Abstract
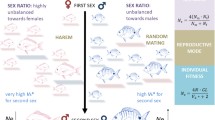
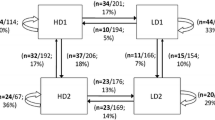
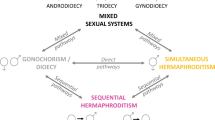
Sequential hermaphroditism (sex change) is understood to be a strategy that maximizes lifetime reproduction in systems where one sex confers highest fitness early in life, and the other later in life. This strategy is evolutionarily stable despite costs to growth, survival, or current reproduction. Few studies have examined advantages of sex change outside of reproduction. The mangrove rivulus fish, Kryptolebias marmoratus, presents a unique system in which to study non-reproductive consequences of sex change because reproductive opportunity decreases significantly with sex change. In natural conditions, individuals develop as self-fertilizing simultaneous hermaphrodites. Some individuals change sex to male at various points after sexual maturity, even in isolation, essentially foregoing future reproductive assurance. In a large-scale experiment that examined fitness differences among individuals exposed to ecologically relevant environmental challenges, we found that individuals that change sex from hermaphrodite to male had overwhelmingly greater chances of survival compared to those that remained hermaphrodite. Furthermore, hermaphrodites derived from lineages with higher propensities to change sex experienced greater survival advantages by changing sex. Our results indicate that sex change may be a survival strategy, one with genotype-dependent consequences.


Similar content being viewed by others
References
Aldenhoven JM (1986) Different reproductive strategies in a sex-changing coral reef fish Centropyge bicolor (Pomacanthidae). Mar Freshw Res 37:353–360
Allsop DJ, West SA (2003) Life history: changing sex at the same relative body size. Nature 425:783–784
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bauer G (1987) Reproductive strategy of the freshwater pearl mussel Margaritifera margaritifera. J Anim Ecol 56:691–704
Breitburg DL (1986) Effect of variability in recruitment on selection for protogynous sex change. Am Nat 128:551–560
Brooks M, Iwasa Y (2010) Size-dependent sex change can be the ESS without any size advantage of reproduction when mortality is size-dependent. Theor Popul Biol 78:183–191
Buston PM, Munday PL, Warner RR (2004) Evolutionary biology—sex change and relative body size in animals. Nature 428:U1
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton
Charnov EL (1986) Size advantage may not always favor sex change. J Theor Biol 119:283–285
Charnov EL (1987) On sex allocation and selfing in higher plants. Evol Ecol 1:30–36
Charnov EL, Anderson PJ (1989) Sex change and population fluctuations in pandalid shrimp. Am Nat 134:824–827
Charnov EL, Skúladóttir U (2000) Dimensionless invariants for the optimal size (age) of sex change. Evol Ecol Res 2:1067–1071
Clifton KE, Rogers L (2008) Sex-specific mortality explains non-sex-change by large female Sparisoma radians. Anim Behav 75:1–10
Cole KS, Noakes DLG (1997) Gonadal development and sexual allocation in mangrove killifish, Rivulus marmoratus (Pisces: Atherinomorpha). Copeia 1997:596–600
Davis WP (1990) Field observations of the ecology and habits of mangrove rivulus. Ichthyol Explor Freshw 1:123–134
Earley RL, Hanninen AF, Fuller A, Garcia MJ, Lee EA (2012) Phenotypic plasticity and integration in the mangrove rivulus (Kryptolebias marmoratus): a prospectus. Integr Comp Biol 52:814–827
Ellison A, Cable J, Consuegra S (2011) Best of both worlds? association between outcrossing and parasite loads in a selfing fish. Evolution 65:3021–3026
Ellison A, Wright P, Taylor DS, Cooper C, Regan K, Currie S, Consuegra S (2012) Environmental diel variation, parasite loads, and local population structuring of a mixed-mating mangrove fish. Ecol Evolut 2:1682–1695
Ellison A, De Leaniz CG, Consuegra S (2013) Inbred and furious: negative association between aggression and genetic diversity in highly inbred fish. Mol Ecol 22:2292–2300
Ellison A, Rodrı CM, Moran P, Breen J, Swain M, Megias M, Hegarty M et al (2015) Epigenetic regulation of sex ratios may explain natural variation in self-fertilization rates. Proc R Soc B 282:20151900
Fisher RF (1930) The genetical theory of natural selection. Oxford University Press, Oxford
