common Blackbird (Turdus merula). Partially migrating populations consist of a mixture of migrating and non-migrating or resident individuals (Berthold 2001). In Common Blackbirds migrating individuals had a lower innate immune function than resident individuals (Eikenaar and Hegemann 2016). Whether trading-off immune function for migratory potential is beneficial may depend on the expected conditions en-route. The act of migration, combined with the utilization of a range of distant sites may also increase infection risk, and in these cases immune function should not be compromised and possibly even be boosted. For instance, in the partially migratory Eurasian Skylark (Alauda arvensis), migrating birds had a higher immune function than resident birds (Hegemann et al. 2015). Interestingly, in Red Knots (Calidris canutus) in captivity this higher immune function was found during the migratory period (Buehler, Piersma, Matson, & Tieleman, 2010). Yet, when challenged and compelled to migrate in a wind tunnel, no difference in immune function between “migrating” and control Red Knots was found (Hasselquist et al. 2007).Yet, upregulating immune function and (getting prepared) to battle parasite infections may not always be the best strategy. More recently, the notion that host tolerance and damage control might be an important and common alternative host strategy to fighting parasite infections has gained appreciation (e.g. Read et al. 2008) and is a strategy that might also benefit actively migrating animals (Klaassen et al. 2012; Lochmiller and Deerenberg 2000). Support that tolerance rather than control of parasitic infections (resistance) can yield a higher fitness in migrants comes from Sockeye Salmon (Oncorhynchos nerka), where smolts that did not survive their first migration had gene expression profiles consistent with a highly upregulated immune response to viral pathogens (Jeffries et al. 2014). In birds, Mallards (Anas platyrhynchos) infected with influenza A virus mainly tolerate the infection, mounting a very limited immune response (Helin et al. 2018; Kuiken 2013) with no detectable negative effect of infection on movement ecology or survival (Bengtsson et al. 2016; van Dijk et al. 2015a). Taken together, regardless of whether animals tolerate or fight a parasitic infection, their defence and response to infection comes at a cost, and it is conceivable that infection risk, status or intensity act as a barrier to migration.
Parasites shaping migration strategies
Owing to their typically large geographic range, migratory animals may be at greater risk of infection compared to resident species, making increased parasite infection risk an obvious potential cost of migratory behaviour (Kopeivnikar and Leung 2014; Poulin and Dutra 2021; Teitelbaum et al. 2018). For example, migratory ungulates have a significantly richer parasite community (Teitelbaum et al. 2018), or migratory Mallards have a higher prevalence of avian influenza compared to residents (van Dijk et al. 2014). Conversely, and supported by a range of empirical and theoretical studies, migration can also confer a number of benefits to migrants in terms of allowing escape from infection or by allowing a respite and/or recovery from infection (Folstad et al. 1991; Kubelka et al. 2021; Piersma 1997; Poulin and Dutra 2021; Shaw and Binning 2016). For example, a higher prevalence of tick infestation was recorded in sedentary and short distance migrating Common Blackbirds as compared to long distance migratory individuals (Klaus et al. 2016). As such, we are increasingly realising that parasites may be a central driver of animal migration strategies Altizer et al. 2011; Klaassen et al. 2012; Kubelka et al. 2021; Poulin & de Angeli Dutra 2021).
Through migration, animals may be able to lower their infection risk by four nonexclusive processes (Altizer et al. 2011; Poulin & de Angeli Dutra 2021). First, migratory escape allows animals to leave an area of temporarily high parasite pressure in favour of a refuge with lower infection risk (Loehle 1995; Piersma 1997). Second, through migration, pathogen pressure may be lowered by removing infected individuals from the population through a process referred to as migratory culling (Altizer et al. 2015; Bartel et al. 2011; Bradley and Altizer 2005). Not only does this comprise animals dying during transit, but diseased animals suffering from the negative consequences of infection are less likely to migrate (Bradley and Altizer 2005). Third, migratory recovery proposes that during or through migration, infected animals may recover from infection because the area to which they migrate is not conducive for the fitness of the parasite (Shaw and Binning 2016). Critically, migratory recovery only applies to infected rather than uninfected individuals and may modulate which individuals do and which do not migrate in partially migratory species (Shaw and Binning 2016). Finally, migratory separation is the process whereby infected individuals are delayed in their migration phenology relative to uninfected individuals, resulting in a period of spatial isolation and reduced transmission (Bauer et al. 2015; Galsworthy et al. 2011). Taken together, these four hypotheses provide explanations for how individuals and populations might reduce parasite burden through migration. Importantly, they also provide candidate mechanisms through which migration, and a diversity of migratory strategies for animals faced with substantial parasite burdens, may have evolved.
