Abstract
Ecological conditions may greatly affect the relative importance of vertical and horizontal transmission, in particular for parasites with a mixed mode of transmission. Resource availability is one important environmental factor, affecting host growth and fecundity, but also the parasite’s own development. The consequences for the potential of vertical and horizontal transmission and for the evolution of transmission mode are largely unknown. We let the mixed-mode microsporidian parasite Edhazardia aedis evolve on its mosquito host Aedes aegypti under high-food or low-food conditions, representing permissive and restricted conditions. These alter the timing of development of infected larvae and thereby the probabilities for the parasites to enter the vertical or horizontal transmission pathways. After 10 generations, evolved parasites were assayed under the two food levels. There was an ecological trade-off between transmission modes, mediated by nutrient effects on host development, resulting in a higher vertical transmission (VT) potential under high-food and a higher horizontal transmission (HT) potential under low-food test conditions. Evolution under high food increased the VT potential of the parasite, particularly if it was tested at low food. This involved higher probability of carrying binucleate spores for the emerging females, greater fecundity and a longer life compared to parasites that were tested in the same conditions but had evolved under low food. The changes are related to the developmental regulation and switch in the production of two spore types, affecting investment in VT or HT. In contrast, the HT potential remained relatively unaffected by the parasite’s evolutionary history, suggesting that, within our experiential design, the VT mode evolved independently of the HT mode. Our work illustrates the possible links between resource availability, within-host developmental processes and the evolution of parasite transmission investment. Future work, theoretical and experimental, should scale up from within-host to between-host levels, including eco-evolutionary and epidemiological dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transmission is a key step in the parasite life cycle. It determines the spread and maintenance of infection in the host population, and therefore represents a main component of the parasite’s fitness (Schmid-Hempel 2021). Vertical and horizontal transmission are two distinct ways of accomplishing the passage from one host to another, with very different epidemiological and evolutionary implications (Sachs et al. 2011a, b; Ebert 2013). The success of vertical transfer of infection from parents to their offspring (e.g., via seeds or eggs) depends on the reproductive output of the infected host. This alignment of host and parasite fitness should therefore favour low levels of virulence (Frank 1996). In contrast, horizontal infectious spread (e.g., via specialised transmission particles) is not limited to individuals that are in a parent–offspring relationship, and potentially reaches any susceptible host in the population. Not necessarily relying on host reproduction, horizontal transmission can therefore be associated with considerable levels of virulence (Frank 1996). While these general considerations are straightforward for the two modes in isolation, we still know little about the evolutionary ecology of parasites that combine horizontal and vertical transmission.
Various types of parasites adopt such a mixed-mode transmission strategy, including phages (Weitz et al. 2019), medically relevant viruses (HIV, hepatitis B and C viruses, Zika, Dengue, Chikungunya) and bacteria (Borrelia miyamotoi, Rickettsia rickettsia) (Burgdorfer and Varma 1967; Narita et al. 1991; Ebert 2013; Contopoulos-Ioannidis et al. 2018; da Costa et al. 2018; Lai et al. 2020; Sambado et al. 2020; Teixeira et al. 2021). If there is no interaction between the two modes of transmission, having both should be better than having one (Lipsitch et al. 1995; Ferdy and Godelle 2005). However, due to physiological or developmental constraints (Ebert and Mangin 1997; Messenger et al. 1999; van den Bosch et al. 2010), investing in one transmission mode often reduces transmission with the other. Thus, the two transmission pathways cannot be optimized independently of each other and evolution will favour combinations that maximize the overall transmission success.
The evolutionary adjustment of mixed-mode strategies likely depends on environmental factors and ecological conditions (e.g., temperature, resource availability, spatial structure, host density, co-infection and competition) because these can alter the opportunities for horizontal and vertical transmission (Antonovics et al. 2017; Su et al. 2019). These environmental factors set the stage for the evolution of vertical versus horizontal transmission, and consequently, the evolution of trade-offs or context-dependent shifts in transmission (Kaltz and Koella 2003). Accordingly, theory has introduced the ecological context by comparing predictions for different contact rates (opportunities for horizontal transmission) or host birth rates (for vertical transmission) (Lipsitch et al. 1995, 1996). Further, a number of experimental evolution studies blocked one or the other transmission pathway in several host-parasite systems, and observed subsequent increases or decreases in virulence or transmission capacity, in line with theoretical predictions. Promoting vertical transmission resulted in lower virulence and shifts from parasitism to commensalism (Bull et al. 1991; Sachs and Wilcox 2006), whereas promoting horizontal transmission induced shifts towards parasitism and higher virulence (Sachs and Wilcox 2006; Le Clec’h et al. 2017).
So far only few studies have employed more realistic ecological scenarios, without a priori enforcing one or the other transmission mode. Using experimental microcosms of an aquatic model system, Magalon et al (2010) mimicked conditions of frequent host population expansion, where periods of high host birth rate provide ample opportunity for vertical transmission. This resulted in an evolutionary shift towards increased investment in vertical transmission of the parasite (Magalon et al. 2010), and eventually led to the near-complete loss of the horizontal pathway (Dusi et al. 2015). In a bacteria-phage system, population mixing increased opportunities for horizontal transmission, and led to more virulent, predominantly horizontally transmitting variants (Berngruber et al. 2015). Yet, experimentally increasing the density of susceptible Escherichia coli cells for 500 generations did not lead to the predicted evolution of higher horizontal transmissibility of a “parasitic” plasmid (Turner et al. 1998). These results suggest that predictions about the evolution of transmission pathways may be complex, especially when eco-epidemiological and evolutionary processes and feedbacks are allowed to act freely.
Here, we focus on the role of resource availability for the evolution of mixed-mode parasites. This environmental component has received surprisingly little attention in this context, even though it has strong effects on host-parasite interactions (Mojica and Brussaard 2014). Resource availability affects the growth of the parasite within its host, the host’s immune response (reviewed in Pike et al. 2019) and the virulence of infections and transmission investment (Bedhomme et al. 2004; Restif and Kaltz 2006; Cornet et al. 2014; Cressler et al. 2014). Nutrient availability can also drive indirect demographic feedbacks, via population density, regulating the extent of horizontal and vertical transmission (Mojica and Brussaard 2014; Jover et al. 2014).