Fox J, Weisberg S (2011) An R companion to applied regression. Sage, Thousand Oaks
Freeman DC, Harper KT, Charnov EL (1980) Sex change in plants: old and new observations and new hypotheses. Oecologia 47:222–232
Furness AI, Tatarenkov A, Avise JC (2015) A genetic test for whether pairs of hermaphrodites can cross-fertilize in a selfing killifish. J Hered 106:749–752
García D, Loureiro M, Tassino B (2008) Reproductive behavior in the annual fish Austrolebias reicherti Loureiro and Garcia 2004 (Cyprinodontiformes: Rivulidae. Neotropical Ichthyology 6:243–248
Ghiselin MT (1969) The evolution of hermaphroditism among animals. Q Rev Biol 44:189–208
Guide for the care and use of laboratory animals (2010) National Academies Press
Harrington RW Jr (1961) Oviparous hermaphroditic fish with internal self-fertilization. Science 134:1749–1750
Harrington RW Jr (1963) Twenty-four-hour rhythms of internal self-fertilization and of oviposition by hermaphrodites of Rivulus marmoratus. Physiol Zool 36:325–341
Harrington RW Jr (1967) Environmentally controlled induction of primary male gonochorists from eggs of the self- fertilizing hermaphroditic fish, Rivulus marmoratus Poey. Biol Bull 132:174–199
Harrington RW Jr (1971) How ecological and genetic factors interact to determine when self-fertlizing hermaphrodites of Rivulus marmoratus change into functional secondary males, with a reappraisal of the modes of intersexuality among fishes. Copeia 1971:389–432
Harrington RW Jr (1975) Sex setermination and differentiation among uniparental homozygotes of the hermaphroditic fish Rivulus marmoratus (Cyprinodontidae: Atheriniformes). In: Reinboth R (ed) Intersexuality in the animal Kingdom. Springer, Berlin, pp 249–262
Harrington RW Jr, Rivas L (1958) The discovery in Florida of the cyprinodont fish, Rivulus marmoratus, with a redescription and ecological notes. Copeia 1958:125–130
Hoch JM, Cahill AE (2012) Variation in size at sex-change among natural populations of the protandrous hermaphrodite, Crepidula fornicata (Gastropoda, Calyptraeidae). Mar Biol 159:897–905
Hoffman SG, Schildhauer MP, Warner RR (1985) The costs of changing sex and the ontogeny of males under contest competition for mates. Evolution 39:915–927
Iwasa Y (1991) Sex change evolution and cost of reproduction. Behav Ecol 2:56–68
JMP®, Version 14 Pro. 2019. SAS Institute Inc., Cary
Kazancıoğlu E, Alonzo SH (2009) Costs of changing sex do not explain why sequential hermaphroditism is rare. Am Nat 173:327–336
Leigh EG, Charnov EL, Warner RR (1976) Sex ratio, sex change, and natural selection. Proc Natl Acad Sci 73:3656–3660
Lenth R (2019) emmeans: estimated marginal means, aka least-squares means. R package, version 1.3.2. https://CRAN.R-project.org/package=emmeans
Lomax JL, Carlson RE, Wells JW, Crawford PM, Earley RL (2017) Factors affecting egg production in the selfing mangrove rivulus (Kryptolebias marmoratus). Zoology 122:38–45
Luke KN, Bechler DL (2010) The role of dyadic interactions in the mixed-mating strategies of the mangrove rivulus Kryptolebias marmoratus. Curr Zool 56:6–17
Mackiewicz M, Tatarenkov A, Perry A, Martin JR, Elder JF, Bechler DL, Avise JC (2006) Microsatellite documentation of male-mediated outcrossing between inbred laboratory strains of the self-fertilizing mangrove killifish (Kryptolebias marmoratus). J Hered 97:508–513
Marson KM, Taylor DS, Earley RL (2019) Cryptic male phenotypes in the mangrove rivulus fish, Kryptolebias marmoratus. Biol Bull 236:13–28
Martin SB (2007) Association behaviour of the self-fertilizing Kryptolebias marmoratus (Poey): The influence of microhabitat use on the potential for a complex mating system. J Fish Biol 71:1383–1392
Maynard Smith J (1978) The evolution of sex. Cambridge Univ Press, Cambridge
Molloy PP, Nyboer EA, Côté IM (2011) Male-male competition in a mixed-mating fish. Ethology 117:586–596
Munday PL, Molony BW (2002) The energetic cost of protogynous versus protandrous sex change in the bi-directional sex-changing fish Gobiodon histrio. Mar Biol 141:1011–1017
Munday PL, Buston PM, Warner RR (2006a) Diversity and flexibility of sex-change strategies in animals. Trends Ecol Evol 21:89–95