Age-related differential migration strategies
As raised above, in order to increase our understanding of drivers of migration, studying within-species variation in migration strategy may be of particular interest. Within partially migratory populations, individuals may be obligate or facultative migrants/residents. Obligate migrants are those that appear “hard-wired” in their migratory behaviour, whereas facultative migrants are individuals that flexibly choose whether to migrate or not. In some cases, partial migration manifests in different migration strategies stratified by age. Prime examples of differential migration strategies in age classes have been described in marine animals, notably fish, and referred to as migratory allopatry, or the spatial separation of juvenile and adult age classes (Krkošek et al. 2007). It has been suggested that this migratory allopatry can mitigate parasite transmission from adults to juveniles and break the life cycle of parasites, with resulting reductions in parasite burden being an important driver for the evolution of ontogenetic migration (i.e. different life-stages migrating differentially) (Krkošek et al. 2007; Strathmann et al. 2002).
Age-related differential migration strategies are, however, not limited to marine animals and, at the same time, migratory allopatry of age classes can also be brought about in an array of ways. Using examples from the avian literature we identified as many as four means by which migratory allopatry between adults and juveniles may occur (Figs. 1 and 2). First, this may happen through age differences in the timing of migration. In shorebirds the timing of outbound migration (i.e. migration from the breeding grounds) of the age classes often differs, where adult birds migrate several weeks earlier than juveniles (Alerstam 1990). In Wood Thrushes (Hylocichla mustelina), juveniles depart 7.9 days (± 2.9 days) later than adults during inbound migration (i.e. migration towards the breeding grounds) from the tropics (McKinnon et al. 2014) (Fig. 2). Second, the routes juveniles and adults take may be different. A prime example of this exists in Sharp-tailed Sandpipers (Calidris acuminata) utilizing the East Asian Australasian Flyway where most juveniles use a spectacularly different migration route compared to their parents (Handel and Gill Jr 2010; Lindstrom et al. 2011). Following breeding in Siberia, adult Sharp-tailed Sandpipers migrate from their breeding grounds on a course due south along the East Asian coast towards their wintering grounds in Australia. Juveniles do not follow adults, but rather embark on a detour east across the Bering Strait to Alaska, where they spend about a month preparing for their migration to Australia, most likely via a nonstop flight across the Pacific Ocean (Handel and Gill Jr 2010; Lindstrom et al. 2011) (Fig. 2). Third, juveniles may conduct only an incomplete migration, particularly during inbound migration. In some raptors spending the non-breeding season in Africa, juveniles may stop short of the European breeding grounds during their first northward migration, dispersing into central Africa or southern Europe (Gschweng et al. 2008; Harrison et al. 1997; Mellon et al. 2011; Osterlof 1977) (Fig. 2). Finally, at the extreme end, juveniles may not migrate at all while adults do, as exemplified by “oversummering” shorebirds. In this case juveniles, sometimes accompanied by the odd adult, remain on the non-breeding grounds when the remainder of the population migrates to the breeding grounds, skipping one-year worth of migration, involving both an inbound and an outbound migration (Fig. 2). Thus, in summary, migration strategies between adults and juveniles may differ in timing, route and distance covered, with, in the most extreme case, juveniles foregoing migration entirely (Fig. 1).
Age and susceptibility to parasites
While migration allopatry between juveniles and adults may reduce the chance of adults infecting juveniles with the parasites they are carrying, as suggested for marine animals (Krkošek et al. 2007; Strathmann et al. 2002), differential migration strategies for adults and juveniles may also relate to increased susceptibility of juveniles to parasite infection compared to adults. The importance of host age for the epidemiology and ecology of host-parasite interactions has been demonstrated in a number of host – parasite systems, from helminths (Chylinski et al. 2009) and bacteria (Dhondt et al. 2012; McDonald et al. 2018) to viruses (Amman et al. 2012; Hayman 2015; Latorre-Margalef et al. 2014; van Dijk et al. 2014). For example, in House Finches (Carpodacus mexicanus), bacterial conjunctivitis caused by Mycoplasma gallisepticum is highly seasonal, with peaks occurring following juveniles’ recruitment into the population (Altizer et al. 2004b). During periods of high prevalence, infection probability is significantly higher in juvenile house finches compared to adult birds (Altizer et al. 2004a). Similarly, the autumnal prevalence peaks of avian influenza A virus in waterfowl in the Northern Hemisphere occurs following juvenile recruitment into the population and coincides with migration, with the vast majority of infections occurring in juvenile birds (Latorre-Margalef et al. 2014; van Dijk et al. 2014). Overall, higher infection rates in juveniles as compared to adults have been largely demonstrated in studies focusing on a single-taxa of parasites. With studies targeting the entire virome having come within reach in recent times, bat caves with larger proportions of juveniles were found to have higher virus diversity than those with few juveniles (Bergner et al. 2019). A study in Ruddy Turnstones (Arenaria interpres) demonstrated that juveniles had higher viral abundance and viral community diversity compared to adults (Wille et al. 2021), again confirming that infection rate in juveniles tends to be higher than in adults.
