Abstract
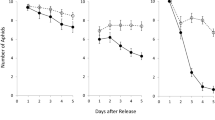
The evolutionary spread of a trait that benefits individuals of one species in their interactions with a second species may be constrained by “ecological costs” if the trait also has detrimental effects on interactions with a third species in the environment. In particular, the identification of ecological costs may help explain why natural plant populations often display less-than-maximal resistance against natural enemies—even when the plant populations possess substantial genetic variation that could serve as raw material for evolving greater resistance. One such resistance trait that confers an obvious fitness advantage, yet is maintained in populations at intermediate levels, is the strategy of resistance-by-ducking displayed by certain species of goldenrods (Solidago). In a ducking individual, the stem temporarily nods near the apex during a time in spring that coincides with the oviposition season of several herbivores. I investigated whether the fitness benefits of ducking in terms of deterring apex-galling insects may be offset by ecological costs posed by a common sap-sucking specialist—the red goldenrod aphid, Uroleucon nigrotuberculatum. In a controlled outdoor experiment, ducking was associated with significantly lower antixenosis (i.e., reduced preference), antibiosis (reduced performance), and indirect resistance against aphids. Specifically, aphids colonized ducking plants at a higher rate than erect-stemmed plants; populations of aphids on bagged ducking plants increased more quickly than on bagged erect-stemmed plants; and aphid populations on erect-stemmed plants were wiped out more quickly by natural enemies than on ducking plants when bags were removed. To the extent that aphid damage reduces goldenrod’s fitness, these ecological costs may be responsible for limiting the ducking trait to intermediate levels in goldenrod populations. Because most plant species are attacked by multiple types of herbivores, ecological costs of resistance mechanisms are likely to be more widespread than is generally appreciated, though their subtlety may make them challenging to identify.




Similar content being viewed by others
References
Abrahamson WG, Weis AE (1997) Evolutionary ecology across three trophic levels: goldenrods, gallmakers, and natural enemies. Monographs in population biology. Princeton University Press, Princeton
Abrahamson WG, Ball Dobley K, Houseknecht HR, Pecone CA (2005) Ecological divergence among five co-occurring species of old-field goldenrods. Plant Ecol 177:43–56
Agrawal AA (2005) Natural selection on common milkweed (Asclepias syriaca) by a community of specialized insect herbivores. Evol Ecol Res 7:651–667
Agrawal AA (2011) Current trends in the evolutionary ecology of plant defence. Funct Ecol 25:420–432
Ando Y, Ohgushi T (2008) Ant- and plant-mediated indirect effects induced by aphid colonization on herbivorous insects on tall goldenrod. Popul Ecol 50:181–189
Bode RF, Kessler A (2012) Herbivore pressure on goldenrod (Solidago altissima L., Asteraceae): its effects on herbivore resistance and vegetative reproduction. J Ecol 100:795–801
Cappuccino N (1987) Comparative population dynamics of two goldenrod aphids: spatial patterns and temporal consistency. Ecology 68(6):1634–1646
Cappuccino N (1988) Spatial patterns of goldenrod aphids and the response of enemies to patch density. Oecologia 76:607–610
Carter MC, Sutherland D, Dixon AFG (1984) Plant structure and the searching efficiency of coccinellid larvae. Oecologia 63:394–397
Cipollini D, Walters D, Voelckel C (2014) Costs of resistance in plants: from theory to evidence. Annu Plant Rev 47:263–308
Dorchin N, McEvoy MV, Dowling TA, Abrahamson WG, Moore JG (2009a) Revision of the goldenrod-galling Rhopalomyia species (Diptera: Cecidomyiidae) in North America. Zootaxa 2152:1–35
Dorchin N, Scott ER, Clarkin CE, Luongo MP, Jordan S, Abrahamson WG (2009b) Behavioural, ecological and genetic evidence confirm the occurrence of host-associated differentiation in goldenrod gall-midges. J Evol Biol 22:729–739