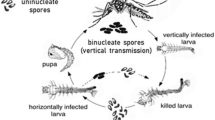
The microsporidian Edhazardia aedis is an obligate parasite of the mosquito Aedes aegypti. It has a relatively complex, developmentally programmed mixed-mode strategy, with horizontal and vertical transmission occurring at different stages of the host life cycle. Edhazardia aedis is transmitted horizontally among mosquito larvae or vertically through the eggs of infected females (Becnel et al. 1989). During its development within a host, the parasite has a fixed program alternating the production of two types of spores associated with vertical or horizontal transmission (Fig. 1a). Shortly after horizontal infection of a larvae via ingestion of uninucleate spores, binucleate spores that spread from the gut into different parts of the body are produced at relatively low density. If the infected larva develops fast enough, this stage of infection allows the transition from the juvenile to the adult stage. In females, infection of gonads by binucleate spores then gives rise to vertically infected eggs and subsequently to infected larvae, which again produce uninucleate spores that are released for horizontal transmission upon larval death (Fig. 1a). Another horizontal transmission pathway is possible when larval development is slow, giving the parasite the time to go through its developmental sequence from uninucleate to binucleate to again uninucleate spores within the same larval individual, bypassing vertical transmission (Fig. 1a). This “shortcut” horizontal pathway may be frequent in natural habitats, typically characterised by low resources (Christophers 1960), whereas the above alternating horizontal-vertical pathway requires more resource-rich conditions, allowing fast enough larval development.

(modified from Koella and Agnew 1999). The parasite has a fixed developmental program alternating the production of two spore types. The production of a new batch of uninucleate spores after the infection of a juvenile requires first the production of binucleate spores. “Shortcut” horizontal transmission occurs when juveniles (larvae or pupae) die in the aquatic environment; vertical transmission occurs via infected females laying infected eggs. Once the new generation of vertically infected larvae emerges, they either die and new events of horizontal transmission occur, or they emerge to become adults with the potential for vertical transmission. b Protocol of the experimental evolution part of the study. At each generation, uninfected larvae from the stock colony were horizontally infected with uninucleate spores obtained by hatching eggs and harvesting the vertically infected larvae from the previous generation. Infected larvae were raised under permissive high-food conditions (red, Evo HF) or restricted low-food conditions (blue, Evo LF), which resulted in fast or slow development into adults and thereby changing the match with parasite development. The rearing of the vertically infected larvae and the infection of the next generation followed a standardized protocol (main text)
Model system and experimental protocol. a Life cycle of the microsporidian parasite Edhazardia aedis and of its host Aedes aegypti
The fixed developmental program of the parasite may represent a strategy to deal with unpredictable and ephemeral ecological conditions which are likely characterised by ample variation in the resources available for the mosquito. This kind of parasite development ensures some level of vertical transmission under good conditions, when host fecundity is high, and avoids the risk of relying on vertical transmission from hosts with low fecundity under poor conditions. Indeed, previous work demonstrated that investment in either vertical or "shortcut" horizontal transmission can be modulated by the experimental manipulation of resource condition, via the modification of rates of host development (Agnew and Koella 1999; Zilio et al. 2018; Zilio and Koella 2020). While these experiments typically span a single infected host generation, the present study explored the scope for evolutionary modification of the parasite's strategy set when facing the same resource conditions over multiple generations.
Using a controlled serial infection protocol, we passaged the parasite over several mosquito generations in treatments with either high or low resource levels, while precluding evolution of the host. Resource levels were manipulated in the aquatic environment in which infected larvae developed. The focus of our experiment was on resource-induced changes in one particular segment of the infection life cycle: juvenile development and the transition to the adult stage (Fig. 1b). The goal was to assess whether these predictable changes in the rate of the host’s development produced evolutionary responses in the parasite's vertical and horizontal transmission potential. In particular, high food levels lead to faster development of infected larvae, a higher chance to reach the adult stage and higher adult fecundity (Zilio et al. 2018), thereby potentially increasing the prospects of vertical transmission. Hence, our main prediction was that if the parasite evolves in an environment with ample resources, it can predict effective vertical transmission; it should therefore adjust the developmental switch from uni- to binucleate spores that occurs within the first days of infection. This evolution would be visible as an increase of vertical transmission in particular when the parasite is tested in an environment with low food. In contrast, in the low-food evolutionary treatment, larval development is slow, so the parasite's developmental switch may become delayed. Consequently, we expected evolution at low food to lead to less vertical transmission potential when the parasite is tested at high food.
After 10 host generations, we assayed evolved parasites under high-food and low-food test conditions, by measuring several components of vertical and horizontal transmission (Fig. 2). This allowed us to test the above predictions about the evolution of the vertical pathway, but also to analyse possible correlated responses to selection in the horizontal pathway, which was a priori not under direct selection in our experiment.
Transmission components of the parasite. a For the horizontal transmission we measured four transmission proxies: The number of juveniles not emerging, the probability of these individuals carrying uninucleate spores, the uninucleate spore load and the juvenile survival. b For vertical transmission we measured five transmission proxies: The number of females emerging, the probability of these females carrying binucleate spores, binucleate spore load, wing size (fecundity) and longevity
Materials and methods
Experimental system
The yellow fever mosquito Aedes aegypti (obtained from Patrick Guérin, University of Neuchâtel) is found throughout the tropics and subtropics. It is an important vector of several human diseases and its genetics, physiology, and ecology of A. aegypti are well known (Christophers 1960; Nene et al. 2007). The larvae grow in natural or artificial containers and during their development are often challenged by periods of nutrient restriction and competition. The eggs can be stored for some months at standard lab conditions (Munstermann 1997).
The microsporidian parasite Edhazardia aedis (provided by J. J. Becnel, USDA, Gainesville, USA) can complete its development only in A. aegypti. It has a complex infection life cycle (Fig. 1a), with mixed-mode transmission (Desjardins et al. 2015; Grigsby et al. 2020). It produces uninucleate spores for horizontal transmission and binucleate spores for vertical transmission; these spores are morphologically distinguishable (see Fig. 1a). Mosquito larvae ingest uninucleate spores with their food, resulting in the infection of the gut and epithelial cells. Uninucleate cells eventually give rise to binucleate cells, which subsequently spread to other tissues. Binucleate spores invading the oocytes of a female are responsible for vertical transovarial transmission. Male adults represent a transmission dead-end. On average, 95% of the eggs laid by an infected female result in infected larvae (Becnel et al. 1995). Once the eggs hatch, a new generation of uninucleate spores is produced in the growing larvae. Most of these larvae die before emergence, releasing uninucleate spores in the aquatic environment and starting a new round of horizontal transmission, when ingested by uninfected larvae (the spores remain infective for about 48 h outside the host (Nasci et al. 1992)). Horizontal transmission is also possible when, within a larva, the parasite first produces binucleate spores (initiated c. 4 days post infection) and then uninucleate spores, which kill the host. This “shortcut” horizontal transmission, bypassing the vertical transmission, typically occurs under restricted ecological conditions with low food availability. Low food slows down host development and delays emergence into adults (Agnew and Koella 1999; Zilio et al. 2018). The parasite is then more likely to produce new uninucleate spores. In contrast, permissive conditions, which let the larva develop rapidly, favour host emergence and the parasite life cycle here matches with the one of the host; the parasite produces binucleate spores and the females produces eggs. Thus, whether a new infection kills the juveniles with the release of uninucleate spores or whether the infection proceeds to binucleate spore production depends strongly on the ecological conditions (Agnew and Koella 1999; Duncan et al. 2015; Zilio et al. 2018), but the host genetic background plays also a role (Zilio et al. 2018). We here assay whether, in addition, the parasite can adapt the developmental switch from uninucleate to binucleate spores according to the host’s environment.