The trend of higher parasite loads in juveniles compared to adults is driven, at least in part, by the increased susceptibility of juveniles, for which detailed studies of avian influenza in ducks further provide an excellent example. While juveniles have higher avian influenza viral prevalence than adults, the converse is observed when assessing seroprevalence (the proportion of birds with antibodies). Specifically, the seroprevalence of general influenza antibodies is higher in adults as compared to juvenile birds (Hoye et al. 2012; Tolf et al. 2013). Furthermore, a study by Hill et al. (2016) eloquently showed that specific antibody responses (that is, towards specific subtypes of influenza as compared to general antibodies that detect any subtype of influenza) broaden with age. That is, the number of avian influenza subtypes to which individuals have antibodies increased with bird age (Hill et al. 2016). With time, individuals are challenged with numerous viruses, and in long-lived species antibodies are typically retained for many years (Ramos et al. 2014). In this particular case, the age differences in susceptibility are more likely driven by the adaptive rather than the innate response. Studies of the innate immune response of ducks against avian influenza viruses showed that age did not modulate complement, haptoglobin or heterophils:lymphocytes ratio in the blood (van Dijk et al. 2015b). In addition to differences in immune response being driven by exposure history, low immune responses in young individuals may be attributable to developmental constraints that potentially involve trade-offs between immune function and other growth-related, resource-demanding processes. For example, in order to reach a reproductive state quickly, immature individuals are expected to prioritize sexual maturation over immune function compared to adult individuals (Lozano and Lank 2003; Noreen et al. 2011).
Oversummering as an adaptation to higher parasite infection risk in juveniles
To address how age-dependent differential migration strategies may have evolved in relation to higher susceptible to parasite infections in juveniles compared to adults we focus on the most extreme type of migratory allopatry. Oversummering is the phenomenon by which birds choose not to migrate to their breeding grounds and instead remain in the non-breeding grounds (Loftin 1962; McNeil et al. 1994) (Fig. 2). Oversummering is a form of partial migration, though reversed from the usual pattern seen in partial migrants. In partial migrants, part of the population typically remains on the breeding grounds (Tavera et al. 2020) whereas in oversummering, the juvenile component remains on the non-breeding grounds. The phenomenon of oversummering has been reported in at least 15 bird families and, as raised above, is particularly prevalent in shorebirds (Family Charadriidae and Scolopacidae) (McNeil 1970; McNeil et al. 1994). While not exclusively limited to young birds, the propensity to oversummer is particularly high in one-year-old shorebirds in almost all species in which this phenomenon has been documented (Hockey et al. 1998; Johnson 1979; Summers et al. 1995). Notably in the larger shorebird species, oversummering as a juvenile seems to be a near-universal phenomenon. In smaller shorebird species, such as the Calidris sandpipers, recent examples of oversummering rates include a study on Semipalmated Sandpipers (Calidris pusilla) in Peru reporting 28% of yearlings and 19% of adults oversummering (Tavera et al. 2020), and 57% yearlings and 43% adults Red Knots oversummering in Patagonia, Chile (Martinez-Curci et al. 2020).
Oversummering would only be favoured by natural selection if survival of oversummering individuals was raised sufficiently to compensate for the missed breeding opportunity. Indeed, studies have shown higher survival for oversummering individual Semipalmated Sandpipers and Western Sandpipers (Calidris mauri) in Peru (Tavera 2020). That migration may involve a fitness cost was also demonstrated by a meta-analysis in partial migrants finding that in most (but not all) studies, residents had a higher survival than migrants (Buchan et al. 2019). In the case of oversummering, examples do exist wherein those individuals that oversummer have lower survival as compared to those that migrate, such as in Sanderling (Calidris alba) oversummering in tropical West Africa (Reneerkens et al. 2019). It is critical to consider that differences found in survival between those that do and do not oversummer may also involve other inherent differences between individuals, not only their propensity to migrate.