Dorchin N, Joy JB, Hilke LK, Wise MJ, Abrahamson WG (2015) Taxonomy and phylogeny of the Asphondylia species (Diptera: Cecidomyiidae) of North American goldenrods: challenging morphology, complex host associations, and cryptic speciation. Zool J Linn Soc 174:256–304
Genung MA, Crutsinger GM, Bailey JK, Schweitzer JA, Sanders NJ (2012) Aphid and ladybird beetle abundance depend on the interaction of spatial effects and genotypic diversity. Oecologia 168:167–174
Grevstad FS, Klepetka BW (1992) The influence of plant architecture on the foraging efficiencies of a suite of ladybird beetles feeding on aphids. Oecologia 92:399–404
Hartnett DC, Abrahamson WG (1979) The effects of stem gall insects on life history patterns in Solidago canadensis. Ecology 60(5):910–917
Johnson RH, Hull-Sanders HM, Meyer GA (2007) Comparison of foliar terpense between native and invasive Solidago gigantea. Biochem Syst Ecol 35:821–830
Kareiva P, Sahakian R (1990) Tritrophic effects of a simple architectural mutation in pea plants. Nature 345:433–434
Kliebenstein DJ (2014) Quantitative genetics and genomics of plant resistance to insects. Annu Plant Rev 47:235–262
Lucas-Barbosa D (2016) Integrating studies on plant-pollinator and plant-herbivore interactions. Trends Plant Sci 21(2):125–133
Maddox GD, Root RB (1987) Resistance to 16 diverse species of herbivorous insects within a population of goldenrod, Solidago altissima: genetic variation and heritability. Oecologia 72:8–14
Meyer GA (1993) A comparison of the impacts of leaf- and sap-feeding insects on growth and allocation of goldenrod. Ecology 74(4):1101–1116
Meyer GA (2000) Effects of insect feeding on growth and fitness of goldenrod (Solidago altissima). Recent Res Dev Entomol 3:29–41
Meyer GA, Root RB (1993) Effects of herbivorous insects and soil fertility on reproduction of goldenrod. Ecology 74(4):1117–1128
Meyer GA, Whitlow TH (1992) Effects of leaf and sap feeding insects on photosynthetic rates of goldenrod. Oecologia 92:480–489
Miller TEX, Tenhumberg B, Louda SM (2008) Herbivore-mediated ecological costs of reproduction shape the life history of an iteroparous plant. Am Nat 171(2):141–149
Moore BD, Andrew RL, Külheim C, Foley WJ (2014) Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol 201:733–750
Ness JH (2006) A mutualism’s indirect costs: the most aggressive plant bodyguards also deter pollinators. Oikos 113:506–514
Ôtake A (1999) Analytical study of fundatrix populations of Uroleucon nigrotuberculatum (Olive) (Hemiptera: Aphididae: Aphidinae) on an observation plot of the goldenrod Solidago altissima L. Appl Entomol Zool 34(4):435–442
Pilson D (1992) Aphid distribution and the evolution of goldenrod resistance. Evolution 46(5):1358–1372
Pilson D, Rausher MD (1995) Clumped distribution patterns in goldenrod aphids: genetic and ecological mechanisms. Ecol Entomol 20:75–83
Puentes A, Ågren J (2014) No trade-off between trichome production and tolerance to leaf and inflorescence damage in a natural population of Arabidopsis lyrata. J Plant Ecol 7(4):373–383
Richardson ML, Hanks LM (2011) Differences in spatial distribution, morphology, and communities of herbivorous insects among three cytotypes of Solidago altissima (Asteraceae). Am J Bot 98(10):1595–1601
Roach DA, Wulff RD (1987) Maternal effects in plants. Annu Rev Ecol Syst 18:209–235
Roche BM, Fritz RS (1997) Genetics of resistance of Salix sericea to a diverse community of herbivores. Evolution 51(5):1490–1498
Rutledge CE, Robinson AP, Eigenbrode SD (2003) Effects of a simple plant morphological mutation on the arthropod community and the impacts of predators on a principal insect herbivore. Oecologia 135:39–50
Sakata Y, Ohgushi T, Isagi Y (2013) Geographic variations in phenotypic traits of the exotic herb Solidago altissima and abundance of recent established exotic herbivorous insects. J Plant Interact 8(3):216–218