Experimental design
All experiments were run in a climate chamber set to 26 °C, 70% humidity and a photoperiod of 12 h light and 12 h dark.
Experimental evolution
We let E. aedis evolve for 10 generations (ca. 1 year, see Fig. 1b) under high-food or low-food conditions. Each food treatment was replicated in five independent cage populations. We started each generation by synchronously (i.e., at reduced air pressure) hatching eggs from our baseline mosquito colony and haphazardly assigning 200 larvae to each selection line. The larvae of each line were reared for the first four days in groups of 50 individuals in four petri dishes (8 cm diameter) containing 30 mL of deionized water. In the high-food treatment, they were fed daily with a standard amount of TetraMin baby™ fish food (age 0 (i.e., day of hatching): 0.06 mg/larva, age 1: 0.08 mg, age 2: 0.16 mg, age 3: 0.32 mg, age 4: 0.64 mg, from age 5 onwards: 0.32 mg); in the low-food treatment, they received half of the high-food diet. Seventy-two hours after hatching, we added 2 × 105 uninucleate spores (in 1 mL water) to each petri dish. This spore dose produces nearly 100% infection without causing high juvenile mortality. The spores had been harvested by crushing and homogenizing 7-day old, vertically infected larvae that had hatched 7 days earlier than the day of infection in an Eppendorf tube with 1 mL of deionized water. The concentration of the spores was determined with a hemocytometer and a phase-contrast microscope (Zeiss Axio Lab.A1). For the first generation, we used spores from our standard batch of parasites; for later generations we used the spores obtained in the previous generation for each line.
Twenty-four hours after exposure to the parasite, all larvae from a given line were transferred to a 200 × 150 × 50 mm plastic tray containing 1 L of deionized water; each tray thus represented a different line of parasite. The pupae were moved to 30 × 30 × 30 cm size cages containing a 10% sugar solution for food and an oviposition cup with a filter paper. No sexual transmission is known, but to avoid any potential bias we removed all infected males with an aspirator just after emergence (males emerge earlier than females) and replaced them with uninfected males from the colony. Thus, infected females only mated with uninfected males. The mosquitoes were blood fed for 10 min on GZ’s arms five and seven days after the day when 75% of the individuals of a given line had pupated. The filter papers with newly laid (vertically infected) eggs were removed every 2 days and stored in a petri dish in a climate chamber until the start of a new parasite generation.
Final test: parasite assay conditions
Using parasites from the last batch of vertically infected eggs obtained at generation 10, we tested each parasite line under high-food and low-food conditions on naive mosquitoes. During the experimental evolution we lost one low-food line (all host females died before laying eggs), leaving 5 high-food selection lines and 4 low-food lines for the test. No uninfected control was included in the final test. This parasite is very virulent and a control would have not added much information; independently of the food treatment the uninfected mosquitoes would have differed strongly in any measure of reproductive success. For example, in another study in which juvenile conditions (without parasite) were the same as in our experiment (Zeller and Koella 2016), uninfected adult females lived, on average, 60 days and more than 80% of 185 females were still alive at day 23 (no difference between HF and LF). In contrast, in this study only 13 out of 300 (4%) of the infected females with Edhazardia aedis were still alive 23 days after emergence.
The final test was split into two blocks starting three weeks apart. Eggs from the naive baseline colony were simultaneously hatched and 2627 deliberately picked larvae (1299 in block 1; 1328 in block 2) were individually reared in 12-well tissue-culture plates filled with 3 mL of deionized water. In each block half of the larvae were assigned to a high-food diet, and the other half to a low-food diet. Forty-eight hours after hatching, each larva received 400 uninucleate spores (in 100 µL water) from one of the 9 parasite lines (from 140 to 154 individuals per line and food test environment). The spores had been obtained by hatching the vertically infected larvae of the evolved parasite lines seven days before the day of infection, as described above. We moved the pupae into 50-ml tubes and provided the emerging adults with cotton-wool soaked with 10% sugar solution, which we replaced every 6 days. We checked the mosquitoes (larvae, pupae and adults) daily, transferred dead mosquitoes into 2 mL Eppendorf tubes and stored them at − 20 °C until further investigation. Female wings were removed and mounted on microscope slides, digitally scanned and measured with the software IMAGEJ. We stopped the tests 23 days after hatching and the remaining individuals were frozen at − 20 °C.
We counted the spores and determined the type of spores in each individual by adding 0.1 mL of deionized water, homogenizing the samples with a TissueLyser LT-QIAGEN, sampling 4 µL of the obtained solution with a hemocytometer and assaying the solution with a phase contrast microscope (Zeiss Axio Lab.A1). The treatment status of the samples was unknown to the observer (GZ) during the counting. We considered and measured 5 traits as components of the vertical transmission potential: The number of females emerging, the probability of these females carrying binucleate spores, binucleate spore load, wing length as proxy for fecundity (Koella and Lyimo 1996; Leisnham and Juliano 2010; Farjana and Tuno 2012) and longevity. For the horizontal transmission we measured 4 traits: The number of juveniles not emerging and dying in the aquatic environment, the probability of these individuals carrying uninucleate spores, the uninucleate spore load and the juvenile survival.
Statistical analysis
We first analysed variation in each individual transmission component (5 vertical and 4 horizontal components; Fig. 2). For these analyses, we used generalized linear models with appropriate error structure (binomial or poisson) and corresponding link functions (logit or log). For analysis of female longevity, we employed parametric survival analysis (Weibull function, right censoring). For the age at death of juveniles, we used standard ANOVA, with least-square fitting. All models included the effects of evolution treatment, test food conditions and parasite selection lines (nested within evolution treatment), and their possible interactions. We further included experimental block in the final test. Analogous to sums of squares, deviances (2 × log-likelihood ratio) were used to perform Quasi-F tests (Schmid and Dolt 1994).