A number of explanations for oversummering in juvenile birds have been proposed. Proximate mechanistic explanations include: sexual immaturity (Eisenmann 1951; Soto-Montoya et al. 2009); less efficient foraging (Puttick 1979); under-developed alternate (breeding) plumage (Johnson and Johnson 1983); flight cost on primary wear (O’Hara 2002); sterility, injuries or illness (Wetmore 1927) and parasite load (McNeil et al. 1994). Ultimate explanations comprise behavioural adaptations to distance-dependant costs (Lank et al. 2003), low chances of successful first breeding (Summers et al. 1995), and the offsetting life history benefit of a higher probability of survival in non-breeding areas (Fernandez et al. 2004). Supporting evidence for McNeil et al’s 1994 parasite load hypothesis as a key driver of oversummering behaviour comes from measurements of trematode-burden in shorebirds spending the non-breeding season in Venezuela, where birds with the highest parasite burden at the end of the non-breeding period show a greater tendency to oversummer (McNeil 1970, as summarized by McNeil et al. 1994). A study of Greater Yellowlegs (Tringa melanoleuca) showed that digenean trematodes increased steadily over the non-breeding season and that there was an inverse relationship between fat loads and parasite loads at the end of the season, potentially delaying or preventing migration (McNeil et al. 1995). Parasite loads were also higher in juvenile compared to adult birds (McNeil et al. 1995). Interestingly, recently arrived adults on the non-breeding grounds were more infested with helminths than recently arrived juveniles, suggesting helminth infection is not a barrier for southward migration in juvenile shorebirds (McNeil et al. 1994, 1995, 1996) (Fig. 1).
Importantly, McNeil et al.’s 1994 parasite load hypothesis is a proximate explanation for oversummering and applicable to both juvenile and adult birds. But there may also be an ultimate infection and disease-risk explanation for oversummering relating to Fernandez et al’s (2004) hypothesis of a higher probability of survival when remaining in the non-breeding area. The latter explanation would notably apply to juveniles. As outlined above, it is well established that juvenile birds tend to have higher parasite prevalence and lower specific immunity to select parasites compared to conspecific adults. Critically, it need not only be the acute parasite burden that may cause juveniles to forego migration, but rather it may be their increased susceptibility and thus increased infection risk, rather than immediate burden that explains offsetting migration. Thus, beyond infection with parasites acting as a proximate reason to defer migration in juvenile and some adult birds, we argue infection risk may also be an ultimate reason for oversummering, as well as the other three migratory allopatry types (Fig. 1), where inherently higher infection risk in juveniles has shaped their ontogenetic migration strategy, i.e., they are hard-wired not to migrate at an early age because they will pay the price in terms of reduced (life-time) fitness if they do.
Where to from here?
To address the role of parasites in shaping migration strategies, it would be beneficial to undertake studies of parasite burden at various stages in the annual cycle in species where partial migration exists amongst juveniles. Indeed, Tavera et al. (2020) found that those juveniles which forewent migration had a higher survival than those that migrated back to the breeding grounds in their first year of life. Yet, the cause of this differential survival and whether it is related to parasite burden remains unknown. Research to address this phenomenon need not be limited to oversummering versus migrating juveniles. Similar studies comparing parasite burden between birds taking different routes (e.g. juvenile Sharp-tailed Sandpipers flying via Alaska and across the Pacific to Australia versus those flying in “the wake” of their parents along the east Asian coast towards their non-breeding destination) could also be undertaken. And there are more examples that we summarised in Fig. 2, where the extent of parasite burden could explain the differential migration strategies, but where we as yet lack the data to confirm or refute that possibility.
There are still many discoveries to be made regarding (differential) migration strategies. Notably with the advent of increasingly smaller, more powerful and affordable tracking techniques, we are progressively in a better position to study and discover differential migration strategies among migrants. Once identified and when attempting to explain their occurrence, including hypotheses relating to the avoidance or minimisation of parasite burden should be widely incorporated.
Overall, parasites can greatly impact the lives of animals, however their role in explaining the proximate and ultimate reasons for the great diversity in migration behaviour is still limited (Pedersen et al. 2007; Shaw et al. 2019). We argue that disease risk may be an important explanation for migration allopatry, particularly for the most extreme case of migration allopatry, i.e. oversummering. While the hypothesis that disease is a driver for oversummering, albeit proximate, is not new, we argue that it may also be an ultimate driver given the known increased disease risk in juveniles compared to adults. Critically, with ongoing climate change, the burden of parasites that may have been responsible for shaping migration behaviours is projected to increase (Kubelka et al. 2021), integrating parasite burden into studies of bird migration is imperative.