Sato Y, Ito K, Kudoh H (2017) Optimal foraging by herbivores maintains polymorphism in defence in a natural plant population. Funct Ecol. https://doi.org/10.1111/1365-2435.12937
Schlaepfer DR, Edwards PJ, Semple JC, Billeter R (2008) Cytogeography of Solidago gigantea (Asteraceae) and its invasive ploidy level. J Biogeogr 35(11):2119–2127
Strauss SY, Rudgers JA, Lau JA, Irwin RE (2002) Direct and ecological costs of resistance to herbivory. Trends Ecol Evol 17(6):278–285
Szymura M, Szymura TH, Wolski K (2016) Invasive Solidago species: how large [an] area do they occupy and what would be the cost of their removal? Pol J Ecol 64:25–34
Triplehorn CA, Johnson NF (2005) Borror and DeLong’s introduction to the study of insects, 7th edn. Brooks/Cole, Belmont
Utsumi S, Ando Y, Craig TP, Ohgushi T (2011) Plant genotypic diversity increases population size of a herbivorous insect. Proc R Soc Lond B 278(1721):3108–3115
Voigt D (2019) Foothold matters: attachment on plant surfaces promotes the vitality of omnivorous mirid bugs Dicyphus errans. Arthropod–Plant Interact 13(6):819–834
Weber E (2001) Current and potential ranges of three exotic goldenrods (Solidago) in Europe. Conserv Biol 15(1):122–128
Williams RS, Avakian MA (2015) Colonization of Solidago altissma by the specialist aphid Uroleucon nigrotuberculatum: effects of genetic identity and leaf chemistry. J f Chem Ecol 41:129–138
Williams RS, Howells JM (2018) Effects of intraspecific genetic variation and prior herbivory in an old-field plant on the abundance of the specialist aphid Uroleucon nigrotuberculatum (Hemiptera: Aphididae). Environ Entomol 47(2):422–431
Wise MJ (2009) To duck or not to duck: resistance advantages and disadvantages of the candy-cane stem phenotype in tall goldenrod, Solidago altissima. New Phytol 183:900–907. https://doi.org/10.1111/j.1469-8137.2009.02879.x
Wise MJ (2018a) Defense with benefits? Ducking plants outperformed erect plants in the goldenrod Solidago gigantea in the absence of herbivory. Am J Bot 105(6):1096–1103
Wise MJ (2018b) Field frequency and pattern of inheritance of the herbivory-defense trait “resistance by ducking” in the giant goldenrod (Solidago gigantea Asteraceae). Plant Ecol Evolut 151(2):271–277
Wise MJ, Abrahamson WG (2008) Ducking as a means of resistance to herbivory in tall goldenrod, Solidago altissima. Ecology 89(12):3275–3281
Wise MJ, Rausher MD (2013) Evolution of resistance to a multiple-herbivore community: genetic correlations, diffuse coevolution, and constraints on the plant’s response to selection. Evolution 67(6):1767–1779
Wise MJ, Yi CG, Abrahamson WG (2009) Associational resistance, gall-fly preferences, and a stem dimorphism in Solidago altissima. Acta Oecol 35:471–476
Wise MJ, Abrahamson WG, Cole JA (2010a) The role of nodding stems in the goldenrod-gall-fly interaction: a test of the ducking hypothesis. Am J Bot 97(3):525–529
Wise MJ, Cole JA, Carr DE (2010b) A field study of potential ecological costs of resistance by ‘stem ducking’ in tall goldenrod, Solidago altissima. Entomol Exp Appl 136:271–280
Yip EC, Sowers RP, Helms AM, Mescher MC, De Moraes CM, Tooker JF (2019) Trade-offs between defenses against herbivores in goldenrod (Solidgo altissima). Arthropod–Plant Interact 13:279–287
Zovi D, Stastny M, Battisti A, Larsson S (2008) Ecological costs on local adaptation of an insect herbivore imposed by host plants and enemies. Ecology 89(5):1388–1398
Acknowledgements
The Department of Biology at Roanoke College provided logistical support for this study, D. E. Carr provided statistical guidance and advice on working with aphids, and S. E. Wise and two anonymous reviewers provided constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wise, M.J. Ecological costs of goldenrod’s ducking strategy in the currency of antixenosis, antibiosis, and indirect resistance to aphids. Evol Ecol 34, 273–287 (2020). https://doi.org/10.1007/s10682-020-10032-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-020-10032-6