Second, we used multivariate techniques to summarise the individual transmission components into a combined measure, referred to as vertical or horizontal “transmission potential”. To this end, we averaged each transmission component per parasite selection line and test food treatment (9 lines × 2 food levels = 18 means) and performed MANOVAs of vertical and horizontal transmission potential, with evolution treatment and test food treatment as factors. We further performed Principal Component Analyses (PCA) to visualise the differences between treatments in multi-trait space. The values obtained for the first PC axis were then taken as estimates of the vertical and horizontal transmission potential, respectively; in an additional MANOVA we used these vertical and horizontal PC1 values to test whether the relationship between vertical and horizontal transmission potential differed between evolution and test food treatments. For example, a significant evolution treatment effect could indicate an evolutionary trade-off between the two transmission modes. All analyses were conducted with the JMP 14 statistical package (SAS Institute Inc. 2018).
Results
In the final test, 1957 out of 2627 individuals (74%) died as juveniles, with mortality being higher under low-food than high-food test conditions (87 vs. 61%). Of the remaining 670 individuals emerging to adulthood 300 were females (44% of the emerging adults), 243 reared under high food, and 57 under low food. A summary of the results from univariate analyses can be found in Tables 1 and 2.
Vertical transmission components
High-food test conditions resulted in increased values of the vertical transmission components (significant Test food effect, Table 1). Compared to low-food conditions, there was a four-fold increase in the number of emerging females (Fig. 3a), an up to twofold increase in the fraction of these females carrying binuclear spores for vertical transmission (Fig. 3b), and a doubling of the binucleate spore load (Fig. 3c). Furthermore, high-food conditions produced a c. 10% increase in wing size of females with binucleate spores, which corresponds to an increase in their fecundity, i.e., the number of (potentially) vertically infected eggs.
Means of the a–e vertical and f–i horizontal parasite transmission components. a The number of adul females emerging, b the probability of the females carrying binucleate spores, c the binucleate spore load (spores/0.9μL), d the wing size (in cm; proxy for fecundity and number of eggs laid) and e the longevity (in days). f The number of juveniles not emerging and dying in the water, g the probability of the dead juveniles carrying uninucleate spores, h the uninucleate spore load of the juveniles (spores/0.9μL) and i their survival (in days). Big symbols and bars are the overall mean and standard errors, small symbols are the mean for each parasite selection line. Red and blue symbols represent evolution in permissive (Evo HF) and restricted ecological (Evo LF) conditions respectively. Full and empty symbols are the conditions of the final test, corresponding to high (Test HF) and low (Test LF) food levels
Evolution treatments explained some of the observed variation in vertical transmission components, through statistically significant interactions with test environment (Test food × Evo treat interaction; Table 1). Specifically, high-food parasite selection lines tended to enhance vertical transmission under low-food conditions. Thus, females infected with high-food parasites tended to be more likely to carry vertical binucleate spores (Fig. 3b, although Test food × Evo treat interaction non-significant), and allowed these females to become larger and live longer than did low-food parasites (Fig. 3d, e). In contrast, high- and low-food parasites showed very similar effects under high-food test conditions (except for longevity; Fig. 3e).
Horizontal transmission components
Low-food test conditions resulted in increased values of three of the four horizontal transmission components (significant Test food effect, Table 2). Compared to high food conditions, there was a 40% increase in the number of juveniles that failed to emerge and died at the aquatic stage, where horizontal transmission occurs (Fig. 3f), a three times larger fraction of these individuals carried uninucleate, horizontally transmitted spores (Fig. 3g), and uninucleate spore loads were about three times higher (Fig. 3h).
Evolutionary treatments explained relatively little of the observed variation in the horizontal transmission components. If anything, under high-food test conditions, some high-food selection lines prevented more larvae to reach the adult stage (Fig. 3f), increased uninucleate spore loads (Fig. 3h) and kept the larvae alive for longer (Fig. 3i), compared to low-food parasites. However, none of these individual trends were statistically significant (Test food × Evo treat interactions; Table 2).
Multivariate analysis: vertical and horizontal transmission potential
Principle Component Analysis (PCA; Figs. 4 and 5) showed that all components contributed roughly equally and in a concerted fashion to the description of the explained variance (similar length and direction of arrows in Fig. 4; see also Table S1–4 for PC axes loadings). For both horizontal and vertical transmission, there was a near-complete separation of points defined by the test food conditions (open vs. closed symbols; Fig. 4). Accordingly, the MANOVA revealed a highly significant Test food effect (Table 3). Thus, using the PC1 values as an index of “transmission potential”, we found that low-food test conditions strongly increased horizontal transmission potential (Fig. 4b), whereas high-food test conditions strongly increased vertical transmission potential (Fig. 4a).
PCA for the a vertical and b horizontal transmission components. The first two principal component axes (PC1 and PC2) are shown. Each arrow represents a single trait/component used for the PCA. The length and the direction of the arrow indicate which vertical and horizontal transmission traits/components are driving the separation between treatments. Each point represents the average value of a given parasite line in multivariate space: Evolved in permissive (Evo HF, red) or restricted (Evo LF, blue) ecological conditions; tested in the high (Test HF, full circles and solid lines) or low (Test LF, empty circles and dashed lines) food. The ellipses are the 95% containment probability region for the four treatments
Reduction of the several traits measured for the horizontal and vertical transmission components to one dimension (PC1) after PCA and multivariate analysis. Synthetic measurement of a parasite vertical and b horizontal transmission potential. For visualization, PC1 vertical transmission values were multiplied by − 1, in order to have positive values corresponding to higher transmission. c Relationship between the synthetic measurement of horizontal and vertical transmission. Red and blue symbols represent evolution in permissive (Evo HF) and restricted (Evo LF) ecological conditions respectively. Full and empty symbols are the conditions of the final test, corresponding to high (Test HF) and low (Test LF) food levels. Big symbols and bars are the overall mean and standard errors, small symbols are the mean for each parasite selection line
For vertical transmission, visual inspection of the PCA (Fig. 4a) showed that high-food parasites (red symbols) were grouped in-between low-food parasites (blue symbols). This is supported by the MANOVA, revealing a significant Evo treat x Test food interaction (Table 3). Thus, while there was considerable overlap under high-food test conditions, the vertical transmission potential of high-food parasites exceeded that of low-food parasites when tested under low-food conditions (Fig. 5a).
For horizontal transmission, the PCA did not show a clear-cut grouping of high-food and low-food parasite selection lines (red vs. blue symbols; Fig. 4b), although the MANOVA yielded a trend for overall evolutionary treatment effect (Table 3). This indicates a higher horizontal transmission potential of high-food parasites, despite considerable scatter produced by the individual selection lines (Fig. 5b).
A second MANOVA analysed the relationship between horizontal and vertical transmission potentials. A significant Test food effect (Table 4) indicated an environmental trade-off between transmission potentials: low-food conditions increased the horizontal transmission potential and reduced the vertical transmission potential, while conversely high-food conditions increased the vertical transmission potential and decreased the horizontal transmission potential (Fig. 5c). This negative relationship held for both high-food and low-food parasites. In contrast, the evolutionary relationship varied with the test conditions (marginally significant Evo treat x Test food interaction; Table 4): Under low-food test conditions, the sign of the relationship tends to be positive, with high-food parasites having both higher horizontal and vertical transmission potential than low-food parasites. Under high-food test conditions, the sign tended to be negative, with high-food parasites having a higher horizontal potential and a lower vertical potential than low-food parasites (Fig. 5c).