References
Alerstam T (1990) Bird Migration. Cambridge University Press, Cambridge, England.
Alerstam T, Hedenstrom A, Akesson S (2003) Long-distance migration: evolution and determinants. Oikos 103:247–260
Altizer S, Bartel R, Han A (2011) Animal migration and infectious disease risk. Science 331:296–302
Altizer S, Davis AK, Cook KC et al (2004a) Age, sex, and season affect the risk of mycoplasmal conjunctivitis in a southeastern house finch population. Can J Zool 82(5):755–763
Altizer S, Hobson KA, Davis AK et al (2015) Do Healthy Monarchs Migrate Farther? Tracking Natal Origins of Parasitized vs. Uninfected Monarch Butterflies Overwintering in Mexico. PLoS ONE, 10: e0141371. 0141310.0141371/journal.pone.0141371
Altizer S, Hochachka WM, Dhondt AA (2004b) Seasonal dynamics of mycoplasmal conjunctivitis in eastern North American house finches. J Anim Ecol 73(2):309–322
Amman BR, Carroll SA, Reed ZD et al (2012) Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog, 8(10): e1002877. doi: 1002810.1001371/journal.ppat.1002877
Avgar T, Street G, Fryxell JM (2013) On the adaptive benefits of mammal migration. Cann J Zool 92:481–490
Bartel RA, Oberhauser KS, de Roode JC et al (2011) Monarch butterfly migration and parasite transmission in eastern North America. Ecology 92(2):342–351
Battley PF, Warnock N, Tibbitts TL et al (2012) Contrasting extreme long-distance migration patterns in Bar-tailed Godwits Limosa lapponica. J Avian Biol 43:21–32
Bauer S, Lisovski S, Hahn S (2015) Timing is crucial for consequences of migratory connectivity. Oikos 125:605–612
Bengtsson D, Safi K, Avril A et al (2016) Does influenza A virus infection affect movement behaviour during stopover in its wild reservoir host? R Soc Open Sci:. doi: https://doi.org/10.1098/rsos.150633
Bergner LM, Orton RJ, Benavides JA et al (2019) Demographic and environmental drivers of metagenomic viral diversity in vampire bats. Mol Ecol 29:26–39
Berthold P (2001) Bird migration: a general survey. Oxford University Press, Oxford, United Kingdom
Binning SA, Shaw AK, Roche DG (2017) Parasites and host performance: Incorporating infection in our understanding of animal movement. Integr Comp Biol 57:267–280
Bradley CA, Altizer S (2005) Parasites hinder monarch butterfly flight: implications for disease spread in migratory hosts. Ecol Lett 8:290–300
Browner LP (1995) Understanding and misunderstanding the migration of the Monarch Butterfly (Nymphalidae) in North America. J Lep Soc 49:304–385
Buchan C, Gilroy JJ, Catry I et al (2019) Fitness consequences of different migratory strategies in partially migratory populations: A multi-taxa meta-analysis. J Anim Ecol 89:678–690
Buehler DM, Tieleman BI, Piersma T (2010) How do migratory species stay healthy over the annual cycle? A conceptual model for immune function and for resistance to disease. Integr Comp Biol 50:346–357
Chapman JW, Reynolds DR, Wilson K (2015) Long-range seasonal migration in insects: mechanisms, evolutionary drivers and ecological consequences. Ecol Lett 18:287–302
Chylinski C, Boag B, Stear MJ et al (2009) Effects of host characteristics and parasite intensity on growth and fecundity of Trichostrongylus retortaeformis infections in rabbits. Parasitology 136(1):117–123
Dhondt AA, States SL, Dhondt KV et al (2012) Understanding the origin of seasonal epidemics of mycoplasmal conjunctivitis. J Anim Ecol 81(5):996–1003
Egevang C, Stenhouse IJ, Phillips RA et al (2010) Tracking of Arctic terns Sterna paradisaea reveals longest animal migration. Proc Natl Acad Sci USA 107(5):2078–2081
Eikenaar C, Hegemann A (2016) Migratory common blackbirds have lower innate immune function during autumn migration than resident conspecifics. Biol Letters, 12(3): e20160078. doi: https://doi.org/10.1098/rsbl.2016.0078
Eisenmann E (1951) Northern birds summering in Panama. Wilson Bull 63:181–185
Fernandez G, O’Hara PD, Lank DB (2004) Tropical and subtropical Western sandpipers (Calidris mauri) differ in life history strategies. Onithol Neotrop 15:385–394