Munday PL, Wilson White J, Warner RR (2006b) A social basis for the development of primary males in a sex-changing fish. Proc R Soc B Biol Sci 273:2845–2851
Nakamura Y, Suga K, Sakakura Y, Sakamoto T, Hagiwara A (2008) Genetic and growth differences in the outcrossings between two clonal strains of the self-fertilizing mangrove killifish. Can J Zool 86:976–982
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rodgers EW, Earley RL, Grober MS (2007) Social status determines sexual phenotype in the bi-directional sex changing bluebanded goby Lythrypnus dalli. J Fish Biol 70:1660–1668
RStudioTeam (2016) RStudio: integrated development for R. RStudio Inc, Boston
Sakakura Y, Soyano K, Noakes DLG, Hagiwara A (2006) Gonadal morphology in the self-fertilizing mangrove killifish, Kryptolebias marmoratus. Ichthyol Res 53:427–430
Scarsella GE, Gresham JD, Earley RL (2018) Relationships between external sexually dimorphic characteristics and internal gonadal morphology in a sex-changing fish. J Zool 305:133–140
Tatarenkov A, Gao H, Mackiewicz M, Taylor DS, Turner BJ, Avise JC (2007) Strong population structure despite evidence of recent migration in a selfing hermaphroditic vertebrate, the mangrove killifish (Kryptolebias marmoratus). Mol Ecol 16:2701–2711
Tatarenkov A, Lima SMQ, Earley RL, Berbel-Filho WM, Vermeulen FBM, Taylor DS, Marson K et al (2017) Deep and concordant subdivisions in the self-fertilizing mangrove killifishes (Kryptolebias) revealed by nuclear and mtDNA markers. Biol J Lin Soc 122:558–578
Taylor DS (2012) Twenty-four years in the mud: What have we learned about the natural history and ecology of the mangrove rivulus, Kryptolebias marmoratus? Integr Comp Biol 52:724–736
Taylor DS, Turner BJ, Davis WP, Chapman BB (2008) A novel terrestrial fish habitat inside emergent logs. Am Nat 171:263–266
Todd EV, Ortega-Recalde O, Liu H, Lamm MS, Rutherford KM, Cross H, Black MA et al (2019) Stress, novel sex genes and epigenetic reprogramming orchestrate socially-controlled sex change. Sci Adv 5:eaaw7006
Turner BJ, Fisher MT, Taylor DS, Davis WP, Jarrett BL (2006) Evolution of “maleness” and outcrossing in a population of the self-fertilizing killifish, Kryptolebias marmoratus. Evol Ecol Res 8:1475–1486
Warner RR (1975) The adaptive significance of sequential hermaphroditism in animals. Am Nat 109:61–82
Warner RR (1988a) Sex change in fishes: hypotheses, evidence, and objections. Environ Biol Fishes 22:81–90
Warner RR (1988b) Sex change and the size-advantage model. Trends Ecol Evol 3:133–136
Warner RR, Robertson DR, Leigh EG (1975) Sex change and sexual selection. Science 190:633–638
Yamaguchi S (2016) Time required for sex change in teleost fishes: hormonal dynamics shaped by selection. J Theor Biol 407:339–348
Acknowledgements
We are grateful to two anonymous reviewers and an editor for valuable comments that significantly improved the manuscript. This work was supported by E. O. Wilson Fellowship (University of Alabama), Edward C. Raney Fund (American Society of Ichthyologists and Herpetologists), Graduate Council Fellowship (University of Alabama), and Research Grants Committee (University of California, Irvine). All procedures were approved by the University of Alabama Institutional Animal Care and Use Committee (protocols #13-10-0048 and #15-04-0102). Field activities, including collection methods and processing of animals, were approved by a Florida Fish and Wildlife Conservation Commission Special Activity License (SAL-15-1132A-SR), Florida Department of Environmental Protection State Park permits (06051410, 06261510, and 06231610), a Brevard County Environmentally Endangered Lands Program Research permit, and a Pinellas County Parks and Conservation Resources research permit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Online appendix (as one document) includes: Study organism, Experimental design, Seasonal variation in sex change, Relationship between sex change and fecundity, Identifying males, and Extended statistical model output. Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gresham, J.D., Marson, K.M., Tatarenkov, A. et al. Sex change as a survival strategy. Evol Ecol 34, 27–40 (2020). https://doi.org/10.1007/s10682-019-10023-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-019-10023-2