Discussion
Environmental factors and ecological conditions can set the stage for the evolution of parasite transmission modes (Antonovics et al. 2017; Drew et al. 2021). We allowed the microsporidian parasite Edharzardia aedis to evolve in its mosquito host Aedes aegypti reared at high or low food conditions. Resource availability alters the development of infected larvae and thereby the probabilities of entering the vertical or horizontal transmission pathways. After passaging the parasite for 10 host generations, we detected signatures of evolutionary responses to the contrasting environments. This namely concerned the vertical transmission potential, while the horizontal component remained relatively unaffected. Some of these changes are related to the developmental regulation of the production of two spore types, affecting investment in vertical or horizontal transmission. One of the main results was the corroboration of our main prediction: When the parasite evolved in an environment enabling rapid development, it should adjust the developmental switch from uni- to binucleate spores within the first days of infection. As expected, this was visible as an increase of vertical transmission, in particular when the parasite was tested in an environment with low food.
Evolution of vertical transmission (VT) potential
When food is abundant, juvenile mosquitoes have higher survival and reach the adult stage ca. 2 days earlier than under low-food conditions (Zilio et al. 2018). This should provide ample opportunity for vertical transmission, as infected hosts rapidly leave the aquatic environment, have higher fecundity and lay eggs with a high probability of being vertically infected (Becnel et al. 1995). Corroborating previous results (Zilio et al. 2018; Zilio and Koella 2020), we show here that infected individuals raised under high-food conditions had a higher VT potential compared to those raised under low food. We therefore expected selection on the parasite to match the fast host development and facilitate vertical transmission. Indeed, parasites evolved in permissive conditions showed generally high VT potential (Fig. 5a). Even when tested at low food, these parasites maintained intermediate trait values (open-red symbols, Figs. 4a and 5a), higher than those of parasites evolved and tested in low-food conditions (open-blue symbols) and more similar to values observed under high-food test conditions (filled symbols). This mainly involved a higher probability of carrying binucleate spores for the emerging females (Fig. 3b), higher fecundity (Fig. 3d), and increased longevity (Fig. 3e).
Developmental mechanisms and within-host dynamics, such as changes in the fidelity of VT or delayed switch from VT to HT type of cells (Magalon et al. 2010), may explain how the high-food parasites achieved better VT potential. During host development, E. aedis moves through several tissues relying on specific protein modification and trafficking to finally arrive in the ovaries, where binucleate spores accomplish the infection of the eggs for vertical transmission (Desjardins et al. 2015). During evolution, these different steps might be fine-tuned and optimised, thereby increasing the VT potential. Inspection of the individual VT components suggests indeed an adjustment of the developmental switch from uni- to binucleate spores, as we observed here for the high food evolution treatment under low food test (Fig. 3b). Higher adult female fecundity and longevity (Fig. 3d and e, red open symbols) may also be a consequence of this earlier switch: it could reduce the accumulation of uninucleate spores, which are known to be the more virulent forms (Agnew and Koella 1997).
We would have expected high-food parasites to have some advantage in their "own" high-food environment. However, there was no clear sign of differentiation between high-food and low-food parasites when tested at high food (Fig. 3b). It could be that, in our final assay, the highly permissive conditions masked the expression of genetic variation, a common observation in mosquito-parasite systems (Koella and Offenberg 1999; Bedhomme et al. 2004; Fellous and Koella 2010). Possibly, differences in the developmental switch of the parasite could be detected through high-resolution time series, tracking the onset of binucleate spore production during juvenile development. Additional adaptation might be seen in an assay covering the complete vertical pathway from egg production to the survival of the vertically infected offspring. This could also include measurements of behavioural components, such as blood feeding and oviposition, which are known to be affected by this parasite (Zettel Nalen et al. 2013).
Evolution of horizontal transmission (HT) potential
Low-food test conditions slowed down juvenile development and increased the death of infected hosts before reaching the adult stage. We show here that the delayed emergence was associated with a higher HT potential (Fig. 3a). This immediate (ecological) effect is due to the fixed developmental program of the parasite. In slowly developing juveniles (larvae or pupae), the parasite has already gone through the binucleate stage and is producing uninucleate HT spores (Agnew and Koella 1999; Zilio et al. 2018).
There was no evidence for responses to selection in the horizontal transmission components, as parasites from high-food and low-food treatments did not significantly differ in HT potential. Unlike other studies (Stewart et al. 2005; Sachs and Wilcox 2006; Le Clec’h et al. 2017), our experiment was not designed to impose direct selection on the HT pathway. The “shortcut” horizontal transmission was blocked, and all infections had to go vertically in order to reach next cycle of selection. Contributions from this horizontal pathway could be included, for example, by collecting uninucleate (HT) spores from naturally killed juveniles and adding these to the inocula used to start a new infected cohort. Future experiments should aim at protocols allowing the interplay between food conditions and epidemiological processes over the entire infection life cycle (Fig. 1b). The ultimate (but challenging) solution would be to let long-term populations self-maintain in cages, only provisioning them with regular blood meals and letting females oviposit in miniaturised ponds placed inside the cage, with high or low food levels.
Trade-off between horizontal and vertical transmission
Our multivariate analyses indicate an ecological trade-off between HT and VT potential, with high HT potential under low food test conditions and high VT potential under high food test conditions. However, there was no corresponding evolutionary trade-off, such that an evolutionary increase in VT potential would cause a correlated decrease in the HT mode, which is commonly assumed in theoretical models (Lipsitch et al. 1996) and found in certain selection experiments (Magalon et al. 2010; Dusi et al. 2015). In fact, if anything, an increase in VT potential of high-food parasites was accompanied by a higher HT potential (at least in tests under low-food conditions; Fig. 5c), a pattern also observed in other studies (van Frankenhuyzen et al. 2007; de Roode et al. 2009).
As discussed above, the absence of an evolutionary trade-off could be explained by the fact that our experiment did not allow direct evolutionary responses of the HT pathway. Nevertheless, our data show such a trade-off also does not arise from negative correlated responses to selection on the VT mode. This suggests that the VT mode can evolve independently of the (“shortcut”) HT mode, despite the VT vs HT decision the parasite "needs to make" in each individually infected larva. Interestingly, the infection life cycle in this system further suggests that increased vertical transmission (i.e., more vertically infected offspring) is also positively associated with rates of HT via the second pathway, which is accomplished through the killing of the vertically infected offspring (Fig. 1).