Folstad I, Nilssen AC, Halvorsen O et al (1991) Parasite avoidance: the cause of post-calving migrations in Rangifer? Can J Zool 69:2423–2429
Galsworthy SJ, ten Bosch QA, Hoye BJ et al (2011) Effects of infection-induced migration delays on the epidemiology of avian influenza in wild Mallard populations. PLoS ONE 6(10):e26118. https://doi.org/10.1371/journal.pone.0026118
Gnanadesikan GE, Pearse WD, Shaw AK (2017) Evolution of mammalian migrations for refuge, breeding, and food. Ecol Evol 7:5891–5900
Gschweng M, Kalko E, Querner U et al (2008) All across Africa: highly individual migration routes of Eleonoras’s falcon. Proc Roy Soc B 275:2887–2896
Hahn S, Bauer S, Liechti F (2009) The natural link between Europe and Africa – 2.1 billion birds on migration. Oikos 118:624–626
Hake M, Kjellen N, Alerstam T (2003) Age-dependant migration strategy in Honey Buzzards Pernis apivorus tracked by satellite. Oikos 103:385–396
Handel CM, Gill RE Jr (2010) Wayward youth: trans-Beringian movement and differential southward migration by juvenile Sharp-tailed Sandpipers. Arctic 63:273–288
Harrison JA, Underhill DG, Herremans M et al (1997) The Atlas of Southern African Birds. Volume 1: Non Passerines. BirdLife South Africa, Johannesburg, South Africa
Hasselquist D, Lindstrom A, Jenni-Eiermann S et al (2007) Long flights do not influence immune responses of a long-distance migrant bird: a wind-tunnel experiment. J Exp Biol 210:1123–1131
Hayman DTS (2015) Biannual birth pulses allow filoviruses to persist in bat populations. Proc Royal Soc B, 282(1803): e20142591. doi: https://doi.org/10.1098/rspb.2014.2591
Hegemann A, Marra PP, Tieleman BI (2015) CAuses and consequences of partial migration in a passerine bird. Am Nat 186:531–546
Helin AS, Wille M, Atterby C et al (2018) A rapid and transient innate immune response to avian influenza infection in mallards. Mol Immunol 95:64–72
Hill SC, Manvell RJ, Schulenburg B et al (2016) Antibody responses to avian influenza viruses in wild birds broaden with age. Proc Royal Soc B 283(1845). doi: https://doi.org/10.1098/rspb.2016.2159
Hockey P, Turpie J, Velasquez C et al (1998) What selective pressures have driven the evolution of deferred northward migration by juvenile waders? J Avian Biol 29:325–330
Hoye BJ, Fouchier RAM, Klaassen M (2012) Host behaviour and physiology underpin individual variation in avian influenza virus infection in migratory Bewick’s Swans. Proc Royal Soc B 279(1728):529–534
Jeffries KM, Hinch SG, Gale MK et al (2014) Immune response genes and pathogen presence predict migration survival in wild salmon smolts. Mol Ecol 23:5803–5815
Johnson OW (1979) Biology of shorebirds summering on Enewetak Atoll. Stud Avian Biol 2:193–205
Johnson OW, Johnson PM (1983) Plumage-molt-age relationships in “over-summering” and migratory Lesser Golden Plovers. Condor 85:406–419
Klaassen M, Hoye BJ, Nolet BA et al (2012) Ecophysiology of avian migration in the face of current global hazards. Phil Trans Roy Soc B Sci 367:1719–1732
Klaus C, Gethmann J, Hoffmann B et al (2016) Tick infestation in birds and prevalence of pathogens in ticks collected from different places in Germany. Parasitol Res 115(7):2729–2740
Kopeivnikar J, Leung TLF (2014) Flying with diverse passengers: greater richness of parasitic nematodes in migratory birds. Oikos 124:399–405
Krkošek M, Gottesfeld A, Proctor B et al (2007) Effects of host migration, diversity and aquaculture on sea lice threads to Pacfic salmon. Proc Roy Soc B, 274: 3141–3149. doi: 3110.1098/rspb.2007.1122
Kubelka V, Sandercock BK, Szakely T et al (2021) Animal migration to northen latitutes: envirnemtal changes and increasing threats. Trends Ecol Evol: doi https://doi.org/10.1016/j.tree.2021.1008.1010
Kuiken T (2013) Is low pathogenic avian influenza virus virulent for wild waterbirds? Proc Royal Soc B, 280(1763): 20130990. doi: 20130910.20131098/rspb.20132013.20130990
Lank DB, Butler RW, Ireland J et al (2003) Effects of predation danger on migration stragies of sandpipers. Oikos 103:303–319