More generally, in systems with mixed-mode transmission parasites the evolutionary relationship between the VT and HT modes may be less straightforward (Kover and Clay 1998) than assumed in conceptual models. With a more complex life cycle, trait relationships are not as unequivocal and different aspects of virulence may not provide a “common currency” linking the two transmission modes, especially when the traits are expressed at different developmental stages (here delayed juvenile development vs reduced adult fecundity).
Resource availability and the ecological theatre
Anthropogenic activities and global change have massive impacts on the amount and distribution of organic matter and nutrients in natural communities. For host-parasite interactions, these changes may affect opportunities for parasite transmission and the expression of virulence-related traits, with huge implications for the spread of epidemics and evolution (Becker et al. 2015; Altizer et al. 2018; Civitello et al. 2018). Here, we investigated the role of food availability, an ecological variable so far largely overlooked in the context of transmission mode evolution. Yet, resources have long been recognised as a central element for host-parasite interactions, due to their impact on development and virulence of the parasite, or the immunity or tolerance of the host (Lopez-Pascua et al. 2010; Zeller and Koella 2017; Pike et al. 2019). These internal (within-host) effects, together with more indirect between-host effects via host density, likely feed back on epidemiological dynamics and evolutionary trajectories (Hite and Cressler 2018). For example, in a study on Hamiltosporidium tvaerminnensis and Daphnia magna, combining field work with laboratory experiments, Narr et al. (2019) concluded that nutrient-rich habitats can favour investment in vertical transmission and may ultimately result in the evolution of lower virulence in their parasite. Even though our results are in line with this prediction, we urge caution in making generalisations. Our study was primarily focused on the evolutionary match between parasite infection life cycle and resource-mediated changes in host development. How changes in host demography add to the equation requires further investigation, both theoretically and experimentally. Predicted outcomes likely depend on the biology of the interacting species and the precise ecological scenario. For example, nutrient-rich habitats may not only increase fecundity (and thus VT potential), but also sustain higher host density. Thus, in a population at equilibrium, only a small fraction of the vertically infected offspring may successfully establish and most transmission be horizontal due to high contact rates (Kover and Clay 1998). On the other hand, resources are notoriously variable in time and space. In a metapopulation, parasites evolving in productive environments may flood and replace those adapted to poor environments (Hochberg and Baalen 1998; Koella 2000), so that selection on transmission pathways may ultimately depend on the spatio-temporal distribution of different habitat types (Saikkonen et al. 2002; Su et al. 2019). The metapopulation context could also be highly relevant to our host-parasite system, where oviposition sites and water bodies are highly ephemeral (Agnew and Koella 1999). The “shortcut” horizontal transmission represents events of local transmission for the parasite, whereas the vertical transmission is associated with the dispersal of infected adult mosquitoes to new patches and oviposition sites, thereby contributing to the global transmission of the parasite.
Conclusions
Many relevant parasites have a mixed mode of vertical and horizontal transmission, but we are still lacking a good picture of how their dual strategy allows them to respond to environmental heterogeneity. Here we demonstrate effects of resource availability on host and parasite development. Permissive food conditions appeared to have favour the evolution of increased VT potential in this eukaryotic parasite, and these changes did not seem to come at the expense of the parasite's horizontal transmission potential. Understanding the eco-evolutionary processes affecting vertical and horizontal transmission, and how these can scale up from within-host to population-level dynamics, remains one main challenge.
Data availability
The datasets analysed during the current study are available in the Zenodo repository, https://zenodo.org/record/6376063
References
Agnew P, Koella JC (1997) Virulence, parasite mode of transmission, and host fluctuating asymmetry. Proc Biol Sci 264:9–15
Agnew P, Koella JC (1999) Life history interactions with environmental conditions in a host–parasite relationship and the parasite’s mode of transmission. Evol Ecol 13:67–91. https://doi.org/10.1023/A:1006586131235
Altizer S, Becker DJ, Epstein JH et al (2018) Food for contagion: synthesis and future directions for studying host-parasite responses to resource shifts in anthropogenic environments. Philos Trans R Soc Lond B Biol Sci 373:20170102. https://doi.org/10.1098/rstb.2017.0102
Antonovics J, Wilson AJ, Forbes MR et al (2017) The evolution of transmission mode. Phil Trans R Soc B 372:20160083. https://doi.org/10.1098/rstb.2016.0083
Becker DJ, Streicker DG, Altizer S (2015) Linking anthropogenic resources to wildlife-pathogen dynamics: a review and meta-analysis. Ecol Lett 18:483–495. https://doi.org/10.1111/ele.12428
Becnel JJ, Sprague V, Fukuda T, Hazard EI (1989) Development of Edhazardia aedis (Kudo, 1930) N. G., N. Comb. (Microsporida: Amblyosporidae) in the Mosquito Aedes aegypti (L.) (Diptera: Culicidae). J Protozool 36:119–130. https://doi.org/10.1111/j.1550-7408.1989.tb01057.x
Becnel JJ, Garcia JJ, Johnson MA (1995) Edhazardia aedis (Microspora: Culicosporidae) effects on the reproductive capacity of Aedes aegypti (Diptera: Culicidae). J Med Entomol 32:549–553
Bedhomme S, Agnew P, Sidobre C, Michalakis Y (2004) Virulence reaction norms across a food gradient. Proc R Soc B Biol Sci 271:739–744. https://doi.org/10.1098/rspb.2003.2657
Berngruber TW, Lion S, Gandon S (2015) Spatial structure, transmission modes and the evolution of viral exploitation strategies. PLOS Pathog 11:e1004810. https://doi.org/10.1371/journal.ppat.1004810
Bull JJ, Molineux IJ, Rice WR (1991) Selection of benevolence in a host-parasite system. Evolution 45:875–882. https://doi.org/10.2307/2409695
Burgdorfer W, Varma MG (1967) Trans-stadial and transovarial development of disease agents in arthropods. Annu Rev Entomol 12:347–376. https://doi.org/10.1146/annurev.en.12.010167.002023
Christophers, S (1960) Aëdes aegypti (L.) the Yellow Fever Mosquito: its Life History, Bionomics and Structure.