Latorre-Margalef N, Tolf C, Grosbois V et al (2014) Long-term variation in influenza A virus prevalence and subtype diversity in a migratory Mallards in Northern Europe. Proc Royal Soc B 281. doi: https://doi.org/10.1098/rspb.2014.0098
Lindstrom A, Gill RE, Jamieson SE et al (2011) A puzzling migratory detour: are fueling conditions in Alaska driving the movement of juvenile Sharp-Tailed Sandpipers? The Condor 113(1):129–139
Lochmiller RL, Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88:87–98
Loehle C (1995) Social barriers to pathogen transmission in wild animal populations. Ecology 76:326–335
Loftin H (1962) A study of boreal shorebirds summering on Apalachee Bay, Florida. Bird Band 33:21–42
Lozano GA, Lank DB (2003) Seasonal trade-offs in cell-mediated immunosenescence in ruffs (Philomachus pugnax). Proc Royl Soc B 270(1520):1203–1208
Martinez-Curci N, Isacch JP, D’Amico V et al (2020) To migrate or not: drivers of over-summering in a long distance migratory shorebird. J Avian Biol: e02401. doi:https://doi.org/10.1111/jav.02401
McDonald JL, Robertson A, Silk MJ (2018) Wildlife disease ecology from the individual to the population: Insights from a long-term study of a naturally infected European badger population. J Anim Ecol 87(1):101–112
McKinnon EA, Fraser KC, Stanley CQ et al (2014) Tracking from the tropics reveals behaviour of juvenile songbirds on their first spring migration. PLoS ONE, 9(8): e105605. doi: 105610.101371/journal.pone.0105605
McKinnon L, Smithe PA, Nol E et al (2010) Lower predation risk for migratory birds at high latitudes. Science 327:326–327
McNeil R (1970) Hivernage et estivage d’oiseaux aquatiques nord-americains dans le nord-est du Venezuela (mue, accumulation de graisse, capacite de vol et routes de migration). (In French). Oiseau et R.F.O., 40: 185–302
McNeil R, Diaz MT, Casanova B et al (1995) Trematode parasitism as a possible factor in oversummering of Greater Yellowlegs (Tringa melanoleuca). Ornitologia neotropical, p 6
McNeil R, Diaz MT, Casanova B et al (1996) Trematode infestation as a factor in shorebird oversummering: a case study of the Greater Yellowlegs (Tringa melanoleuca). Bull Scand Soc Parasitol 6:114–117
McNeil R, Diaz MT, Villeneuve A (1994) The Mystery of shorebird over-summering: a new hypothesis. Ardea 82:143–152
Mellon U, Yanez B, Liminana B et al (2011) Summer stating areas of non-breeding Short-toed Snake Eagles Curcaetus gallicus. Bird Study 58:516–521
Meltofte H, Berg TB (2004) Post-breeding phenology of waders in central NE Greenland. Wader Study Group Bulletin 104:22–27
Moller AP, Szep T (2010) The role of parasites in ecology and evolution of migration and migratory connectivity. J Ornith 152:141–150
Noreen E, Bourgeon S, Bech C (2011) Growing old with the immune system: a study of immunosenescence in the zebra finch (Taeniopygia guttata). J Comp Physiol 181(5):649–656
O’Hara PD (2002) The role of feather wear in alternative life history strategies of a long-distance migratory shorebird, the Western Sandpiper. (PhD Thesis), Simon Fraser University, Vancouver, Canada
Osterlof S (1977) Migration, wintering areas, and site tenacity of the European Osprey Pandion h. haliaetus (L.). Ornis Scand 8:61–78
Pedersen AB, Jones KE, Nunn CL et al (2007) Infectious diseases and extinction risk in wild mammals. Conserv Biol 21:1269–1279
Piersma T (1997) Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos 80:623–631
Poulin R, de Dutra A, D (2021) Animal migrations and parasitism: reciprocal effects within a unified framework. Biolog Rev 96:1331–1348
Puttick GM (1979) Foraging behaviour and activity budgets of curlew sandpipers. Ardea 67:111–122
Ramos R, Garnier R, Gonzalez-Solis J et al (2014) Long antibody persistence and transgenerational transfer of immunity in a long-lived vertebrate. Am Nat 184(6):764–776
Read AF, Graham AL, Raberg L (2008) Animal defenses against infectious agents: is damage control more important than pathogen control. PLoS Biol 6(12):e1000004. https://doi.org/10.1371/journal.pbio.1000004