Civitello DJ, Allman BE, Morozumi C, Rohr JR (2018) Assessing the direct and indirect effects of food provisioning and nutrient enrichment on wildlife infectious disease dynamics. Philos Trans R Soc Lond B Biol Sci 373:20170101
Contopoulos-Ioannidis D, Newman-Lindsay S, Chow C, LaBeaud AD (2018) Mother-to-child transmission of Chikungunya virus: a systematic review and meta-analysis. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0006510
Cornet S, Bichet C, Larcombe S et al (2014) Impact of host nutritional status on infection dynamics and parasite virulence in a bird-malaria system. J Anim Ecol 83:256–265. https://doi.org/10.1111/1365-2656.12113
Cressler CE, Nelson WA, Day T et al (2014) Disentangling the interaction among host resources, the immune system and pathogens. Ecol Lett 17:284–293. https://doi.org/10.1111/ele.12229
da Costa CF, da Silva AV, do Nascimento VA et al (2018) Evidence of vertical transmission of Zika virus in field- collected eggs of Aedes aegypti in the Brazilian Amazon. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0006594
de Roode JC, Chi J, Rarick RM, Altizer S (2009) Strength in numbers: high parasite burdens increase transmission of a protozoan parasite of monarch butterflies (Danaus plexippus). Oecologia 161:67–75. https://doi.org/10.1007/s00442-009-1361-6
Desjardins CA, Sanscrainte ND, Goldberg JM et al (2015) Contrasting host–pathogen interactions and genome evolution in two generalist and specialist microsporidian pathogens of mosquitoes. Nat Commun. https://doi.org/10.1038/ncomms8121
Drew GC, Stevens EJ, King KC (2021) Microbial evolution and transitions along the parasite-mutualist continuum. Nat Rev Microbiol. https://doi.org/10.1038/s41579-021-00550-7
Duncan AB, Agnew P, Noel V, Michalakis Y (2015) The consequences of co-infections for parasite transmission in the mosquito Aedes aegypti. J Anim Ecol 84:498–508. https://doi.org/10.1111/1365-2656.12302
Dusi E, Gougat-Barbera C, Berendonk TU, Kaltz O (2015) Long-term selection experiment produces breakdown of horizontal transmissibility in parasite with mixed transmission mode. Evolution 69:1069–1076. https://doi.org/10.1111/evo.12638
Ebert D (2013) The epidemiology and evolution of symbionts with mixed-mode transmission. Annu Rev Ecol Evol Syst 44:623–643. https://doi.org/10.1146/annurev-ecolsys-032513-100555
Ebert D, Mangin KL (1997) The influence of host demography on the evolution of virulence of a microsporidian gut parasite. Evolution 51:1828. https://doi.org/10.2307/2411005
Farjana T, Tuno N (2012) Effect of body size on multiple blood feeding and egg retention of Aedes aegypti (L.) and Aedes albopictus (Skuse) (Diptera: Culicidae). Med Entomol Zool 63:123–131. https://doi.org/10.7601/mez.63.123
Fellous S, Koella JC (2010) Cost of co-infection controlled by infectious dose combinations and food availability. Oecologia 162:935–940. https://doi.org/10.1007/s00442-009-1535-2
Ferdy J, Godelle B (2005) Diversification of transmission modes and the evolution of mutualism. Am Nat 166:613–627. https://doi.org/10.1086/491799
Frank SA (1996) Models of parasite virulence. Q Rev Biol 71:37–78
Grigsby A, Kelly BJ, Sanscrainte ND et al (2020) Propagation of the microsporidian parasite edhazardia aedis in aedes aegypti mosquitoes. J vis Exp JoVE. https://doi.org/10.3791/61574
Hite JL, Cressler CE (2018) Resource-driven changes to host population stability alter the evolution of virulence and transmission. Philos Trans R Soc B Biol Sci 373:20170087. https://doi.org/10.1098/rstb.2017.0087
Hochberg ME, van Baalen M (1998) Antagonistic coevolution over productivity gradients. Am Nat 152:620–634. https://doi.org/10.1086/286194
Jover LF, Effler TC, Buchan A et al (2014) The elemental composition of virus particles: implications for marine biogeochemical cycles. Nat Rev Microbiol 12:519–528. https://doi.org/10.1038/nrmicro3289
Kaltz O, Koella JC (2003) Host growth conditions regulate the plasticity of horizontal and vertical transmission in Holospora undulata, a bacterial parasite of the protozoan Paramecium caudatum. Evolution 57:1535–1542. https://doi.org/10.1111/j.0014-3820.2003.tb00361.x
Koella JC (2000) Coevolution of parasite life cycles and host life-histories. In: Poulin R, Morand S, Skopring A (eds) Evolutionary biology of host-parasite relationships: Theory meets reality. Elsevier Science Publishers Amsterdam, pp 185–200
Koella JC, Lyimo EO (1996) Variability in the relationship between weight and wing length of Anopheles gambiae (Diptera: Culicidae). J Med Entomol 33:261–264. https://doi.org/10.1093/jmedent/33.2.261
Koella JC, Offenberg J (1999) Food availability and parasite infection in¯uence the correlated responses of life history traits to selection for age at pupation in the mosquito Aedes aegypti. J Evol Biol 12(4):760–769
Kover PX, Clay K (1998) Trade-off between virulence and vertical transmission and the maintenance of a virulent plant pathogen. Am Nat 152:165–175. https://doi.org/10.1086/286159
Lai Z, Zhou T, Liu S et al (2020) Vertical transmission of zika virus in Aedes albopictus. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0008776
Le Clec’h W, Dittmer J, Raimond M et al (2017) Phenotypic shift in Wolbachia virulence towards its native host across serial horizontal passages. Proc R Soc B Biol Sci 284:20171076. https://doi.org/10.1098/rspb.2017.1076
Leisnham PT, Juliano SA (2010) Interpopulation differences in competitive effect and response of the mosquito Aedes aegypti and resistance to invasion by a superior competitor. Oecologia 164:221–230. https://doi.org/10.1007/s00442-010-1624-2
Lipsitch M, Nowak MA, Ebert D, May RM (1995) The population dynamics of vertically and horizontally transmitted parasites. Proc R Soc B Biol Sci 260:321–327. https://doi.org/10.1098/rspb.1995.0099
Lipsitch M, Siller S, Nowak MA (1996) The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution 50:1729. https://doi.org/10.2307/2410731
Lopez-Pascua LDC, Brockhurst MA, Buckling A (2010) Antagonistic coevolution across productivity gradients: an experimental test of the effects of dispersal. J Evol Biol 23:207–211. https://doi.org/10.1111/j.1420-9101.2009.01877.x
Magalon H, Nidelet T, Martin G, Kaltz O (2010) Host growth conditions influence experimnental evolution of life history and virulence of a parasite with vertical and horizontal transmission. Evolution. https://doi.org/10.1111/j.1558-5646.2010.00974.x
Messenger SL, Molineux IJ, Bull JJ (1999) Virulence evolution in a virus obeys a trade-off. Proc R Soc B Biol Sci 266:397–404
Mojica KDA, Brussaard CPD (2014) Factors affecting virus dynamics and microbial host–virus interactions in marine environments. FEMS Microbiol Ecol 89:495–515. https://doi.org/10.1111/1574-6941.12343
Munstermann LE (1996) Care and Maintenance of Aedes Mosquito Colonies. In: Crampton JM, Ben Beard C, Louis C (eds) The Molecular Biology of Insect Disease Vectors. Springer, Dordrecht, pp 13–20. https://doi.org/10.1007/978-94-009-1535-0_2
Narita M, Shibata M, Togashi T, Koga Y (1991) Vertical transmission of human T cell leukemia virus type I. J Infect Dis 163:204. https://doi.org/10.1093/infdis/163.1.204
Narr CF, Ebert D, Bastille-Rousseau G, Frost PC (2019) Nutrient availability affects the prevalence of a microsporidian parasite. J Anim Ecol 88:579–590. https://doi.org/10.1111/1365-2656.12945
Nasci RS, Tang KH, Becnel JJ, Fukuda T (1992) Effect of per os Edhazardia aedis (Microsporida: Amblyosporidae) infection on Aedes aegypti mortality and body size. J Am Mosq Control Assoc 8:131–136
Nene V, Wortman JR, Lawson D et al (2007) Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316:1718–1723. https://doi.org/10.1126/science.1138878
Pike VL, Lythgoe KA, King KC (2019) On the diverse and opposing effects of nutrition on pathogen virulence. Proc R Soc B Biol Sci 286:20191220. https://doi.org/10.1098/rspb.2019.1220
Restif O, Kaltz O (2006) Condition-dependent virulence in a horizontally and vertically transmitted bacterial parasite. Oikos 114:148–158. https://doi.org/10.1111/j.2006.0030-1299.14611.x
Sachs JL, Wilcox TP (2006) A shift to parasitism in the jellyfish symbiont Symbiodinium microadriaticum. Proc Biol Sci 273:425–429. https://doi.org/10.1098/rspb.2005.3346
Sachs JL, Essenberg CJ, Turcotte MM (2011a) New paradigms for the evolution of beneficial infections. Trends Ecol Evol 26:202–209. https://doi.org/10.1016/j.tree.2011.01.010
Sachs JL, Skophammer RG, Regus JU (2011b) Evolutionary transitions in bacterial symbiosis. Proc Natl Acad Sci 108:10800–10807. https://doi.org/10.1073/pnas.1100304108
Saikkonen K, Ion D, Gyllenberg M (2002) The persistence of vertically transmitted fungi in grass metapopulations. Proc R Soc Lond B Biol Sci 269:1397–1403. https://doi.org/10.1098/rspb.2002.2006
Sambado S, Salomon J, Crews A, Swei A (2020) Mixed transmission modes promote persistence of an emerging tick-borne pathogen. ECOSPHERE. https://doi.org/10.1002/ecs2.3171
Schmid B, Dolt C (1994) Effects of maternal and paternal environment and genotype on offspring phenotype in solidago altissima l. Evol Int J Org Evol 48:1525–1549. https://doi.org/10.1111/j.1558-5646.1994.tb02194.x
Schmid-Hempel P (2021) Evolutionary Parasitology: The Integrated Study of Infections, Immunology, Ecology, and Genetics. Oxford University Press
Stewart AD, Logsdon JM, Kelley SE (2005) An empirical study of the evolution of virulence under both horizontal and vertical transmission. Evolution 59:730–739. https://doi.org/10.1111/j.0014-3820.2005.tb01749.x
Su M, Chen G, Yang Y (2019) Dynamics of host-parasite interactions with horizontal and vertical transmissions in spatially heterogeneous environment. Phys Stat Mech Its Appl 517:452–458. https://doi.org/10.1016/j.physa.2018.11.034
Teixeira AF, de Brito BB, Correia TML et al (2021) Simultaneous circulation of zakat, dengue, and chikungunya viruses and their vertical co-transmission among Aedes aegypti. ACTA Trop. https://doi.org/10.1016/j.actatropica.2020.105819
Turner PE, Cooper VS, Lenski RE (1998) Tradeoff between horizontal and vertical modes of transmission in bacterial plasmids. Evolution 52:315–329. https://doi.org/10.2307/2411070
van den Bosch F, Fraaije BA, van den Berg F, Shaw MW (2010) Evolutionary bi-stability in pathogen transmission mode. Proc R Soc B Biol Sci 277:1735–1742. https://doi.org/10.1098/rspb.2009.2211
van Frankenhuyzen K, Nystrom C, Liu Y (2007) Vertical transmission of Nosema fumiferanae (Microsporidia: Nosematidae) and consequences for distribution, post-diapause emergence and dispersal of second-instar larvae of the spruce budworm, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). J Invertebr Pathol 96:173–182. https://doi.org/10.1016/j.jip.2007.03.017
Weitz JS, Li G, Gulbudak H et al (2019) Viral invasion fitness across a continuum from lysis to latency†. Virus Evol. https://doi.org/10.1093/ve/vez006
Zeller M, Koella JC (2016) Effects of food variability on growth and reproduction of Aedes aegypti. Ecol Evol 6:552–559. https://doi.org/10.1002/ece3.1888
Zeller M, Koella JC (2017) The role of the environment in the evolution of tolerance and resistance to a pathogen. Am Nat 190:389–397. https://doi.org/10.1086/692759
Zettel Nalen CM, Allan SA, Becnel JJ, Kaufman PE (2013) Oviposition substrate selection by Florida mosquitoes in response to pathogen-infected conspecific larvae. J Vector Ecol 38:182–187. https://doi.org/10.1111/j.1948-7134.2013.12025.x
Zilio G, Koella JC (2020) Sequential co-infections drive parasite competition and the outcome of infection. J Anim Ecol 89:2367–2377. https://doi.org/10.1111/1365-2656.13302
Zilio G, Thiévent K, Koella JC (2018) Host genotype and environment affect the trade-off between horizontal and vertical transmission of the parasite Edhazardia aedis. BMC Evol Biol. https://doi.org/10.1186/s12862-018-1184-3
Acknowledgements
We thank K. Thiévent, G. Hauser, A. Belli, L. Moesch and M. Zeller for their help in the lab and suggestions. We thank P. Schmid-Hempel for early discussion on the design of the study. We also thank two anonymous reviewers for very helpful and constructive criticism. This is publication ISEM-2022-062 of the Institut des Sciences de l'Evolution de Montpellier.
Funding
Open access funding provided by University of Neuchâtel. No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
GZ and JCK: conceived the study and designed the experiments. GZ: performed the experimental work. GZ and OK: performed the statistical analysis. All authors interpreted the results, contributed to the writing, read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zilio, G., Kaltz, O. & Koella, J.C. Resource availability for the mosquito Aedes aegypti affects the transmission mode evolution of a microsporidian parasite. Evol Ecol 37, 31–51 (2023). https://doi.org/10.1007/s10682-022-10184-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-022-10184-7