Reneerkens J, Versluijs TSL, Piersma T et al (2019) Low fitness at low latitudes: Wintering in the tropics incresases migratory delays and mortality rates in an Arctic breeding shorebird. J Anim Ecol 89:691–703
Risely A, Klaassen M, Hoye BJ (2018) Migratory animals feel the cost of getting sick: A meta-analysis across species. J Anim Ecol 87(1):301–314
Schmid Hempel P (2011) Evolutionary parasitology. the integrated study of infections, immunology, ecology, and genetics. Oxford University Press, Oxford. United Kingdom
Shaw AE, Binning SA (2016) Migratory recovery from infection as a selective pressure for the evolution of migration. Am Nat 187:491–501
Shaw AE, Craft ME, Zuk M et al (2019) Host migration strategy is shaped by forms of parasite transmission and infection cost. J Anim Ecol 88:1601–1612
Soto-Montoya E, Carmona R, Gomez M et al (2009) Oversummering and migrant red knots at Golfo de Santa Clara, Gulf of California, Mexico. Wader Study Group Bulletin 116:191–194
Stone G, Florez-Gonzalez L, Katona SK (1990) Whale migration record. Nature 346:705
Strathmann RR, Hughes TP, Kuris AM et al (2002) Evolution of local recruitment and its consequences for marine populations. Bull Mar Sci 70:377–396
Summers RW, Underhill LG, Prys-Jones RP (1995) Why do young waders in southern Africa delay their first return migration to the breeding grounds? Ardea, 83: 351–357
Tavera EA (2020) Survivorship and Life History Strategies in Relation to Migration Distance in Western and Semipalmated Sandpipers in Peru. (PhD Thesis), Simon Fraser University, Vancouver, Canada
Tavera EA, Stauffer GE, Lank DB et al (2020) Oversummering juvenile and adult Semipalmated sandpipers in Peru gain enough survival to compensate for foregone breeding opportunity. Mov Ecol 8:42
Teitelbaum CS, Huang S, Hall RJ et al (2018) Migratory behaviour predicts greater parasite diversity in ungulates. Proc Roy Soc B 285(1875). doi: https://doi.org/10.1098/rspb.2018.0089
Tolf C, Latorre-Margalef N, Wille M et al (2013) Individual variation in influenza A virus infection histories and long-term immune responses in mallards. PLoS ONE, 8(4): e61201. doi: 61210.61371/journal.pone.0061201
van Dijk J, Kleyheeg E, Soons M et al (2015a) Weak negative associations between avian influenza virus infection and movement behaviour in a key host species, the mallard Anas platyrhynchos. Oikos, 10: 1293–1303. doi:1210.1111/oik.01836
van Dijk JGB, Fouchier RAM, Klaassen M et al (2015b) Minor differences in body condition and immune status between avian influenza virus-infected and noninfected mallards: a sign of coevolution? Ecol Evol 5(2):436–449
van Dijk JGB, Hoye BJ, Verhagen JH et al (2014) Juveniles and migrants as drivers for seasonal epizootics of avian influenza virus. J Anim Ecol 83(1):266–275
Wetmore A (1927) Our migrant shorebirds in South America. USDA Technical Bulletin No. 26
Wille M, Shi M, Hurt AC et al (2021) RNA virome abundance and diversity is asociated with host age in a bird species. Virology: doi: https://doi.org/10.1016/j.virol.2021.1006.1007
Acknowledgements
We would like to acknowledge O.C. Wille for assistance with reviewing the literature on winter raptor distribution in southern Africa, G. Méric for translating McNeil et al. 1970 from French into English, D. Rogers for stimulating discussions on the topic of oversummering in shorebirds and two anonymous reviewers for their constructive feedback on an earlier version of this manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
MW is funded by an ARC Discovery Early Career Researcher Award (DE200100977). Work in MK’s lab is funded by an ARC Discovery Project (DP190101861).
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wille, M., Klaassen, M. Should I stay, should I go, or something in between? The potential for parasite-mediated and age-related differential migration strategies. Evol Ecol 37, 189–202 (2023). https://doi.org/10.1007/s10682-022-10190-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-022-10190-9